Abstract
Introduction
Hypermobility spectrum disorders (HSD) and hypermobile Ehlers-Danlos syndrome (hEDS) are often accompanied by varied and complex multisystemic comorbid symptoms/conditions. The Spider questionnaire was developed to evaluate the presence and impact of eight common multisystemic comorbidities. Thirty-one questions across eight symptom domains assess neuromusculoskeletal, pain, fatigue, cardiac dysautonomia, urogenital, gastrointestinal, anxiety, and depression symptoms. This study aimed to evaluate the Spider’s construct validity in adults.
Method
A cross-sectional observational study was conducted over four stages. Three international patient charities aided recruitment of participants through social media and website advertisements. Adults aged 18 to 65 years, with and without HSD/hEDS, were invited to participate. Validated, frequently used comparator questionnaires were used to establish convergent validity of Spider symptom domains. A control group was recruited for known-group validity analysis. Participants answered each Spider domain and the corresponding comparator questionnaire via surveys hosted by REDCap. Anonymous data were analysed using SPSS. Convergent validity was assessed through Spearman’s correlational analysis and known-group validity through Mann–Whitney U analysis.
Results
A total of 11,151 participants were recruited across the four stages. Statistically significant, moderate-to-strong correlations were found between all Spider domains and their comparators (p < 0.001, r = 0.63 to 0.80). Known-group validity analysis showed statistically significant differences (p < 0.001) between the hypermobile and control groups in all eight domains.
Conclusions
Convergent and known-group validity of the Spider was established with adults. These results suggest the Spider can measure the presence and impact of multisystemic comorbid symptoms/conditions in adults with HSD/hEDS, providing a tool which guides multidisciplinary management.
Key Points • The Spider questionnaire is a novel tool assessing the presence and impact of the multisystemic comorbid symptoms/conditions associated with HSD/hEDS. • Convergent and known-group validity of the Spider questionnaire was established in adults aged 18 to 65. • This tool provides a quick and easy method to visualise the symptom profile of those with HSD/hEDS to guide symptom management. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Joint hypermobility (JH) is defined as an increased active and passive range of joint motion, either locally or generally. The presence of JH is thought to be influenced by age, sex, and ethnicity and is often asymptomatic [1]. However, some adults with JH can experience debilitating symptoms across multiple body systems. Symptomatic hypermobility (SH) has been described using varying terminology, but since 2017, diagnostic criteria and guidelines created by the International Ehlers-Danlos Syndrome (EDS) consortium advise use of the terms ‘hypermobility spectrum disorder’ (HSD) and ‘hypermobile EDS’ (hEDS) [2]. Whilst these two conditions are differentiated during diagnosis, they present with similar and often complex multisystemic symptom profiles. Around 90% of patients with hEDS report generalised body or soft tissue pain, 78% report neuropathic pain [3], and up to 89% of individuals with HSD report chronic musculoskeletal pain [4]. Joint instability is common, with 78% of people with HSD/hEDS reporting dislocations [3]. Chronic fatigue affects 77% of individuals with HSD/hEDS [5]. In both patient groups, symptoms often extend beyond the musculoskeletal system. Gastrointestinal issues are prevalent, with symptoms such as abdominal pain, constipation, and diarrhoea reported by 47–73%, of people with HSD/hEDS, compared to 9–27% of individuals without HSD/hEDS [6]. Orthostatic intolerance, including postural orthostatic tachycardia syndrome (POTS), affects 60 to 80% of patients with HSD/hEDS, and around 25% of patients with POTS are also diagnosed with HSD/hEDS [7]. Urinary incontinence has been reported in 68–84% of women with HSD/hEDS compared to 25–45% of women in the general population, and genital pain/discomfort is reported by 60% of women with HSD [8]. Research suggests that approximately 70% of individuals with HSD/hEDS have some type of anxiety disorder and have a 4.1 to 4.4 times greater probability of being affected by depression [9] and anxiety [10], respectively, compared to the general population.
Whilst these symptoms are commonly reported by the HSD/hEDS community, the presence and impact of each symptom/condition vary greatly between individuals and throughout their lifetime, leading to a population of adults with diverse and complex symptom profiles [11]. The impact poor functioning of one body system may have on other systems is an important consideration and has been explored in some areas [11] [12] [13] [14]. It is becoming evident that the presence of these comorbid conditions influences the individual’s disease severity and level of disability [11]. Multisystemic symptoms can greatly impact quality of life (QoL) and people with HSD/hEDS often report poor health-related QoL (HRQoL) [15,16,17,18]. Those with multiple comorbid symptoms may have a worse prognosis and health status, particularly if symptoms are overlooked and left unmanaged [19, 20].
Whilst the exact prevalence of HSD/hEDS is difficult to quantify due to varying terminology and diagnostic criteria, it is thought to affect around 1 in 500 patients in the UK [21]. This suggests HSD/hEDS is not as rare as previously thought, but a common pathology which burdens healthcare services [21]. Patients with HSD/hEDS frequently require healthcare services; in the UK, 39% of patients in pain management and 37% of individuals treated by rheumatologists have HSD/hEDS [22]. Despite these large numbers of patients with HSD/hEDS seeking care, evidence suggests that clinician knowledge around the associated multisystem comorbidities is lacking [23]. Patients may not associate systemic symptoms with their HSD/hEDS, so unless clinicians ask about these symptoms during appointments, they may be missed and remain unmanaged [24]. Awareness of the presence and impact of these symptoms helps ensure care is holistic and patients receive appropriate multidisciplinary management, reducing functional impairment and disability.
To identify each individual’s symptom profile and help direct care, an international multidisciplinary group of researchers and clinicians developed the ‘Spider’ questionnaire. The Spider addresses the eight main symptom domains commonly associated with HSD/hEDS. These include pain, fatigue, neuromusculoskeletal symptoms, cardiac dysautonomia, urogenital and gastrointestinal symptoms, anxiety, and depression (supplementary material). This concise screening tool allows researchers, clinicians, and patients to assess the presence and impact of symptoms, easily identify areas of need, and guide management. The tool was validated for use in adolescents in 2024 [25] with acceptable convergent and known-group validity. The present study aims to establish the construct validity of the Spider domains in an adult population.
Materials and methods
Ethical approval was granted by the University College London Research Ethics Committee (19,629/002). A series of studies were undertaken to examine the construct validity (convergent and known-group validity) of the Spider domains with adults aged 18 to 65. As large numbers of questions were required for the validation of each domain, the validation was completed in four stages between July 2023 and November 2023 to reduce participant fatigue. A cross-sectional questionnaire research design was used. Each stage analysed the validity of one, two, or three Spider domains to evenly distribute the number of questions across the stages. The most appropriate validated comparator patient-rated outcome measures (PROMs) were used to assess the Spider’s convergent validity, chosen based on their concepts and psychometric properties.
-
Stage 1: The Spider neuromusculoskeletal, fatigue, and gastrointestinal domains were validated using select questions of the Bristol Impact of Hypermobility questionnaire (BIoH) [26], the Checklist for Individual Strength (CIS) [27], Pittsburgh Sleep Quality Index (PSQI) [28], and the Gastrointestinal Symptom Rating Scale (GSRS) [29] as comparators retrospectively.
-
Stage 2: The Spider anxiety and depression domains were validated using the General Anxiety Disorder (GAD-7) [30] and Patient Health Questionnaire (PHQ-9) [31] as comparators respectively. The Hospital Anxiety and Depression Scale (HADS) [32] was also used with 300 participants due to funding limitations.
-
Stage 3: The Spider cardiac dysautonomia domain was validated using select questions from the COMPASS-31 [33]. The Spider pain domain was validated using the Multidimensional Pain Inventory (MPI) [34] Sects. 1 and 3 and select questions from the BIoH [26].
-
Stage 4: The Spider urogenital domain was validated using the symptoms of Lower Urinary tract dysfunction Research Network (LURN) Symptom Index-29 (LURN SI-29) [35] as a comparator.
Participants
For all stages, participants were recruited using self-selection and snowball sampling. Three hypermobility charities (Hypermobility Syndromes Association, Ehlers-Danlos Support UK, and the Ehlers-Danlos Society) aided recruitment by posting study advertisements on their website, social media pages, or newsletters. Participants were asked to self-identify guided by inclusion criteria (Table 1) and encouraged to ask hypermobile and non-hypermobile family members or friends to participate. It was made clear in study advertisements that a healthy control group (no hypermobility) was also required for comparison. Participants were included if they had diagnoses of HSD and hEDS or had symptomatic hypermobility (SH), referred to collectively as the ‘SH-group’. The inclusion of those with SH was to ensure individuals who have comorbid symptoms and were awaiting official diagnoses of HSD/hEDS were not excluded from participating. The age range was set at 18 to 65, as after the age of 65, people are considered ‘elderly’ [36] and it becomes more difficult to determine if symptoms are associated with aging or HSD/hEDS. Whilst researchers recognise people do not biologically age at the same rate, as this research was conducted virtually with no means to assess frailty, an age limit of 65 was agreed after researching prevalence of multisystemic symptoms in those aged 65 and above [37,38,39,40,41].
Procedure
If participants self-identified as meeting the inclusion criteria for the SH or control group, they accessed the study questionnaires via an online survey hosting platform REDCap©. They were provided with study information and asked to consent to providing anonymous data. Participants provided demographic information (including age, sex assigned at birth, gender, ethnicity, location, diagnosis, and the clinician who provided the diagnosis) and answered the Spider domain and comparator questions. To differentiate between participants with and without SH, participants were asked to choose their diagnosis and who diagnosed them during demographic data collection. The options provided for diagnosis were ‘HSD’, ‘hEDS’, ‘SH’, and ‘I do not have any of the above conditions’. For the control group, the option of ‘not applicable, I do not have hypermobility’ was available for the question ‘professional who gave diagnosis’. Only participants choosing ‘I do not have any of the above conditions’ and ‘not applicable, I do not have hypermobility’ were included in the control group.
The Spider questionnaire
The Spider consists of 31 questions across eight symptom domains commonly associated with HSD/hEDS, including pain, fatigue, neuromusculoskeletal symptoms, cardiac dysautonomia, urogenital and gastrointestinal symptoms, and anxiety and depression (supplementary material). A six-point Likert scale is used to score answers, with ‘not present’ and ‘no impact on daily life’ scoring 0, and ‘mild’, ‘moderate’, ‘severe’, and ‘disabling’ impact scoring 25, 50, 75, and 100 respectively. Two questions have alternative scoring (q.19 and q.25) scored between 0 and 100. Each domain is scored by averaging the domain questions total, providing an overall domain percentage, with higher scores indicating higher impact on daily living. A radar graph is produced upon completion of the questionnaire, providing a visual overview of a patient’s symptom profile (Fig. 1). Further description of the development and initial validation of the tool are presented in the previous work by Ewer et al. [25]. Face and content validity was established with adults and adolescents and convergent and known-group validity was assessed in adolescents with HSD/hEDS [25].
An example of two completed questionnaires’ radar graphs [25]. Descriptive caption: a picture showing two diagrams, shaped like a Spider’s web, with eight symptom domains as described above. Within each diagram are points mapped to each symptom joined together by lines. The closer to the edge the point is, the larger the impact of this symptom for the individual. Each diagram has different points, demonstrating different symptom profiles
Data management and analysis
The IBM® SPSS® package version 29 was used for descriptive and inferential statistics calculations. Only complete data were analysed. Partially completed questionnaires were omitted from the data analysis. Convergent and known-group validity analyses were used to assess construct validity of each Spider domain. Normality of data was assessed through visual inspection of the histograms and Shapiro–Wilk test. Between-group differences were analysed using chi-square test and independent t-tests for categoric data or Mann–Whitney U for numeric data. Convergent validity was assessed through Spearman’s Rho analysis using data collected from the SH group. Convergent validity is deemed unacceptable if correlations are below r = 0.50, correlations of r > 0.6 are considered moderate, and r > 0.7 are considered strong [42]. A p-value of < 0.05 was considered statistically significant, and bootstrapping was used to calculate 95% confidence intervals for non-parametric tests. Known-group validity was calculated by comparing scores of the Spider domains from the SH and healthy control groups. Mann–Whitney U tests were used due to non-parametric data.
Results
Participants
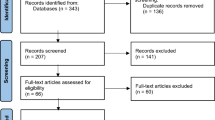
Across the four stages, there were a total of 11,151 participants included from an initial cohort of 14,926. There were 10,853 participants with SH and 298 healthy controls. A Prisma flow diagram (Fig. 2) shows the recruitment flow, including participant numbers across all four stages and per individual stage.
Participant recruitment flow diagram. Descriptive caption: a flow diagram showing the stages of recruitment from participants accessing the questionnaire, consenting (14,841) and completing the survey (11,151). Three thousand six hundred ninety participants were excluded if their responses were missing or incomplete or if they self-identified as the control group but reported symptomatic hypermobility
Demographic data are presented in Table 2. Across all stages, most patients were white, female, and from the United Kingdom (UK) or North America. In stages 1 to 4, there were 3206, 2115, 2934, and 2590 participants recruited in the SH group and 110, 79, 64, and 45 health controls respectively. There were significant between-group differences in sex and gender across all stages. In stage one, there were between-group differences in country of residence, and in stages 1, 2, and 4, there were also between-group differences in age.
Construct validity
Convergent validity results are shown in Table 3. All results were statistically significant (p < 0.001). Strong positive correlations were found between the Spider neuromusculoskeletal domain and select questions of the BIoH questionnaire (r = 0.73), between the Spider gastrointestinal domain and the GSRS questionnaire (r = 0.80), and between the Spider cardiac domain and the COMPASS-31 (r = 0.74). Strong correlations were also demonstrated between the Spider anxiety and depression domains and the GAD-7 and PHQ-9 (r = 0.73 and r = 0.79) respectively and between the Spider urogenital domain and the LURN- SI-29 (r = 0.78). Moderate positive correlations were found between the Spider fatigue domain and the combined scores from the CIS and PSQI (r = 0.63) and between the Spider anxiety and depression domains and the HADS anxiety and depression questions respectively (r = 0.67 and r = 0.69) with 300 of the participants. Moderate positive correlations were also found between the Spider pain domain and select questions from the MPI and BIoH (r = 0.62 and r = 0.68 respectively).
Known-group and descriptive statistics are shown in Table 4, and box and whisker plots showing the known-group analysis are shown in Fig. 3. There were statistically significant differences in all Spider domain scores between participants with SH and non-hypermobile controls (p < 0.001). Data is presented as mean (SD) or median (IQR) depending on normality. Large differences were found between the SH and control groups in the neuromusculoskeletal domain: 45.65 (SD 19.66) and 5 (IQR 0–15), gastrointestinal domain: 41.48 (SD 22.17) and 6.25 (IQR 0–25), and fatigue domain: 66.67 (IQR 50–83.33) and 16 (IQR 8.3–43.75) respectively. Large differences were also seen between the SH and control groups in the depression domain: 41.67 (IQR 25–58.33) and 16.67 (IQR 0–33.33), cardiac domain: 47.5 (IQR 28.75–66.25) and 11.8 (IQR 5–33.75), and pain domain: 51.93 (SD 20.37) and 18.75 (IQR 6.25–37.5). Moderate differences were found between the SH and control groups in the anxiety domain: 45.55 (SD 23.51) and 25 (IQR 8.33–50) and the urogenital domain: 30 (IQR 15.46.67) and 13.33 (IQR 0–30.83).
Box and whisker plots demonstrating differences between domain scores of SH and control groups. Descriptive caption: A figure showing eight box and whisker plots for each domain of the Spider questionnaire. The graph shows the difference between Spider domain scores of the symptomatic hypermobile group and the control group. The whiskers show the minimum and maximum values, the box shows the third quartile as the top line, the median as a line across the middle, and first quartile as a line at the bottom. The mean is represented by a cross, and outliers are represented by circles
Sub-group analysis
The authors recognised that the sample was predominantly white participants, which may not reflect the heterogeneity of the HSD/hEDS population. A secondary analysis was completed excluding white participants to assess the impact of ethnicity on the convergent validity results. The domains retained similar statistically significant (p < 0.001) moderate-to-strong correlations. In stage 1, 320 participants self-identifying as Black, Asian, mixed or multiple, or other ethnicities were included. In the NMSK, fatigue, and gastrointestinal domains, correlations of r = 0.71, r = 0.65, and r = 79 were found with the BIoH, CIS + PSQI, and the GSRS respectively. In stage 2, 198 participants were included in the sub-group analysis and correlations of r = 0.75 and r = 0.79 were found between the Spider anxiety and depression domains and the GAD-7 and PHQ-9. In stage 3, 326 participants were included, with correlations of r = 0.72, r = 0.72, and r = 0.69 found between the Spider cardiac domains and the COMPASS-31 and the Spider pain domain the MPI and the BIoH respectively. In stage 4, 291 participants were included in the sub-group analysis. A strong correlation of r = 0.76 was found between the Spider urogenital domain and the LURN-SI-29.
Discussion
This paper is the second in a series validating the Spider questionnaire, a novel, hypermobility-specific tool whose results provide insight into the multisystemic symptom profile of individuals with HSD/hEDS. This study found acceptable convergent and known-group validity for all Spider domains in a population of adults with SH. The results are in keeping with the previous validation study with adolescents, which found acceptable convergent validity in all domains and known-group validity in seven domains [25]. When correlating the Spider domains with validated, appropriate comparator questionnaires, the 95% confidence intervals remained above the recommended correlation of r > 0·5 in all domains [43]. These studies collectively demonstrate that the Spider can accurately capture the concept of the multisystemic symptoms/comorbidities in individuals aged 13 to 65 and in most domains distinguish between individuals with and without SH.
Strengths of the Spider
To the best of our knowledge, the Spider questionnaire is the first disease-specific tool to provide a broad, visual overview of the presence and impact of multisystemic symptoms and comorbidities that have consistently been associated with JH. The Spider is concise, capturing a broad clinical overview through only 31 questions, which were developed collaboratively by specialist clinicians, researchers, and patients. The questionnaire takes around 10 min to complete, without the need for multiple, time-consuming, and lengthy PROMs. This reduces the burden on both patients and clinicians and can be used without compromising patient energy or appointment time, which is particularly limited in government healthcare. It ensures that the individual is assessed holistically and that the non-musculoskeletal symptoms, which are often neglected during assessment, are considered when deciding multidisciplinary management. There is a lack of recognition of the multisystemic nature of HSD/hEDS, leading to unmet medical needs and impacting QoL greatly [24]. The Spider can be used by any member of the multidisciplinary team to guide the conversation between patients and clinicians, ensuring the presence and impact of multiple comorbid symptoms and conditions are considered when deciding and prioritising care. The Spider can also be completed independently by patients, which will help them in voicing their concerns and management priorities succinctly during healthcare appointments. Upon completion, a radar graph providing a visual overview of the symptom profile is created, which is easy to interpret.
Study strengths and limitations
Sampling procedure
As with the adolescent study, the use of international charities during recruitment resulted in a very large sample of international participants. Over 11,000 participants from various locations and ethnicities participated in total, with 2000 to 3000 participants per stage. Whilst there are often concerns with large sample sizes, such as increased study costs and researcher burden, syntax formulas created prior to data collection were used to complete data analysis without increasing analysis time. The larger sample size increases the likelihood that the study sample represents the HSD/hEDS population and improves generalisability of results. As with previous studies, the sample was largely white and female. Whilst HSD/hEDS are more common in females, the disparity is larger than reported in the literature [44]. The choice of sampling through self-selection may have introduced bias and limited the ethnic diversity of the sample. Researchers also encouraged snowball sampling, which may have increased homogeneity of the sample through referrals to friends or family with similar demographic characteristics. However, this recruitment did allow for inclusion of a broad, international participant sample which would not have been practical or feasible with other recruitment methods. To mitigate the impact of sample homogeneity on the results, a secondary sub-group analysis excluding white participants was completed and showed similar convergent validity correlations to the whole sample analysis. This suggests the findings of this research are still generalisable to other ethnic groups, despite the reduced representation in this sample.
Validation procedure
To establish convergent validity, comparator questionnaires measuring similar concepts are used and results are compared using correlational analysis. Whilst this is an accepted method of establishing validity, it must be acknowledged that there are currently no gold standard PROMs recommended as a comparator in hypermobile patient populations [45]. The choice of comparator questionnaires was guided by the recommendations and the Common Data Elements developed by the Ehlers-Danlos Society Internal Consortium, expertise of researchers and clinicians specialising in SH, and assessment of the psychometric properties of available PROMs [46]. As the nature of SH is complex, diverse, and varied, the research team used multiple measures to ensure all concepts measured in the Spider domains were captured. Instead of the full MPI, BIoH, and COMPASS-31 questionnaires, specific questions were selected to validate the pain, neuromusculoskeletal, and cardiac dysautonomia domains respectively. This decision was made due to the lack of validated comparator questionnaires which captured the specific symptoms that hypermobile patients present. In several domains, multiple PROMs were used as comparators. In the pain domain, the MPI sections A and C were used as a general measure of overall pain but select questions from the BIoH were required to assess the more specific symptoms associated with hypermobility. In the Spider anxiety and depression domain validation, the HADS questionnaire was used in conjunction with the GAD-7 and PHQ-9. The HADS has been used widely in hypermobility research, but as there are licensing costs, study funding could only accommodate its use with 300 participants. Large participant numbers were expected, and researchers did not want to limit the assessment of the anxiety and depression domains, so the GAD-7 and PHQ-9 were used as additional comparators. Whilst these are less commonly used in hypermobility research, they are validated with strong psychometric properties in other chronic health conditions [47, 48]. Finally, in the fatigue domain, the CIS was the preferred comparator, being widely used in hypermobility research [49,50,51], but it did not contain a question about sleep. As such, the PSQI was added to evaluate sleep quality and fatigue and the scores combined with the CIS.
Developments and further research
A cost-free digital application is being developed which can be accessed by clinicians and researchers internationally. It is anticipated that the Spider will allow monitoring of the natural history and interindividual variability of the multisystemic comorbidities associated with HSD/hEDS. This may enable future studies to identify determinates that influence prognosis and highlight treatment priorities. Future research possibilities include reliability analysis, including internal consistency, test–retest reliability, and factor structure analysis with adolescents and adults. Following this, minimal clinically important differences will be established so the Spider can be used to track meaningful symptom change and assess the effects of treatment. The Spider could be used to monitor the effect of multidisciplinary interventions and rehabilitation programmes on the whole symptom profile. The Spider will be translated using forward-backwards translation to ensure it is accessible internationally. Finally, the research team predict the Spider has the potential to be developed with other heritable connective tissue disorders.
Conclusion
This study adds to a body of work establishing psychometric properties of the Spider, a unique and symptom-specific multisystemic questionnaire for people with SH. Statistically significant, moderate-to-strong convergent validity and statistically significant known-group validity were shown in all questionnaire domains. The Spider is a novel, concise, efficient, and valid tool, freely accessible to clinicians and researchers to assess the multisystemic symptom profiles of people aged 13 to 65 with SH. The questionnaire enables clinicians to ensure patient care is individualised, holistic, and tailored, to meet the specific needs of the individual with SH.
Data availability
Authors had full control over all primary data. The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Beighton P, Grahame R, Bird H (1989). In: Grahame R (ed) Hypermobility of joints, 2nd edn. Springer, London
Castori M, Tinkle B, Levy H, Grahame R, Malfait F, Hakim A (2017) A framework for the classification of joint hypermobility and related conditions. Am J Med Genet C Semin Med Genet 175(1):148–157. https://doi.org/10.1002/ajmg.c.31539
Chopra P, Tinkle B, Hamonet C, Brock I, Gompel A, Bulbena A et al (2017) Pain management in the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet 175(1):212–219. https://doi.org/10.1002/ajmg.c.31554
Scheper MC, de Vries JE, Verbunt J, Engelbert RH (2015) Chronic pain in hypermobility syndrome and Ehlers-Danlos syndrome (hypermobility type): it is a challenge. J Pain Res 8:591–601. https://doi.org/10.2147/jpr.S64251
Ruiz Maya T, Fettig V, Mehta L, Gelb BD, Kontorovich AR (2021) Dysautonomia in hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders is associated with exercise intolerance and cardiac atrophy. Am J Med Genet A 185(12):3754–3761. https://doi.org/10.1002/ajmg.a.62446
Thwaites PA, Gibson PR, Burgell RE (2022) Hypermobile Ehlers-Danlos syndrome and disorders of the gastrointestinal tract: what the gastroenterologist needs to know. J Gastroenterol Hepatol 37(9):1693–1709. https://doi.org/10.1111/jgh.15927
Tai FWD, Palsson OS, Lam CY, Whitehead WE, Sperber AD, Tornblom H et al (2020) Functional gastrointestinal disorders are increased in joint hypermobility-related disorders with concomitant postural orthostatic tachycardia syndrome. Neurogastroenterol Motil 32(12):e13975. https://doi.org/10.1111/nmo.13975
Gilliam E, Hoffman JD, Yeh G (2020) Urogenital and pelvic complications in the Ehlers-Danlos syndromes and associated hypermobility spectrum disorders: a scoping review. Clin Genet 97(1):168–178. https://doi.org/10.1111/cge.13624
Smith TO, Easton V, Bacon H, Jerman E, Armon K, Poland F et al (2014) The relationship between benign joint hypermobility syndrome and psychological distress: a systematic review and meta-analysis. Rheumatology 53(1):114–122. https://doi.org/10.1093/rheumatology/ket317
Pasquini M, Celletti C, Berardelli I, Roselli V, Mastroeni S, Castori M et al (2014) Unexpected association between joint hypermobility syndrome/Ehlers-Danlos syndrome hypermobility type and obsessive-compulsive personality disorder. Rheumatol Int 34(5):631–636. https://doi.org/10.1007/s00296-013-2901-2
National Academies of Sciences EaM (2022). In: Wedge RA, Cartaxo T, Spicer CM, Volberding PA (eds) Selected heritable disorders of connective tissue and disability. National Academies Press, Washington (DC)
Krahe AM, Adams RD, Nicholson LL (2018) Features that exacerbate fatigue severity in joint hypermobility syndrome/Ehlers-Danlos syndrome - hypermobility type. Disabil Rehabil 40(17):1989–1996. https://doi.org/10.1080/09638288.2017.1323022
Wasim S, Suddaby JS, Parikh M, Leylachian S, Ho B, Guerin A et al (2019) Pain and gastrointestinal dysfunction are significant associations with psychiatric disorders in patients with Ehlers-Danlos syndrome and hypermobility spectrum disorders: a retrospective study. Rheumatol Int 39(7):1241–1248. https://doi.org/10.1007/s00296-019-04293-w
Fikree A, Aziz Q, Sifrim D (2017) Mechanisms underlying reflux symptoms and dysphagia in patients with joint hypermobility syndrome, with and without postural tachycardia syndrome. Neurogastroenterol Motil. 29(6) https://doi.org/10.1111/nmo.13029.
Orenius T, Kautiainen H, Louhi M, Montin L, Bulbena A, Lindgren K-A (2022) Health-related quality of life and psychological distress in patients with hypermobility type Ehlers-Danlos syndrome. SAGE Open 12(2):21582440221091236. https://doi.org/10.1177/21582440221091237
Pacey V, Tofts L, Adams RD, Munns CF, Nicholson LL (2015) Quality of life prediction in children with joint hypermobility syndrome. J Paediatr Child Health 51(7):689–695. https://doi.org/10.1111/jpc.12826
Berglund B, Pettersson C, Pigg M, Kristiansson P (2015) Self-reported quality of life, anxiety and depression in individuals with Ehlers-Danlos syndrome (EDS): a questionnaire study. BMC Musculoskelet Disord 16(1):89. https://doi.org/10.1186/s12891-015-0549-7
Sahin E, Gulbahar S, Baydar M, OzcanSoylev G, Bircan C, El O et al (2015) Impaired quality of life and functional status in patients with benign joint hypermobility syndrome. MYOPAIN 23(1–2):28–33. https://doi.org/10.3109/10582452.2016.1143074
De Wandele I, Rombaut L, Malfait F, De Backer T, De Paepe A, Calders P (2013) Clinical heterogeneity in patients with the hypermobility type of Ehlers-Danlos syndrome. Res Dev Disabil 34(3):873–881. https://doi.org/10.1016/j.ridd.2012.11.018
Scheper MC, Pacey V, Rombaut L, Adams RD, Tofts L, Calders P et al (2017) Generalized hyperalgesia in children and adults diagnosed with hypermobility syndrome and Ehlers-Danlos syndrome hypermobility type: a discriminative analysis. Arthritis Care Res (Hoboken) 69(3):421–429. https://doi.org/10.1002/acr.22998
Demmler JC, Atkinson MD, Reinhold EJ, Choy E, Lyons RA, Brophy ST (2019) Diagnosed prevalence of Ehlers-Danlos syndrome and hypermobility spectrum disorder in Wales, UK: a national electronic cohort study and case–control comparison. BMJ Open 9(11):e031365. https://doi.org/10.1136/bmjopen-2019-031365
To M, Simmonds J, Alexander C (2017) Where do people with joint hypermobility syndrome present in secondary care? The prevalence in a general hospital and the challenges of classification. Musculoskeletal Care 15(1):3–9. https://doi.org/10.1002/msc.1147
Russek LN, LaShomb EA, Ware AM, Wesner SM, Westcott V (2016) United States physical therapists’ knowledge about joint hypermobility syndrome compared with fibromyalgia and rheumatoid arthritis. Physiother Res Int 21(1):22–35. https://doi.org/10.1002/pri.1613
Russek LN, Stott P, Simmonds J (2019) Recognizing and effectively managing hypermobility-related conditions. Phys Ther 99(9):1189–1200. https://doi.org/10.1093/ptj/pzz078
Ewer E, Kazkaz H, Ninis N, Rowe P, De Pauw R, Tang E et al (2024) The Spider; a multisystemic symptom impact tool for people with hypermobility related disorders. Initial validation in adolescents. The J Pediatr: Clin Pract 12:200098. https://doi.org/10.1016/j.jpedcp.2024.200098
Palmer S, Cramp F, Lewis R, Gould G, Clark EM (2017) Development and initial validation of the Bristol Impact of Hypermobility questionnaire. Physiotherapy 103(2):186–192. https://doi.org/10.1016/j.physio.2016.04.002
Vercoulen JH, Swanink CM, Fennis JF, Galama JM, van der Meer JW, Bleijenberg G (1994) Dimensional assessment of chronic fatigue syndrome. J Psychosom Res 38(5):383–392. https://doi.org/10.1016/0022-3999(94)90099-x
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28(2):193–213. https://doi.org/10.1016/0165-1781(89)90047-4
Svedlund J, Sjödin I, Dotevall G (1988) GSRS–a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci 33(2):129–134. https://doi.org/10.1007/bf01535722
Spitzer RL, Kroenke K, Williams JB, Löwe B (2006) A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 166(10):1092–1097. https://doi.org/10.1001/archinte.166.10.1092
Kroenke K, Spitzer RL, Williams JB (2001) The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 16(9):606–613. https://doi.org/10.1046/j.1525-1497.2001.016009606.x
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67(6):361–370. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x
Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W (2012) COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc 87(12):1196–1201. https://doi.org/10.1016/j.mayocp.2012.10.013
Kerns RD, Turk DC, Rudy TE (1985) The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain 23(4):345–356. https://doi.org/10.1016/0304-3959(85)90004-1
Cella D, Smith AR, Griffith JW, Flynn KE, Bradley CS, Gillespie BW et al (2019) A new outcome measure for LUTS: symptoms of Lower Urinary Tract Dysfunction Research Network Symptom Index-29 (LURN SI-29) questionnaire. Neurourol Urodyn 38(6):1751–1759. https://doi.org/10.1002/nau.24067
Institute of Medicine Committee to Design a Strategy for Quality R, Assurance in M, (1990). In: Lohr KN (ed) Medicare: a strategy for quality assurance, vol 1. National Academies Press, Washington (DC)
Barbour KE, Helmick CG, Boring M (2017) Brady TJ (2017) Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation — United States, 2013-2015. Morb Mortal Wkly Rep 66:246–253. https://doi.org/10.15585/mmwr.mm6609e1External
Verdejo C, Méndez S, Salinas J (2016) Urinary tract dysfunction in older patients. Medicina Clínica (English Edition) 147(10):455–460. https://doi.org/10.1016/j.medcle.2016.12.006
Parashar R, Amir M, Pakhare A, Rathi P, Chaudhary L (2016) Age related changes in autonomic functions. J Clin Diagn Res. 10(3):Cc11–Cc15. https://doi.org/10.7860/jcdr/2016/16889.7497
Sushma S, Rao MY, Aslam SM (2021) Assessment of functions of the autonomic nervous system in the elderly with different comorbid factors. J Neurosci Rural Pract 12(1):80–87. https://doi.org/10.1055/s-0040-1718854
Lin W-Q, Jing M-J, Tang J, Wang J-J, Zhang H-S, Yuan L-X et al (2015) Factors associated with fatigue among men aged 45 and older: a cross-sectional study. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph120910897
Carlson KD, Herdman AO (2010) Understanding the impact of convergent validity on research results. Organ Res Methods 15(1):17–32. https://doi.org/10.1177/1094428110392383
Abma IL, Rovers M, van der Wees PJ (2016) Appraising convergent validity of patient-reported outcome measures in systematic reviews: constructing hypotheses and interpreting outcomes. BMC Res Notes 9:226. https://doi.org/10.1186/s13104-016-2034-2
Castori M, Camerota F, Celletti C, Grammatico P, Padua L (2010) Ehlers-Danlos syndrome hypermobility type and the excess of affected females: possible mechanisms and perspectives. Am J Med Genet A 152a(9):2406–2408. https://doi.org/10.1002/ajmg.a.33585
Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL et al (2010) The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res 19(4):539–549. https://doi.org/10.1007/s11136-010-9606-8
TheEhlersDanlosSociety (2017) The international consortium on Ehlers-Danlos syndromes and related disorders. https://www.ehlers-danlos.com/2018-cdes/. Accessed 03/01/2024
Bisby MA, Karin E, Scott AJ, Dudeney J, Fisher A, Gandy M et al (2022) Examining the psychometric properties of brief screening measures of depression and anxiety in chronic pain: the Patient Health Questionnaire 2-item and Generalized Anxiety Disorder 2-item. Pain Pract 22(4):478–486. https://doi.org/10.1111/papr.13107
Hitchon CA, Zhang L, Peschken CA, Lix LM, Graff LA, Fisk JD et al (2020) Validity and reliability of screening measures for depression and anxiety disorders in rheumatoid arthritis. Arthritis Care Res (Hoboken) 72(8):1130–1139. https://doi.org/10.1002/acr.24011
Liaghat B, Skou ST, Sondergaard J, Boyle E, Søgaard K, Juul-Kristensen B (2022) Clinical characteristics of 100 patients with hypermobility spectrum disorders and shoulder complaints with or without mechanical symptoms: a cross-sectional study. Arch Phys Med Rehabil 103(9):1749–57.e4. https://doi.org/10.1016/j.apmr.2021.12.021
De Wandele I, Rombaut L, De Backer T, Peersman W, Da Silva H, De Mits S et al (2016) Orthostatic intolerance and fatigue in the hypermobility type of Ehlers-Danlos syndrome. Rheumatology (Oxford) 55(8):1412–1420. https://doi.org/10.1093/rheumatology/kew032
De Wandele I, Calders P, Peersman W, Rimbaut S, De Backer T, Malfait F et al (2014) Autonomic symptom burden in the hypermobility type of Ehlers-Danlos syndrome: a comparative study with two other EDS types, fibromyalgia, and healthy controls. Semin Arthritis Rheum 44(3):353–361. https://doi.org/10.1016/j.semarthrit.2014.05.013
Acknowledgements
The research team would like to sincerely thank Ehlers-Danlos Support UK, the Ehlers-Danlos Society, and the Hypermobility Syndromes Association for aiding recruitment at each stage of this research. Secondly, we would like to thank the Ehlers-Danlos Society, the Association of Paediatric Chartered Physiotherapists (APCP), and the Private Physiotherapy Education Fund (PPEF) for continuing to support ongoing research. Finally, we want to express our gratitude to all patients and participants who were involved in the creation and validation of the Spider questionnaire, which we hope will have a positive impact on future symptom management for people with hypermobility-related conditions.
Funding
This research was funded by the Ehlers-Danlos Society, the Association of Paediatric Chartered Physiotherapists (APCP), and the Private Physiotherapy Education Fund (PPEF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Ethics approval statement
Ethical approval was granted by the University College London Research Ethics Committee for all research projects.
Patient consent statement
Informed consent was gained from all participants prior to accessing the questionnaire.
Permission to reproduce material from other sources
Permission was granted for use of all comparator outcome measures by the creators of the tools for use within this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ewer, E.R., De Pauw, R., Kazkazk, H. et al. The Spider: a visual, multisystemic symptom impact questionnaire for people with hypermobility-related disorders—validation in adults. Clin Rheumatol 43, 3005–3017 (2024). https://doi.org/10.1007/s10067-024-07071-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-024-07071-7