Abstract
Periodic fever syndromes (PFS) are a group of autoinflammatory diseases characterized by repeated febrile episodes and systemic inflammation. The most common monogenic periodic fever syndromes are familial Mediterranean fever, mevalonate kinase deficiency/hyper immunoglobulin D syndrome, cryopyrin-associated periodic syndrome, and tumor necrosis factor receptor-associated periodic syndrome. Although fever is the predominant feature of PFS, other systems, including the cardiovascular system, may be involved in the disease process. This review focuses on cardiovascular risks and issues in monogenic PFS. Cardiovascular involvement may occur as a disease manifestation, association, or result of complications or a drug’s adverse effects in monogenic PFS. Pericarditis seems to be a feature of PFS. Patients with recurrent pericarditis or pericarditis resistant to conventional treatment should be evaluated for PFS. Amyloidosis is the most severe complication of PFS, increasing the risk of cardiac morbidity. Furthermore, ongoing inflammation may result in early atherosclerosis. Therefore, assessing cardiovascular risks in PFS patients should be considered a part of routine care.
Key points • Pericarditis is the most common cardiac involvement of monogenic periodic fever syndromes (PFS), while some forms may present with myocarditis. • Amyloidosis, the most significant complication of PFS, may lead to deterioration in cardiac functions. • Ongoing inflammation in PFS may result in endothelial dysfunction and atherosclerosis. • Effective control of inflammation and reducing concomitant risk factors such as obesity, diabetes mellitus, and hypertension could improve cardiovascular outcomes in PFS patients. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodic fever syndromes (PFS) are a group of autoinflammatory disorders that are mediated by the overactivation of the innate immune system without the presence of autoreactive antibodies or antigen-specific T cells [1]. Periodic fever syndromes are a diagnostic spectrum that includes illnesses with Mendelian inheritance and diseases with complex modes of inheritance [1]. The clinical manifestations of PFS consist mainly of repeated febrile episodes lasting for a few days to a few weeks, accompanied by systemic inflammation.
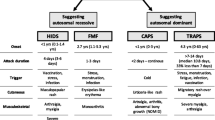
The most common monogenic PFS is familial Mediterranean fever (FMF) [2]. The other relatively more common ones are mevalonate kinase deficiency (MKD)/hyper immunoglobulin D syndrome (HIDS), cryopyrin-associated periodic syndrome (CAPS), and tumor necrosis factor receptor-associated periodic syndrome (TRAPS). Clinical features of these syndromes differ; however, genetic analysis is generally required to verify the ultimate diagnosis.
Cardiovascular findings are commonly present in PFS. However, there are limited data on cardiovascular issues in PFS. Therefore, this review aims to provide an overview of cardiovascular involvement and cardiovascular risk factors in monogenic PFS. We will focus on the more common PFS such as FMF, MKD/HIDS, CAPS, and TRAPS.
Search strategy
A review of the literature was retrieved from Web of Science, Scopus, and MEDLINE/PubMed databases until December 2022, according to the published guidance on narrative reviews [3], by using the following keywords: “familial Mediterranean fever,” “mevalonate kinase deficiency,” “hyper immunoglobulin D syndrome,” “cryopyrin-associated periodic syndrome,” “tumor necrosis factor receptor-associated periodic syndrome,” “cardiac involvement,” and “cardiovascular disease.” The search was restricted to English-language articles. The publications that provided data about cardiovascular issues in monogenic PFS were included. The reference lists of the included articles were also examined in detail. Priority was frequently given to the papers published within the last decade.
Familial Mediterranean fever
Familial Mediterranean fever (FMF), which is due to gain-of-function mutations of the MEditerranean FeVer (MEFV) gene, is the prototype of PFS [4]. The pyrin protein encoded by the MEFV gene is involved in the activation of the caspase-1 enzyme and the production of interleukin (IL)-1-β [4]. Pyrin is expressed in monocytes, granulocytes, and dendritic cells in the serosal membranes, such as peritoneal, pleural, and pericardial. Pyrin plays a role in converting pro-IL-1β molecule into its active form IL-1β, which is a significant mediator of fever and inflammation. Despite being categorized as an autosomal recessive condition, the FMF phenotype can be present in people carrying just one mutation [5].
Colchicine forms the mainstay of FMF treatment [6]. It prevents febrile episodes, subclinical inflammation, and secondary amyloidosis. Most of the patients with FMF demonstrate a complete response, while 5–10% are unresponsive or intolerant to colchicine therapy. Colchicine resistance is generally defined as perpetuating disease activity as frequent attacks (≥1 attack per month) or subclinical inflammation despite a maximum tolerated dose of colchicine for 3–6 months [7]. Since interleukin-1 (IL-1) is the main cytokine responsible for the inflammation in FMF, anti-IL-1 agents emerge as a potential therapeutic alternative in colchicine-resistant FMF cases [7].
Patients with FMF usually manifest with recurrent, unprovoked, and self-limiting episodes of fever and serositis [8]. Cardiovascular issues in FMF include cardiac involvement of FMF as pericardial disease, increased frequency of comorbid vascular disorders (mainly vasculitis), cardiovascular effects secondary to inflammation such as atherosclerosis, cardiovascular effects due to secondary amyloidosis, and side effects of FMF treatment (Fig. 1) (Table 1).
Cardiac involvement of FMF
Pericardial disease
Pericarditis is the inflammation of the pericardium. In the presence of pericarditis, patients present with sudden onset of sharp chest pain worsening by lying down or deep breathing. The diagnosis of pericarditis is based on symptoms. In case of pericarditis, the pain is characteristically retrosternal, which is different from unilateral chest pain due to pleuritis in FMF attacks [9]. The electrocardiogram reveals elevated ST, and a chest X-ray or echocardiography may confirm the evidence of pericardial fluid accumulation.
Compared with other serositis types, recurrent pericarditis is infrequent in FMF [10]. Usually, pericarditis is seen in the late course of FMF [10]. A study including children and adults showed that the mean age at the first pericardial attack and the first FMF attack was 30 ± 10.8 years and 13 ± 7.96 years, respectively [10]. However, recurrent pericarditis may be FMF’s initial or sole manifestation [11]. FMF has even been reported in pediatric cases who had cardiac tamponade at disease onset [12,13,14].
Many studies have confirmed that pericarditis is more common in FMF patients than in healthy individuals [15]. A nationwide multicenter study from Turkey evaluating 2468 pediatric and adult FMF patients reported 60 (2.4%) cases, presenting with at least one episode of pericarditis during their disease course. Among these 60 patients, 34 had definite, and 26 had probable pericarditis [16]. In another study including pediatric and adult FMF patients (n=4000) from Israel, the rate of pericarditis was reported as 0.6% [10]. Kilic et al. [17] evaluated the association between clinical findings and genetic variants in pediatric FMF patients. They showed an increased frequency of chest pain among patients carrying homozygous M694V and heterozygous E148Q variants. Pericardial effusion was detected by using echocardiography in 10.9% of patients suffering from chest pain. Another pediatric study by Salah et al. [18] reported an association between pericardial effusions and the presence of E148Q, P369S, and V726A variants. Tutar et al. [19] found that the frequency of pericardial effusion during the FMF attacks was 3.6% in their study including adult and pediatric patients. With the increase in the usage of echocardiography as a diagnostic tool in clinical practice, pericardial disease may be more frequently diagnosed. Most recently, the rate of pericarditis in FMF patients was 0.6% in a large pediatric cohort, and an increased frequency of chest pain among children with homozygous or compound heterozygous mutations in exon 10 was observed [20]. In patients with recurrent attacks of pericarditis or pericarditis refractory to standard treatment, FMF should be sought for, especially in endemic regions.
Increased frequency of comorbid vascular diseases
Vasculitides
Increased susceptibility to various systemic inflammatory diseases has been reported in FMF patients [21]. The high prevalence of vasculitides, such as polyarteritis nodosa (PAN) and immunoglobulin-A vasculitis (IgAV), was demonstrated in pediatric and adult FMF patients [21]. A systematic review showed that the most prevalent vasculitis in FMF patients was IgAV (prevalence: 2.7–7%), followed by PAN (prevalence: 0.9–1.4%) [22]. Patients with IgAV and FMF share the same phenotype with isolated IgAV except for intussusception. Increased frequency of intussusception in patients with IgAV and FMF (8.7%) was reported compared to the patients with isolated IgAV [22]. Also, recurrent IgAV suggests FMF comorbidity [23]. Polyarteritis nodosa is a necrotizing vasculitis, predominantly affecting medium-sized vessels. Patients with FMF-associated PAN and patients with isolated PAN express some distinctive features. FMF-associated PAN is associated with a younger age at disease onset, increased frequency of central nervous system involvement, and perirenal hematoma [22]. Cardiac involvement, including coronary vasculitis, is a significant feature of PAN. However, cardiac involvement is less common in patients with FMF-associated PAN than in patients with PAN alone (6.6% vs. 20.4%) [22, 24]. Behçet’s disease (BD) is a vasculitis affecting arteries and veins of any size. The association between FMF and BD is not clear. Ben-Chetrit et al. [25] suggested that BD and FMF were two distinct diseases with a mildly high trend that cannot be verified to have a definite relationship. However, interestingly, carriage of MEFV mutation was found to cause a 2.5-fold increase in BD risk in the Turkish population [26].
Cardiovascular effects of inflammation in FMF
Endothelial dysfunction and atherosclerosis
Inflammatory diseases may display predisposition factors for early atherosclerosis [15]. Atherosclerosis is a form of chronic inflammation caused by the interaction between macrophages, T cells, modified lipoproteins, and the cellular elements of the arterial wall [27]. Ongoing low-grade inflammation in FMF may result in endothelial dysfunction and vascular damage. The atherogenic index is calculated by dividing plasma high-density lipoprotein (HDL) to triglyceride (TG) levels, and some studies showed an increased atherogenic index in adult FMF patients [28,29,30]. Furthermore, elevated mean platelet volume, a risk marker for atherosclerosis, was also observed in pediatric and adult FMF patients [31]. A pediatric study by Yel et al. [32] showed increased endothelial microparticles (EMPs) as a marker of endothelial dysfunction in FMF attacks, while EMPs were similar to healthy controls in the attack-free period. They concluded that uncontrolled disease might be the source of endothelial dysfunction and early atherosclerosis [32]. Correspondingly, previous adult studies showed elevated endothelial dysfunction markers such as asymmetric dimethyl arginine (ADMA) and endocan in patients with FMF [33, 34].
Akdogan et al. [35] reported impaired flow-mediated dilation of the brachial artery and increased carotid intima-media thickness (CIMT) in FMF patients compared with healthy controls. They noticed an increased atherosclerosis risk in FMF patients [35]. Similarly, increased CIMT was demonstrated in several studies, including children with FMF [36, 37]. It is hypothesized that persistent inflammation results in a rapid proliferation of low-density lipoprotein (LDL) and cholesterol in the intima of arterial lumen and lipid plaque formation. Increased CIMT levels were positively correlated with serum amyloid A (SAA), the most sensitive laboratory test for detecting subclinical inflammation in FMF patients [36, 37]. However, some conflicting results have also been published. Sari et al. [38] showed that CIMT measurements in adult FMF patients did not differ from healthy controls. Different results could be due to the diversity in disease severity among study participants.
Arterial stiffness is the rigidity of the arterial wall, which may be the early sign of atherosclerosis. Pulse wave velocity is a technique that evaluates arterial elasticity. Pulse wave velocity measurement may help the detection of impaired arterial elasticity. Some studies concluded that patients with FMF showed increased pulse wave velocity compared to the control group [39, 40]. Evaluation of epicardial adipose thickness is another sensitive method for detecting subclinical atherosclerosis. The myocardium is surrounded by epicardial adipose tissue, which positively correlates with cholesterol and TG levels. The thickness of this tissue increases in many diseases, such as obesity, metabolic syndrome, and diabetes mellitus. A recent meta-analysis by Motawea et al. [41] showed that epicardial adipose tissue thickening and atherosclerosis risks were elevated in patients with FMF. Basar et al. [42] suggested that carrying MEFV mutations may predispose to early coronary heart disease. Caliskan et al. [43] reported impaired coronary microvascular function and left ventricular diastolic function in adult patients with FMF. However, data on the acute coronary syndrome in FMF patients have been restricted to case reports and autopsy studies [44, 45].
Colchicine, the mainstay treatment of FMF, has an anti-atherosclerotic effect that may be attributed to slowing vascular damage down. Correspondingly, a study from Israel [46] showed that ischemic heart disease prevalence in adult FMF patients was lower compared to the age-matched general population. In this study, the authors highlighted the cardioprotective effect of colchicine. In contrast to this study, Gendelman et al. [47] showed an elevated risk for ischemic heart disease and mortality in FMF patients.
Conduction system disorders
In healthy individuals, the autonomic nervous system (ANS) has a significant role in regulating the cardiovascular system by ensuring optimal function during various activities. Several non-invasive methods are used to assess ANS, such as heart rate turbulence, heart rate variability (HRV), heart rate recovery index, and QT dynamics. Systemic inflammation may cause dysautonomia [48]. Since FMF is an inflammatory disorder, evaluating the ANS in FMF patients has become an area of interest. Autonomic dysfunction without clinical symptoms has been reported in adult FMF patients [49, 50]. However, the association between FMF and ANS is controversial. Rozenbaum et al. [50] evaluated the autonomic responses of adult patients with FMF by tilt table testing. Patients with FMF showed significantly different heart rate and blood pressure responses to postural challenges compared to healthy individuals. Another study by the same group showed an increased cardiovascular reactivity score among adult FMF patients related to abnormal ANS [49].
Furthermore, deterioration in the heart rate recovery index in both children and adults with FMF has been reported [51,52,53]. In contrast to these studies, Sahin et al. [54] did not find any difference in heart rate recovery between pediatric FMF patients and healthy controls. Nussinovitch et al. [55, 56] demonstrated normal HRV parameters in both colchicine-resistant and colchicine-responsive adult FMF patients without amyloidosis, while they detected HRV abnormalities in adult FMF patients complicated with amyloidosis [57]. The discrepancy between studies on ANS function in FMF may be related to the different characteristics of patients and the degree of inflammation.
Researchers also evaluated the conduction system abnormalities in FMF patients. The QT dispersion is a value measurement to predict the risk for ventricular arrhythmia. However, studies focusing on QT dispersion in FMF patients showed debatable results. Some studies revealed that cardiac repolarization indices were similar regardless of colchicine response between adult FMF patients and healthy controls [58,59,60]. In contrast, some others demonstrated that QT interval was longer in both pediatric and adult FMF patients than healthy subjects [61, 62]. In a recent study, Farag et al. [62] have demonstrated that FMF patients had an increased risk of arrhythmia [62]. In their study, some cardiac repolarization abnormalities were associated with FMF disease severity markers, suggesting that better inflammation control may help prevent arrhythmia in these patients [62]. Despite these findings, current knowledge does not lead to conclusion on a definite interaction between the FMF and the conduction system.
Cardiovascular effects due to FMF-associated secondary (AA) amyloidosis
Secondary AA amyloidosis, which could cause chronic renal failure, is the most severe consequence of FMF. With increased awareness and better medical care, the frequency of secondary amyloidosis has gradually decreased during the last two decades [63]. A recent pediatric study from Turkey reported the amyloidosis rate as 0.3% in a large FMF cohort [20]. FMF-related AA amyloidosis also increases the prevalence of cardiac complications and mortality. Patients with AA amyloidosis may have cardiomyopathy due to amyloid deposition in the myocardium.
Furthermore, renal failure due to amyloidosis may also assist the progression of cardiovascular involvement. Amyloid deposition in the myocardium and heart valves may lead to heart failure, while accumulation in coronary arteries may result in myocardial infarction [44]. Yılmaz et al. [64] showed elevated ADMA levels and impaired brachial flow-mediated dilatation in adult patients with FMF-related amyloidosis. They concluded that amyloidosis related to FMF resulted in endothelial dysfunction and increased the cardiovascular disease event risk [64]. In a study by Ambartsymian [44], 68 FMF patients aged 15–65 years who died from amyloid-induced congestive heart failure were included, and the autopsy materials were investigated. They suggested that cardiac failure might have developed before renal amyloidosis and uremia. Therefore, early detection of subclinical changes in myocardial tissue is quite essential. Bozaci and Tatar [65] proposed that azurocidin might be a predictor of both inflammatory state and risk of cardiovascular disease in adult FMF patients with amyloidosis. Recently, M694V homozygosity has been related to cardiovascular disease risk elevation in FMF patients with amyloidosis [66].
Doppler imaging or strain echocardiography studies may be more sensitive than conventional echocardiography studies while evaluating cardiac functions. For instance, Ceylan et al. [67] confirmed that Doppler and strain echocardiography might detect subclinical changes when conventional echocardiography was normal. Erken Pamukcu et al. [68] also assessed ventricular systolic and diastolic functions with speckle-tracking echocardiography in adult FMF patients. They reported lower values of right ventricular global longitudinal strain and higher myocardial performance index in FMF patients suggesting subclinical right ventricular deterioration. A study by Celik et al. [69] also showed impaired Doppler-derived diastolic index in FMF patients aged 29 ±12 years. However, all these studies are limited by a small sample size.
Side effects of FMF treatment on the cardiovascular system
Colchicine, which prevents inflammatory flares and the development of amyloidosis, is the primary drug in the treatment of FMF. It is metabolized by cytochrome P450. In addition to its use in rheumatology, colchicine has been used in the treatment of many cardiovascular diseases, such as recurrent pericarditis, coronary artery disease, atherosclerosis, vascular restenosis, myocardial infarction, and heart failure [70, 71]. Furthermore, a definite benefit of colchicine has been demonstrated in the cardiovascular outcomes of adults who had a myocardial infarction during the last 30 days in the COLCOT (Colchicine Cardiovascular Outcomes Trial) [72]. However, colchicine itself may also lead to an increased occurrence of ventricular tachyarrhythmias [73]. Frommeyer et al. [73] showed the pro-arrhythmic effect of colchicine in rabbits. They found that colchicine treatment increased ventricular fibrillation inducibility in a dose-dependent way. Interestingly, unlike the animal models, Ocal et al. [74] showed that colchicine treatment had a favorable effect on ventricular repolarization, while another study by Nussinovitch et al. [75] reported that FMF patients receiving colchicine showed normal total cosine R to T (TCRT) (a repolarization marker) analysis.
In patients with a resistance or intolerance to colchicine, anti-IL-1 drugs are used in the management [6]. To date, there is no relevant data on side effects of anti-IL-1 drugs on cardiac system. In systemic juvenile idiopathic arthritis patients treated with anti-IL-1 drugs, fatal lung disease and pulmonary hypertension were reported [76]. A recent study has shown that this could be the result of a severe delayed hypersensitivity reaction named “drug reaction with eosinophilia and systemic symptoms” (DRESS), due to exposure to anti-IL-1 or anti-IL-6 drugs [77]. Furthermore, a specific HLA haplotype, HLA-DRB1*15, is associated with an increased risk of this reaction in case of biologic exposure. Checking for the mentioned haplotype could be considered in PFS patients before initiating anti-IL-1 therapies.
Other monogenic periodic fever syndromes and cardiovascular involvement
Mevalonate kinase deficiency/hyper immunoglobulin D syndrome (MKD/HIDS)
Mevalonate kinase deficiency (MKD) is an autosomal recessive disease caused by mutations in the MVK gene. This gene encodes mevalonate kinase (MVK), which takes a role in isoprenoid and cholesterol synthesis. Residual enzyme activity determines the phenotype [78, 79]. The milder phenotype of HIDS/MKD is usually characterized by febrile attacks lasting for 3–7 days. Infections, vaccination, or stress could trigger attacks. The signs and symptoms include aphthous stomatitis, cervical lymphadenopathy, abdominal pain, nausea/vomiting, diarrhea, and maculopapular or urticarial skin rash. On the other hand, mevalonate kinase is deficient in mevalonic aciduria, which forms the severe phenotype. Patients with mevalonic aciduria usually have severe cognitive impairment along with complications such as macrophage activation syndrome due to hyperinflammation. The elevated urinary mevalonic acid level is an important clue for diagnosis [80].
Pericarditis has also been described in the setting of MKD/HIDS, although it is not part of the typical disease features [81]. In a cohort of 1910 individuals with monogenic autoinflammatory diseases, acute pericarditis was observed in 3.7% of MKD/HIDS patients [82]. Prominent systemic involvement was observed in all patients at diagnosis, but pericardial chest pain was not indicated as a first symptom by any of these patients. Only one patient with MKD/HIDS needed pericardiocentesis due to cardiac tamponade, and no case with myocardial complication was recorded [82].
Thors et al. [78] reported a case of a young female patient with fever of unknown origin, who was diagnosed with and treated for incomplete Kawasaki disease. However, observation of recurrent febrile attacks leads to the correct diagnosis as MKD/HIDS. With cardiac ultrasonography on the tenth day of fever, mild dilation of the right and left coronary arteries was detected. The dilation of the proximal coronary arteries had resolved spontaneously within five months. Coronary artery dilation was thought to be a part of the systemic inflammatory response [78].
In 2008, Willer et al. [83] performed a genome-wide association study in European populations to analyze genetic variants affecting plasma lipid concentrations. They discovered a novel loci at chromosome 12q24, which includes the MVK gene influencing HDL concentrations. Epidemiological and clinical studies have demonstrated that low levels of HDL in plasma increase the risk of coronary heart disease [83, 84]. Therefore, the MVK gene may be a candidate as a susceptibility gene modulating HDL concentrations affecting dyslipidemia and coronary heart disease risk. However, different ethnic backgrounds and lifestyle changes across populations could affect the influences of single-nucleotide polymorphisms on dyslipidemia [85]. For instance, no significant associations between dyslipidemia and polymorphisms in MVK gene were shown in the Chinese population [85] (Table 2).
Cryopyrin-associated periodic syndromes (CAPS)
Cryopyrin-associated periodic syndromes (CAPS) or NLRP3-associated autoinflammatory diseases (NLRP3-AIDs) are rare autosomal dominant autoinflammatory diseases associated with gain-of-function mutations in NLRP3. CAPS is a spectrum including mild to severe NLRP3-AID phenotypes, familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and neonatal-onset multisystem inflammatory disease/chronic infantile neurological cutaneous and articular syndrome (NOMID/CINCA) [86]. In FCAS, attacks of fever, urticarial, and arthralgia occur, usually triggered by a generalized exposure to cold [87]. Patients with MWS usually have progressive sensorineural hearing loss. The febrile attacks are more severe than FCAS attacks, and arthritis and severe fatigue could also be observed [88]. Patients with NOMID/CINCA, the most severe phenotype, usually have disease onset during the first years of life, and the clinical picture is characterized by inflammatory central nervous system involvement among many severe organ manifestations.
Cardiovascular involvement may also occur in CAPS patients [89]. In a family study including 13 MWS patients with heterozygous E311K mutation in NLRP3, a single pericarditis attack was seen long before the diagnosis of MWS in three patients (23%). Pericarditis was presented with typical symptoms like shortness of breath and chest pain, and patients were treated with nonsteroidal anti-inflammatory drugs and corticosteroids. Recurrence of pericarditis was not seen with or without IL-1 inhibition [88]. In a study from China investigating 15 Chinese children with CAPS, two patients with coronary artery ectasia were diagnosed with Kawasaki disease before admission [90]. Endo et al. [91] reported sudden cardiac death in a 39-year-old woman with CAPS. In coronary angiography, there was no stenosis in cardiac vessels, and amyloidosis was detected in cardiac biopsy [91].
Severe CAPS may be associated with premature atherosclerosis even during childhood. Yamamura et al. [92] examined three young NOMID patients (aged 5, 7, and 15 years) and age-matched healthy controls. Early signs of atherosclerosis were observed in NOMID patients after ultrasonographic evaluations, including CIMT, ankle-brachial index, the stiffness parameter β, and pressure wave velocity [92] (Table 2).
Tumor necrosis factor receptor-associated periodic syndrome (TRAPS)
Tumor necrosis factor receptor-associated periodic syndrome (TRAPS) is an autosomal dominantly inherited periodic fever syndrome associated with TNFRSF1A mutations [93]. Clinical symptoms of TRAPS include recurrent fever, arthralgia or arthritis, severe myalgia, muscle tenderness, migratory rash, conjunctivitis, periorbital edema, and serositis. Like other chronic inflammatory diseases, some patients may experience systemic amyloidosis, a potentially fatal condition that typically manifests with nephropathy [94].
Cardiac involvement can mainly occur as pericarditis in TRAPS [93]. Pericarditis during attacks, often as a component of polyserositis, is common in TRAPS. Furthermore, there have been reports of patients who present with recurrent pericardial involvement as a distinct clinical symptom of inflammatory attacks [95]. Therefore, some idiopathic recurrent acute pericarditis (IRAP) cases may eventually be diagnosed with TRAPS. In a study by Cantarini et al. [96], TNFRSF1A gene mutations were examined in 131 Caucasian IRAP patients. Eight (6.1%) of them had a mutation in this gene. Six of these eight patients were heterozygous for the R121Q variant. The R121Q variant (previously known as R92Q) is classified as a variant of unknown significance [97]. In a study by Peet et al. [82] in a cohort of non-Finnish European ancestry, the allele frequency of R121Q was 2.5% (5/200), which was not significantly different from ancestry-matched healthy controls. This result argues against the pathogenic role of R121Q in IRAP, but this issue is an area of debate currently. TNFRSF1A mutations should be sought in patients with recurrent pericarditis if they have a positive family history of pericarditis or PFS, a poor response to colchicine, recurrences in a year after the initial attack, or while taking colchicine, steroid dependency, or if they require immunosuppressive medications.
Patients with TRAPS may also manifest with myocarditis [98]. Myocarditis was reported in two patients with TRAPS, accompanied by an acute dilated cardiomyopathy in one [98, 99].
Although TRAPS is an inflammatory disease with attacks usually longer than other PFS, no studies evaluate the incidence of atherosclerosis in TRAPS patients compared to the healthy controls.
The R121Q variant has been associated with myocardial infarction in a cohort of 95 individuals with premature myocardial infarction and familiarity with myocardial infarction [100]. Stojanov et al. [101] reported the novel V173D TNFRSF1A mutation (involving the receptor cleavage site) in an Austrian family. Two members of this family developed an arterial thrombosis and a myocardial infarction caused by the hypothetical atherogenic effect of the mutation [101]. Stojanov et al. [101] also postulated that patients with the TNFRSF1A V173D cleavage site mutation responded well to etanercept which may be a good therapeutic option for cardiovascular complications of TRAPS [101]. The atherogenic effect of V173D TNFRSF1A mutation may be due to prolonged inflammation. TNFRSF1A mutations may increase the risk of atherosclerosis by impairing the endothelial TNF receptor-mediated stimulation of nitric oxide synthetase [102]. Thus, young patients who present with heart attacks could be screened for TNFRSF1A mutations [103] (Table 2).
Conclusion
This review summarized cardiovascular issues in PFS. Cardiovascular involvement in PFS may occur as a disease manifestation, association, or result of complications or a drug’s adverse effects (Table 3). Cardiac involvement, especially pericarditis, may be a feature of PFS. In the presence of recurrent attacks of pericarditis or pericarditis refractory to conventional treatment, PFS should be considered in the differential diagnosis. Furthermore, uncontrolled inflammation may result in early endothelial damage and increase the risk of early atherosclerosis. Also, PFS complications such as amyloidosis and drugs used in the treatment could cause cardiovascular problems. Since long-term survival is provided in PFS with the improvement of therapeutic options, secondary complications such as endothelial dysfunction and atherosclerosis may be increasingly encountered in clinical practice. Therefore, screening for cardiovascular diseases in patients with PFS appears reasonable, and assessment of cardiovascular risk in these patients should be a part of routine care. Controlling the disease activity and subclinical inflammation may prevent early atherosclerosis and amyloidosis. Also, a further focus on reducing concomitant factors increasing the risk of atherosclerosis, such as obesity, diabetes mellitus, dyslipidemia, and hypertension could provide better control for cardiovascular risk in patients with PFS. Clinicians should be alert about cardiovascular issues during the follow-up of patients with PFS. Increasing knowledge will guide physicians to better care for cardiovascular problems in PFS patients. However, prospective and well-planned studies are required to increase the evidence-based data.
Abbreviations
- ADMA:
-
Asymmetric dimethyl arginine
- ANS:
-
Autonomic nervous system
- BD:
-
Behçet’s disease
- CAPS:
-
Cryopyrin-associated periodic syndrome
- CIMT:
-
Carotid intima-media thickness
- EMPs:
-
Endothelial microparticles
- FCAS:
-
Familial cold autoinflammatory syndrome
- FMF:
-
Familial Mediterranean fever
- HIDS:
-
Hyper immunoglobulin D syndrome
- HDL:
-
High-density lipoprotein
- HRV:
-
Heart rate variability
- IgAV:
-
Immunoglobulin-A vasculitis
- IL:
-
Interleukin
- IRAP:
-
Idiopathic recurrent acute pericarditis
- LDL:
-
Low-density lipoprotein
- MEFV:
-
Mediterranean fever
- MKD:
-
Mevalonate kinase deficiency
- MVK:
-
Mevalonate kinase
- MWS:
-
Muckle-Wells syndrome
- NLRP3-AIDs:
-
NLRP3-associated autoinflammatory diseases
- NOMID/CINCA:
-
Neonatal-onset multisystem inflammatory disease/chronic infantile neurological cutaneous and articular syndrome
- PAN:
-
Polyarteritis nodosa
- PFS:
-
Periodic fever syndromes
- SAA:
-
Serum amyloid A
- TG:
-
Triglyceride
- TRAPS:
-
Tumor necrosis factor receptor-associated periodic syndrome
References
Gattorno M, Hofer M, Federici S et al (2019) Classification criteria for autoinflammatory recurrent fevers. Ann Rheum Dis 78:1025–1032
Batu ED, Basaran O, Bilginer Y, Ozen S (2022) Familial Mediterranean fever: how to interpret genetic results? How to treat? A quarter of a century after the association with the Mefv gene. Curr Rheumatol Rep 24:206–212
Sonmez HE, Batu ED, Ozen S (2016) Familial Mediterranean fever: current perspectives. J Inflamm Res 9:13–20
Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD (2011) Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int 31:1409–1417
Park YH, Remmers EF, Lee W et al (2020) Ancient familial Mediterranean fever mutations in human pyrin and resistance to Yersinia pestis. Nat Immunol 21:857–867
Sonmez HE, Batu ED, Bilginer Y, Ozen S (2017) Discontinuing colchicine in symptomatic carriers for MEFV (Mediterranean FeVer) variants. Clin Rheumatol 36:421–425
Kharouf F, Tsemach-Toren T, Ben-Chetrit E (2022) IL-1 inhibition in familial Mediterranean fever: clinical outcomes and expectations. Clin Exp Rheumatol 40:1567–1574
Ozen S, Batu ED (2015) The myths we believed in familial Mediterranean fever: what have we learned in the past years? Semin Immunopathol 37:363–369
Erken E, Erken E (2018) Cardiac disease in familial Mediterranean fever. Rheumatol Int 38:51–58
Kees S, Langevitz P, Zemer D, Padeh S, Pras M, Livneh A (1997) Attacks of pericarditis as a manifestation of familial Mediterranean fever (FMF). QJM 90:643–647
Okutur K, Seber S, Oztekin E, Bes C, Borlu F (2008) Recurrent pericarditis as the initial manifestation of familial Mediterranean fever. Med Sci Monit 14:CS139-141
Yoldas T, Kayali S, Ertugrul I, Dogan V, Orun UA, Karademir S (2017) Massive pericardial effusion and tamponade can be a first sign of familial Mediterranean fever. Pediatr Emerg Care 33:e48–e51
Zimand S, Tauber T, Hegesch T, Aladjem M (1994) Familial Mediterranean fever presenting with massive cardiac tamponade. Clin Exp Rheumatol 12:67–69
Malek A, Zeraati T, Sadr-Nabavi A, Vakili N, Abbaszadegan MR (2022) Cardiac tamponade: a rare manifestation of familial Mediterranean fever. Case Rep Rheumatol 2022:8334375
Malik J, Shabbir A, Nazir A (2021) Cardiovascular sequelae and genetics of familial Mediterranean fever: a literature review. Pulse (Basel) 8:78–85
Turkish FMF Study Group (2005) Familial Mediterranean fever (FMF) in Turkey: results of a nationwide multicenter study. Medicine (Baltimore) 84:1–11
Kilic A, Varkal MA, Durmus MS, Yildiz I, Yildirim ZN, Turunc G et al (2015) Relationship between clinical findings and genetic mutations in patients with familial Mediterranean fever. Pediatr Rheumatol Online J 13:59
Salah S, Hegazy R, Ammar R, Sheba H, Abdelrahman L (2014) MEFV gene mutations and cardiac phenotype in children with familial Mediterranean fever: a cohort study. Pediatr Rheumatol Online J 12:5
Tutar E, Yalcinkaya F, Ozkaya N, Ekim M, Atalay S (2003) Incidence of pericardial effusion during attacks of familial Mediterranean fever. Heart 89:1257–1258
Ozturk K, Coskuner T, Baglan E et al (2021) Real-life data from the largest pediatric familial mediterranean fever cohort. Front Pediatr 9:805919
Ozdogan H, Arisoy N, Kasapcapur O et al (1997) Vasculitis in familial Mediterranean fever. J Rheumatol 24:323–327
Abbara S, Grateau G, Ducharme-Benard S, Saadoun D, Georgin-Lavialle S (2019) Association of vasculitis and familial Mediterranean fever. Front Immunol 10:763
Karadag SG, Tanatar A, Sonmez HE, Cakmak F, Kiyak A, Yavuz S, Cakan M, Ayaz NA (2019) The clinical spectrum of Henoch-Schonlein purpura in children: a single-center study. Clin Rheumatol 38:1707–1714
Pagnoux C, Seror R, Henegar C, Mahr A, Cohen P, Le Guern V, Bienvenu B, Mouthon L, Guillevin L, French Vasculitis Study G (2010) Clinical features and outcomes in 348 patients with polyarteritis nodosa: a systematic retrospective study of patients diagnosed between 1963 and 2005 and entered into the French Vasculitis Study Group Database. Arthritis Rheum 62:616–626
Ben-Chetrit E, Cohen R, Chajek-Shaul T (2002) Familial Mediterranean fever and Behcet's disease--are they associated? J Rheumatol 29:530–534
Kirino Y, Zhou Q, Ishigatsubo Y, Mizuki N, Tugal-Tutkun I, Seyahi E et al (2013) Targeted resequencing implicates the familial Mediterranean fever gene MEFV and the toll-like receptor 4 gene TLR4 in Behcet disease. Proc Natl Acad Sci U S A 110:8134–8139
Ilyas I, Little PJ, Liu Z, Xu Y, Kamato D, Berk BC, Weng J, Xu S (2022) Mouse models of atherosclerosis in translational research. Trends Pharmacol Sci
Keles N, Aksu F, Aciksari G, Yilmaz Y, Demircioglu K, Kostek O, Cekin ME, Kalcik M, Caliskan M (2016) Is triglyceride/HDL ratio a reliable screening test for assessment of atherosclerotic risk in patients with chronic inflammatory disease? North Clin Istanb 3:39–45
Icli A, Cure E, Uslu AU, Sakiz D et al (2017) The relationship between atherogenic index and carotid artery atherosclerosis in familial Mediterranean fever. Angiology 68:315–321
Acay A, Ulu MS, Ahsen A, Ozkececi G, Demir K, Ozuguz U, Yuksel S, Acarturk G (2014) Atherogenic index as a predictor of atherosclerosis in subjects with familial Mediterranean fever. Medicina (Kaunas) 50:329–333
Uluca U, Demir F, Ece A, Sen V, Gunes A, Aktar F, Tan I, Karabel D, Yazgan U, Sabaz MN (2015) Assessment of epicardial adipose tissue thickness and the mean platelet volume in children with familial Mediterranean fever. Ital J Pediatr 41:15
Yel S, Dursun I, Cetin F, Bastug F, Tulpar S, Dusunsel R, Gunduz Z, Poyrazoglu H, Yilmaz K (2018) Increased circulating endothelial microparticles in children with FMF. Biomarkers 23:558–562
Terekeci HM, Oktenli C, Ozgurtas T, Nalbant S, Top C, Celik S et al (2008) Increased asymmetric dimethylarginine levels in young men with familial Mediterranean fever (FMF): is it early evidence of interaction between inflammation and endothelial dysfunction in FMF? J Rheumatol 35:2024–2029
Ozalper V, Kara M, Tanoglu A, Cetindagli I, Ozturker C, Hancerli Y, Hira S, Kara K, Beyazit Y, Yazgan Y (2017) Evaluation of endothelial dysfunction in patients with familial Mediterranean fever: the relationship between the levels of asymmetric dimethylarginine and endocan with carotid intima-media thickness and endothelium-dependent vasodilation. Clin Rheumatol 36:2071–2077
Akdogan A, Calguneri M, Yavuz B et al (2006) Are familial Mediterranean fever (FMF) patients at increased risk for atherosclerosis? Impaired endothelial function and increased intima media thickness are found in FMF. J Am Coll Cardiol 48:2351–2353
Bilginer Y, Ozaltin F, Basaran C, Duzova A, Besbas N, Topaloglu R, Ozen S, Bakkaloglu A (2008) Evaluation of intima media thickness of the common and internal carotid arteries with inflammatory markers in familial Mediterranean fever as possible predictors for atherosclerosis. Rheumatol Int 28:1211–1216
Peru H, Altun B, Dogan M, Kara F, Elmaci AM, Oran B (2008) The evaluation of carotid intima-media thickness in children with familial Mediterranean fever. Clin Rheumatol 27:689–694
Sari I, Karaoglu O, Can G, Akar S, Gulcu A, Birlik M, Akkoc N, Tunca M, Goktay Y, Onen F (2007) Early ultrasonographic markers of atherosclerosis in patients with familial Mediterranean fever. Clin Rheumatol 26:1467–1473
Kozan M, Ozan ZT, Demir V, Ede H (2019) The relation of novel cardiovascular risk parameters in patients with familial Mediterranean fever. JRSM Cardiovasc Dis 8:2048004018823856
Yildiz M, Masatlioglu S, Seymen P, Aytac E, Sahin B, Seymen HO (2006) The carotid-femoral (aortic) pulse wave velocity as a marker of arterial stiffness in familial Mediterranean fever. Can J Cardiol 22:1127–1131
Motawea KR, Kandil OA, Varney J, Aboelenein M, Ibrahim N, Shaheen A et al (2022) Association of familial Mediterranean fever and epicardial adipose tissue: a systematic review and meta-analysis. Health Sci Rep 5:e693
Basar N, Kisacik B, Ercan S, Pehlivan Y, Yilmaz S, Simsek I et al (2017) Familial Mediterranean fever gene mutations as a risk factor for early coronary artery disease. Int J Rheum Dis 20:2113–2117
Caliskan M, Gullu H, Yilmaz S, Erdogan D, Unler GK, Ciftci O, Topcu S, Kayhan Z, Yucel E, Muderrisoglu H (2007) Impaired coronary microvascular function in familial Mediterranean fever. Atherosclerosis 195:e161–e167
Ambartsymian SV (2012) Myocardial infarction in patients with familial Mediterranean fever and cardiac lesions. Georgian Med News 62-66
Uyarel H, Karabulut A, Okmen E, Cam N (2006) Familial Mediterranean fever and acute anterior myocardial infarction in a young patient. Anadolu Kardiyol Derg 6:272–274
Langevitz P, Livneh A, Neumann L, Buskila D, Shemer J, Amolsky D, Pras M (2001) Prevalence of ischemic heart disease in patients with familial Mediterranean fever. Isr Med Assoc J 3:9–12
Gendelman O, Shapira R, Tiosano S, Pras E, Comaneshter D, Cohen A, Amital H (2020) Familial Mediterranean fever is associated with increased risk for ischaemic heart disease and mortality-perspective derived from a large database. Int J Clin Pract 74:e13473
Rupprecht S, Finn S, Hoyer D, Guenther A, Witte OW, Schultze T, Schwab M (2020) Association between systemic inflammation, carotid arteriosclerosis, and autonomic dysfunction. Transl Stroke Res 11:50–59
Rozenbaum M, Naschitz JE, Yudashkin M, Sabo E, Shaviv N, Gaitini L, Zuckerman E, Yeshurun D, Rosner I (2004) Cardiovascular reactivity score for the assessment of dysautonomia in familial Mediterranean fever. Rheumatol Int 24:147–152
Rozenbaum M, Naschitz JE, Yudashkin M, Rosner I, Sabo E, Shaviv N, Gaitini L, Zuckerman E, Yeshurun D (2002) Cardiovascular autonomic dysfunction in familial Mediterranean fever. J Rheumatol 29:987–989
Canpolat U, Dural M, Aytemir K et al (2012) Evaluation of various cardiac autonomic indices in patients with familial Mediterranean fever on colchicine treatment. Auton Neurosci 167:70–74
Ardic I, Kaya MG, Yarlioglues M, Dogdu O, Celikbilek M, Akpek M, Akyol L, Torun E (2011) Assessment of heart rate recovery index in patients with familial Mediterranean fever. Rheumatol Int 31:121–125
Evrengul H, Yuksel S, Dogan M, Gurses D, Evrengul H (2017) Deteriorated systolic blood pressure recovery and heart rate recovery after graded exercise in children with familial Mediterranean fever. Arch Rheumatol 32:244–249
Sahin M, Kir M, Makay B, Keskinoglu P, Bora E, Unsal E, Unal N (2016) Cardiac autonomic functions in children with familial Mediterranean fever. Clin Rheumatol 35:1237–1244
Nussinovitch N, Livneh A, Katz K, Langevitz P, Feld O, Nussinovitch M, Volovitz B, Lidar M, Nussinovitch U (2011) Heart rate variability in familial Mediterranean fever. Rheumatol Int 31:39–43
Nussinovitch N, Esev K, Lidar M, Nussinovitch U, Livneh A (2015) Normal Heart rate variability in colchicine-resistant familial Mediterranean fever patients. The I Isr Med Assoc J 17:306-309.
Nussinovitch U, Volovitz B, Nussinovitch M, Lidar M, Feld O, Nussinovitch N, Livneh A (2011) Abnormal heart rate variability in AA amyloidosis of familial Mediterranean fever. Amyloid 18:206–210
Nussinovitch U, Kaminer K, Nussinovitch M, Volovitz B, Lidar M, Nussinovitch N, Livneh A (2012) QT interval variability in familial Mediterranean fever: a study in colchicine-responsive and colchicine-resistant patients. Clin Rheumatol 31:795–799
Nussinovitch U, Livneh A, Volovitz B, Nussinovitch M, Ben-Zvi I, Lidar M, Nussinovitch N (2012) Normal QT dispersion in colchicine-resistant familial Mediterranean fever (FMF). Clin Rheumatol 31:1093–1096
Giese A, Ornek A, Kurucay M, Kara K, Wittkowski H, Gohar F, Menge BA, Schmidt WE, Zeidler C (2014) P wave dispersion and QT dispersion in adult Turkish migrants with familial Mediterranean fever living in Germany. Int J Med Sci 11:1140–1146
Ahbap E, Sakaci T, Kara E, Sahutoglu T, Ozturk S, Basturk T et al (2015) Familial Mediterranean fever is associated with abnormal ventricular repolarization indices. Rev Med Chil 143:1560–1568
Farag Y, Sayed S, Mostafa FA, Marzouk H, Mohamed RH, Sobhy R (2022) Cardiac repolarization abnormalities in children with familial Mediterranean fever. Pediatr Rheumatol Online J 20:38
Akse-Onal V, Sag E, Ozen S, Bakkaloglu A, Cakar N, Besbas N, Gucer S (2010) Decrease in the rate of secondary amyloidosis in Turkish children with FMF: are we doing better? Eur J Pediatr 169:971–974
Yilmaz MI, Demirkaya E, Acikel C et al (2014) Endothelial function in patients with familial Mediterranean fever-related amyloidosis and association with cardiovascular events. Rheumatology (Oxford) 53:2002–2008
Bozaci I, Tatar E (2021) The role of azurocidin in patients with familial Mediterranean fever and AA amyloidosis and its association with cardiovascular risk factors. Int Urol Nephrol 53:531–538
Sahin S, Romano M, Guzel F, Piskin D, Poddighe D, Sezer S, Kasapcopur O, Appleton CT, Yilmaz I, Demirkaya E (2022) Assessment of surrogate markers for cardiovascular disease in familial Mediterranean fever-related amyloidosis patients homozygous for M694V mutation in MEFV gene. Life (Basel) 12(5)
Ceylan O, Ozgur S, Orun UA, Dogan V, Yilmaz O, Keskin M, Ari ME, Erdogan O, Karademir S (2015) Assessment of left ventricular functions with tissue Doppler, strain, and strain rate echocardiography in patients with familial Mediterranean fever. Anatol J Cardiol 15:663–668
Erken Pamukcu H, Dogan M, Ozisler C, Sunman H, Pamukcu M, Dinc Asarcikli L (2019) Effects of familial Mediterranean fever on cardiac functions in adults: a cross-sectional study based on speckle tracking echocardiography. Arch Rheumatol 34:204–210
Celik MM, Buyukkaya E, Ustun N, Nacar AB, Kurt M, Karakas MF, Bilen P, Duru M, Sen N, Akcay AB (2015) Relation of fragmented QRS to tissue Doppler-derived parametersin patients with familial Mediterranean fever. Wien Klin Wochenschr 127:185–190
Casula M, Andreis A, Avondo S, Vaira MP, Imazio M (2022) Colchicine for cardiovascular medicine: a systematic review and meta-analysis. Futur Cardiol 18:647–659
Whayne TF (2021) Inflammation may be the future of cardiovascular risk reduction: does colchicine have a current indication? Am J Cardiovasc Drugs 21:1–10
Tardif JC, Kouz S, Waters DD et al (2019) Efficacy and Safety of low-dose colchicine after myocardial infarction. N Engl J Med 381:2497–2505
Frommeyer G, Krawczyk J, Dechering DG, Kochhauser S, Leitz P, Fehr M, Eckardt L (2017) Colchicine increases ventricular vulnerability in an experimental whole-heart model. Basic Clin Pharmacol Toxicol 120:505–508
Ocal AG, Ocal L, Kup A, Eren H, Tezcan ME (2020) Colchicine's effects on electrocardiographic parameters in newly diagnosed familial Mediterranean fever patients: colchicine may have favourable effects on parameters related to ventricular arrhythmias in new diagnosed familial Mediterranean fever. Z Rheumatol 79:210–215
Nussinovitch U, Stahi T, Livneh A (2020) Evaluation of a proarrhythmic repolarization marker (total cosine R to T) in patients with uncomplicated familial Mediterranean fever. J Clin Rheumatol 26:334–337
Saper VE, Chen G, Deutsch GH et al (2019) Emergent high fatality lung disease in systemic juvenile arthritis. Ann Rheum Dis 78:1722–1731
Saper VE, Ombrello MJ, Tremoulet AH et al (2022) Severe delayed hypersensitivity reactions to IL-1 and IL-6 inhibitors link to common HLA-DRB1*15 alleles. Ann Rheum Dis 81:406–415
Thors VS, Vastert SJ, Wulffraat N, van Royen A, Frenkel J, de Sain-van der Velden M, de Koning TJ (2014) Periodic fever in MVK deficiency: a patient initially diagnosed with incomplete Kawasaki disease. Pediatrics 133:e461–e465
van der Burgh R, Ter Haar NM, Boes ML, Frenkel J (2013) Mevalonate kinase deficiency, a metabolic autoinflammatory disease. Clin Immunol 147(3):197–206
Jeyaratnam J, Ter Haar NM, de Sain-van der Velden MG, Waterham HR, van Gijn ME, Frenkel J (2016) Diagnostic value of urinary mevalonic acid excretion in patients with a clinical suspicion of mevalonate kinase deficiency (MKD). JIMD Rep 27:33–38
Breda L, Nozzi M, Di Marzio D, De Sanctis S, Gattorno M, Chiarelli F (2009) Recurrent pericarditis in hyper-IgD syndrome. Clin Exp Rheumatol 27:695
Peet CJ, Rowczenio D, Omoyinmi E, Papadopoulou C, BRR M, Wood MR, Capon F, Lachmann HJ (2022) Pericarditis and autoinflammation: a clinical and genetic analysis of patients with idiopathic recurrent pericarditis and monogenic autoinflammatory diseases at a national referral center. J Am Heart Assoc 11:e024931
Willer CJ, Sanna S, Jackson AU et al (2008) Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet 40:161–169
Willer CJ, Schmidt EM, Sengupta S et al (2013) Discovery and refinement of loci associated with lipid levels. Nat Genet 45:1274–1283
Sun J, Qian Y, Jiang Y et al (2016) Association of KCTD10, MVK, and MMAB polymorphisms with dyslipidemia and coronary heart disease in Han Chinese population. Lipids Health Dis 15:171
Romano M, Arici ZS, Piskin D et al (2022) The 2021 EULAR/American College of Rheumatology points to consider for diagnosis, management and monitoring of the interleukin-1 mediated autoinflammatory diseases: cryopyrin-associated periodic syndromes, tumour necrosis factor receptor-associated periodic syndrome, mevalonate kinase deficiency, and deficiency of the interleukin-1 receptor antagonist. Ann Rheum Dis 81:907–921
Welzel T, Kuemmerle-Deschner JB (2021) Diagnosis and management of the cryopyrin-associated periodic syndromes (CAPS): what do we know today? J Clin Med 10
Kuemmerle-Deschner JB, Lohse P, Koetter I, Dannecker GE, Reess F, Ummenhofer K, Koch S, Tzaribachev N, Bialkowski A, Benseler SM (2011) NLRP3 E311K mutation in a large family with Muckle-Wells syndrome-description of a heterogeneous phenotype and response to treatment. Arthritis Res Ther 13:1–9
Hintenberger R, Falkinger A, Danninger K, Pieringer H (2018) Cardiovascular disease in patients with autoinflammatory syndromes. Rheumatol Int 38:37–50
Li C, Tan X, Zhang J, Li S, Mo W, Han T, Kuang W, Zhou Y, Deng J (2017) Gene mutations and clinical phenotypes in 15 Chinese children with cryopyrin-associated periodic syndrome (CAPS). Sci China Life Sci 60:1436–1444
Endo K, Suzuki A, Sato K, Shiga T (2015) Sudden cardiac arrest secondary to cardiac amyloidosis in a young woman with cryopyrin-associated periodic syndrome. BMJ Case Rep 2015
Yamamura K, Takada H, Uike K, Nakashima Y, Hirata Y, Nagata H et al (2014) Early progression of atherosclerosis in children with chronic infantile neurological cutaneous and articular syndrome. Rheumatology (Oxford) 53:1783–1787
Rigante D, Cantarini L, Imazio M, Lucherini OM, Sacco E, Galeazzi M, Brizi MG, Brucato A (2011) Autoinflammatory diseases and cardiovascular manifestations. Ann Med 43:341–346
Stojanov S, McDermott MF (2005) The tumour necrosis factor receptor-associated periodic syndrome: current concepts. Expert Rev Mol Med 7:1–18
Cantarini L, Lucherini O, Cimaz R, Baldari C, Bellisai F, Paccani SR, Pasini FL, Capecchi P, Sebastiani G, Galeazzi M (2009) Idiopathic recurrent pericarditis refractory to colchicine treatment can reveal tumor necrosis factor receptor-associated periodic syndrome. Int J Immunopathol Pharmacol 22:1051–1058
Cantarini L, Lucherini OM, Brucato A et al (2012) Clues to detect tumor necrosis factor receptor-associated periodic syndrome (TRAPS) among patients with idiopathic recurrent acute pericarditis: results of a multicentre study. Clin Res Cardiol 101:525–531
Aksentijevich I, Galon J, Soares M et al (2001) The tumor-necrosis-factor receptor-associated periodic syndrome: new mutations in TNFRSF1A, ancestral origins, genotype-phenotype studies, and evidence for further genetic heterogeneity of periodic fevers. Am J Hum Genet 69:301–314
Trost S, Rosé CD (2005) Myocarditis and sacroiliitis: 2 previously unrecognized manifestations of tumor necrosis factor receptor associated periodic syndrome. J Rheumatol 32:175–177
Roubille F, Cayla G, Gahide G, Mourad G, Macia JC (2009) Acute myocarditis and tumor necrosis factor receptor-associated periodic (TRAP) syndrome: first case described and discussion. Eur J Intern Med 20:e25–e26
Poirier O, Nicaud V, Gariepy J et al (2004) Polymorphism R92Q of the tumour necrosis factor receptor 1 gene is associated with myocardial infarction and carotid intima-media thickness--the ECTIM, AXA, EVA and GENIC studies. Eur J Hum Genet 12:213–219
Stojanov S, Dejaco C, Lohse P, Huss K, Duftner C, Belohradsky BH, Herold M, Schirmer M (2008) Clinical and functional characterisation of a novel TNFRSF1A c.605T>A/V173D cleavage site mutation associated with tumour necrosis factor receptor-associated periodic fever syndrome (TRAPS), cardiovascular complications and excellent response to etanercept treatment. Ann Rheum Dis 67:1292–1298
Amoura Z, Dode C, Hue S, Caillat-Zucman S, Bahram S, Delpech M, Grateau G, Wechsler B, Piette JC (2005) Association of the R92Q TNFRSF1A mutation and extracranial deep vein thrombosis in patients with Behcet's disease. Arthritis Rheum 52:608–611
Chia S, Qadan M, Newton R, Ludlam CA, Fox KA, Newby DE (2003) Intra-arterial tumor necrosis factor-alpha impairs endothelium-dependent vasodilatation and stimulates local tissue plasminogen activator release in humans. Arterioscler Thromb Vasc Biol 23:695–701
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Part of the Topical Collection entitled ‘Cardiovascular Issues in Rheumatic Diseases’
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sönmez, H.E., Bayındır, Y. & Batu, E.D. Cardiovascular manifestations of monogenic periodic fever syndromes. Clin Rheumatol 42, 2717–2732 (2023). https://doi.org/10.1007/s10067-023-06504-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-023-06504-z