Abstract
A 62-year-old healthy male presents with leg weakness and fever. Imaging revealed leptomeningeal enhancement (LE). After cerebrospinal fluid (CSF) cultures were negative, he was discharged with a diagnosis of aseptic meningitis, but was readmitted due to worsening symptoms. Brain biopsy suggested rheumatoid leptomeningitis associated with elevated serum rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibodies (ACPA). Following discharge, the New York State Department of Health (NYSDOH) reported a polymerase chain reaction (PCR) on CSF and brain DNA consistent with Naegleria fowleri (NF). After dramatic improvement on steroids, the patient declined antimicrobial treatment. Upon prednisone taper, symptoms recurred which responded to rituximab (RTX). This case highlights a possible association between rheumatoid leptomeningitis (RM) onset and infection, in a patient without a history of rheumatoid arthritis (RA). Our goal is to assess whether this association is present in 69 RM cases reported since 2000. We also describe diagnosis and treatment of 31 new cases (January 2017 to March 2020). We did not identify evidence of active/latent infection in patients with RM and previous RA; however, patients without RA history appeared to have a significantly higher rate. This finding could demonstrate the necessity of evaluating for infection in de novo RM cases without antecedent RA history. We also describe characteristic clinical patterns for each group. More studies are needed to corroborate these results and expand into a possible distinct natural history of RM in each group, which might have an impact upon the clinical outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
RA is a systemic inflammatory disease triggered by the interaction between genetic and environmental factors [1]. Generally, RA presents with joint pain, but can be associated with extra-articular manifestations [2]. In these atypical cases, the diagnosis can be delayed impacting morbidity and mortality. Some of the main target organs for extra-articular RA manifestations include lungs, blood vessels, heart, and brain [2]. RM is a rare RA extra-articular complication lacking clear guidelines for diagnosis and treatment. The main sources for understanding the disease are previous systematic reviews. Two of the most extensive reviews included 21 cases before 2000 [3] and 38 cases from 2000 to 2017 [4]. We describe clinical features and treatment of 31 new cases from January 2017 to March 2020, in addition to some cases not previously described by Qin et.al. We also reviewed 69 cases (38 from Qin et. al., plus 31 new cases) searching for infections at the moment of RM diagnosis.
Case report
First admission
A 62-year-old American male, residing in Shanghai, presented with 1-month of fever, headache, and a 3-day history of bilateral leg weakness. The patient had used neti pot nasal irrigation for sinus congestion for 2 months prior to symptom onset. Physical examination demonstrated right lower limb weakness, with a strength of 4/5, right foot drop, difficulties with dorsiflexion/eversion, and inability to walk on toes/heels. No active synovitis was detected.
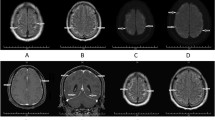
Laboratory studies exhibited an erythrocyte sediment rate (ESR) of 16 mm/h and a positive indirect antinuclear antibody (ANA) (1:80, speckled pattern). CSF demonstrated a white blood cell count (WBC) of 56 cells/ul (97% lymphocytes), intrathecal IgG synthesis index of 1.92, and 3 oligoclonal bands; myelin basic protein was absent. The infectious workup was negative and included CSF gram stain and culture (Haemophilus influenzae B, Neisseria meningitidis serogroup B, Escherichia coli K1, and Streptococcus pneumoniae), tuberculosis (TB) QuantiFERON, Syphilis panel, Lyme serology, Aspergillus antigen, and viral panel (varicella-zoster virus, and hepatitis A, B, and C). Contrast MRI showed abnormal LE in the bilateral frontoparietal lobes at the interhemispheric fissure (Fig. 1). Due to concern about neti pot use, CSF samples were sent to the NYSDOH to test for free-living amoeba (NF, Balamuthia, and Acanthamoeba); results were pending at the time of discharge. The patient received 10-days antimicrobial therapy (vancomycin, cefepime, metronidazole, and acyclovir). He was diagnosed with aseptic meningitis and discharged.
Temporal course of the disease symptoms, MRI findings, laboratory results, and treatment. Our patient presented with headaches, fever, and focal weakness. The symptoms fluctuated over the course of months during flares. Contrast MRI revealed leptomeningeal enhancement in the bilateral frontal and parietal lobes, the interhemispheric fissure, and enlargement and hyperenhancement of the left choroid plexus (not shown). On day 152, a second MRI showed resolution findings, normal ESR (elevated at presentation), and CSF negative for NF (PCR). During the last flare (day 182) he responded very well to RTX, IVIG, steroid pulse. Abbreviations: ABX, antibiotics; CSF, cerebrospinal fluid; DEX, dexamethasone; ESR, erythrocyte sedimentation rate; IVIG, intravenous immunoglobulin; LE, lower extremity; MTX, methotrexate; NF, Naegleria fowleri; RTX, rituximab; PCR, polymerase chain reaction; Valproic ac, valproic acid
Second admission
Six days after discharge, the patient was readmitted with joint pain without synovitis, progressive lower extremity weakness, and paranoia. Once admitted he had a partial seizure, treated with valproic acid. Examination revealed decreased strength in the right dorsal and plantar flexion (0/5 and 1/5), and normal reflexes (2/4) in the 4 extremities.
Blood work revealed elevated RF (579 U/mL), ACPA (> 150 U), and ESR (45 mm/h). Anti-phospholipid panel, anti-dsDNA, and extractable nuclear antigen antibodies were negative. The CSF exhibited lymphocytosis (WBC of 10.96 K/uL), protein of 27.7 mg/dl, glucose levels of 70 mg/dL, cytology with atypical cells (monocytic), and no evidence of leukemia/lymphoma by flow cytometry. Granulomatous leptomeningitis with multinucleated giant cells, necrosis, and neutrophilic inflammation was evident in brain biopsy. Amoebas were not present. Microorganisms were not identified on special stains (bacteria, fungi, spirochetes). There was no significant pathology in the brain parenchyma or pachymeningitis in dural biopsy (Fig. 2). Repeat brain MRI was unchanged from the previous admission.
Brain biopsy. Biopsy demonstrates a dense and solid appearance of the leptomeninges due to the presence of granulomatous inflammation, necrosis, acute inflammation and fibrosis. Arrows (a, b, d, e) identify multinucleated giant cells; necrosis is identified by an asterisk (a). Other than a giant cell (b), the majority of tissue in this panel (b) is comprised of fibrotic leptomeninges. In (c) the inflammation contains numerous plasma cells that form a perivascular infiltrate around a non-inflamed arteriole that is located in the center of this image; larger cells with pale pink cytoplasm are macrophages. Neutrophils are present in some areas of inflammatory infiltration (d). Microorganisms are not found. Biopsies of brain and dura (not shown) did not demonstrate significant pathology. Hematoxylin and eosin; scale bar = 100 μm (a) and 20 μm (b, c, d, e)
The patient’s symptoms improved with dexamethasone, 8 mg IV for 3 days, then 4 mg for 3 days. Afterwards, he received prednisone 60 mg daily with subsequent dramatic clinical improvement. He was discharged on day 36 with a diagnosis of rheumatoid leptomeningitis. He did not meet the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) diagnostic criteria for RA [5].
Interim follow-up
CSF samples sent during the first admission to NYSDOH came back positive for NF. A sample from the brain biopsy (2nd admission) was also sent. NYSDOH ran a conventional PCR (CSF and brain tissue) using Naegleria-specific primers, and a real-time PCR with DNA isolated from brain tissue. All tests were positive for NF (Fig. 3). The real-time PCR was run to 45 cycles and the required reagents were used as mentioned by the center for disease control and prevention (CDC) [6]. A test for Naegleria lovaniensis, the closest species to NF, was negative (supplemental data). The conventional PCR was performed by two different laboratory staff members, on two different days with identical results. To test whether the patient’s DNA sample had been contaminated with positive control (plasmid containing NF); the sample was tested using primers specific for the plasmid vector (Ampicillin resistance gene target). This was negative, ruling out contamination. Although results were positive for NF, the patient declined treatment with amphotericin B due to his remarkable improvement with steroids.
PCR. a Conventional PCR on CSF and brain tissue positive for NF. b Real-time PCR on brain tissue positive for NF. Line 1 shows positive control for NF being detected at cycle 30, 47. Line 2 shows the patient’s brain tissue sample positive for NF at cycle 39, 61. PCR analysis found a 100% identity with partial sequences of NF strains and 99% identity with strains including Mato Forroba 18S ribosomal RNA gene, Empada 18S ribosomal RNA gene, Palche Lala 18S ribosomal RNA gene, Watine 18S ribosomal RNA gene, and N. fowleri 18S ribosomal RNA gene. Sequence analysis was also tested for NL, which is the next closest species but there are numerous mismatches in sequence (supplemental data)
The patient returned to Shanghai and we continued to provide recommendations in collaboration with a local rheumatologist. A MRI on day 111 exhibited resolution of cerebral inflammation and prednisone was tapered to a lower dose. By day 120 (Fig. 1), symptoms recurred, now with significant synovitis, which met standard diagnostic criteria for RA [5]. He was started on methotrexate (MTX) (12.5 mg/week) plus prednisone taper. CSF was re-tested to assess NF infection, with negative results.
Third admission
On day 152 the patient was readmitted due to a relapse, with seizures, dysarthria, and bilateral leg weakness. Laboratories showed a normal ESR (11 mm/h) and a brain MRI demonstrated cerebral edema. RTX and intravenous immunoglobulin were initiated, and the patient dramatically improved (decreased joint swelling and improved mobility). The patient was discharged with a regimen of MTX, prednisone taper, and continued standard RTX (17.5 mg weekly, 2 cycles every 6 months) over the course of 2 years (Fig. 1). The patient has remained asymptomatic since.
Discussion
The terms “rheumatoid meningitis” and “rheumatoid leptomeningitis” were used in PubMed identifying 30 cases in the English literature, published from 2000 to 2020. Cases reviewed by Qin et. al. were excluded from the analysis, except to calculate infection rate in RM patients (31 new cases plus those reviewed by Qin et. al.). The information of the 30 patients, plus our case, is listed in Tables 1 and 2. We use chi-square to compare frequencies, alpha < 0.05 was considered significant.
We reviewed a total of 31 [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31] RM cases from 1/2017 to 3/2020. The proportion of females, 55% (17 women out of 31 patients) is lower than expected for RA, where a greater female predominance (77–79%) is expected [1]. The mean age for men was lower than for women (58.5 vs 66.3 years), which has been previously described, supporting the onset of RM in men at an earlier age [4]. Our findings are similar to Qin et. al. in the number of patients presenting with RM during the 5 first years of RA diagnosis (14/30). Out of 69 reviewed cases (38 from Qin et. al. and our 31 cases), 3 had laboratory results consistent with an active/latent infection (4.3%).
Natural history of RM, diagnosis, and treatment
RM can manifest in long-standing RA [8, 10] or herald the disease [7, 24]. Our patient presented with LE and without synovitis, he met RA diagnostic criteria 120 days after RM diagnosis. Only 20% of the reviewed patients had synovitis at the time of RM diagnosis (Table 1), emphasizing the lack of association of RM with classic articular RA flares/onset [32]. This makes the RM diagnosis challenging. In our review, the diagnosis was supported by symptoms/signs, serology, imaging, and brain biopsy.
Most RM cases (17/31) had a 5-year history of RA or longer at onset [4], and 14 out 31 (46.6%) had less than 5 years. Within the second group, 10 patients completely lack RA antecedents (71%). Our percentage of patients with RM and without a history of RA is higher than previously reported [4]. Common symptoms were focal weakness in 77%, followed by headache (58%), seizures (42%), and rheumatoid meningitis stroke-like attack (RMSA) (35%). Four out of 31 had lung involvement including 1 patient with interstitial lung disease, the other 3 had lung nodules and increased metabolic activity in the lungs in positron emission tomography [12, 17, 18, 26]. Only 1 patient out of 31 (3%) had another autoimmune disease besides RA (myasthenia gravis) [19].
Eighty-six percent of patients were double positive (RF/ACPA), only 1% (3/26) was single positive (RF/ACPA). Seventy-five percent of the sample had RF > 50 IU/mL, and 81% had ACPA > 150 μ/ml. An ESR > 40 mm/h and/or C-reactive protein (CRP) > 10 mg/dl was present in 64%. In the CSF, WBC > 15 cells with lymphocytic predominance was present in 61% of the total, and protein > 45 mg/dl in 68%. Instead, glucose was only less than 30 mg/dl in 8.7%. These results are consistent with previous data [3, 4]. Increased CSF-protein might be explained by intrathecal IgG synthesis and oligoclonal bands (OB), reported in 50% of our cases. Overall, the differential diagnosis included malignancy, neurosarcoidosis, IgG4 disease, other autoimmune diseases, and infections. The angiotensin convertase enzyme (ACE) was elevated (> 53 UI/L) in 18% of the cases. ACE could be elevated in RA due to the intrinsic capacity of granulomas (present in RA/sarcoidosis) to produce ACE [33]. The presence of necrosis in biopsy ruled out neurosarcoidosis [34]. Serum IgG4 levels were normal in 10 cases tested out of 31. The ANA titer was positive above 1:80 in 42%, as described in RA [35]. The use of anti-tumor necrosis factor alpha (anti-TNF alpha) increases ANA seroconversion in RA patients [36]. In our cohort, only 2 patients used anti-TNF alpha therapy.
Gadolinium-MRI was indicated in 30 cases out of 31 (Table 2). Post-contrast enhancement is present in all cases in leptomeninges (pia/arachnoid layers) and/or pachymeninges (dura-mater). Lepto- and pachymeninges were involved at the same time in 37%. Leptomeninges and pachymeninges were individually affected in 50% and 13% respectively. Post-contrast enhancement is bilaterally distributed in 57% of all the cases. The most frequent localization for LE and/or PE is close to the frontoparietal lobes (29%), parietal lobes (23%), interhemispheric fissure (19%), or frontal lobes (13%). In a vertical plane, the enhancement affected more the superior pole of the brain (81%). Only 18% (1 supratentorial, 1 infratentorial, 2 occipital) have findings in the basilar areas. Twenty-three out of 31 cases had a brain biopsy. Meningeal inflammation is present in 21 cases (91%), and is commonly characterized by infiltrates of CD3-positive T cells, B lymphocytes, plasma cells, multinucleated giant cells, macrophages, necrotizing granulomas, and fibrosis, with astrocytosis in the adjacent neural parenchyma [7, 29]. Rheumatoid nodules were described in 35% of the cases (8/23), and vasculitis in 3 out of 23 (13%). Previous authors describe rheumatoid nodules in half of brain biopsies in RM [3, 4] and vasculitis in 36% to 50% rates [4].
MRI enhancement can be extra and intra-axial. Extra-axial involves PE and LE [37]. PE can manifest against the bone, or involve the dural reflections of the falx cerebri, tentorium cerebelli, falx cerebelli, and cavernous sinus [37]. This type of enhancement is seen in many conditions, including granulomatous disease such as tuberculosis, sarcoidosis, and RA [37]. A similar enhancement distribution would be expected in neurosarcoidosis and RM, but the evidence proves otherwise. In neurosarcoidosis, PE typically affects the basilar meninges, but no involvement of the convexities of the cerebral hemispheres [37]. Our RM cases show the opposite, as described above. LE is present in the pia mater and the subarachnoid spaces of the sulci and cisterns, and it is usually associated with infectious leptomeningitis [37].
Gadolinium-MRI is used as a marker in multiple sclerosis [37] because it accumulates in neuroinflammatory lesions. During neuroinflammation, the brain-blood barrier (BBB) permeability increases. Upon administration, gadolinium in the blood rises creating a concentration gradient across the BBB with contrast leakage into CSF [37]. Gadolinium is more attracted to inflammatory pockets because (1) the interstitial space from inflammatory lesions expands, and (2) washout time of contrast is delayed in inflammatory lesions. The interstitial space increases due to hypercellularity and there is altered production of glycosaminoglycans during neuroinflammation [38]. The delayed in contrast-washout could be explain by the formation of gadolinium-GAG complexes retaining the contrast [39]. Interestingly, glycosaminoglycans are elevated in serum and synovial fluid in RA patients [40].
In RM, the meninges (sparing parenchyma) are the main area of inflammation identified through brain biopsy and contrast-MRI [16]. In our reviewed cases, the meningeal enhancement is often bilateral, and mostly situated in areas overlying the interhemispheric fissure, and the frontal-parietal lobes. This distribution locates close to the superior sagittal sinus, instead of basilar areas. This leads us to speculate whether the exclusive inflammation of meninges sparing parenchyma, and the distribution of gadolinium enhancement associates with pathogenic mechanisms in RM. Stromal meningeal cells, blood vessels, and lymphatics are the principal components within meninges [41]. Lymphatics are the main structure able to source and recruit immune cells. Recently, a network of lymphatic vessels within the human meninges was confirmed via contrast-MRI and immunostaining [41]. The exact location of lymphatics within the meninges is still controversial. Central nervous system (CNS) lymphatics are bilateral and in close proximity to the brain sinuses, the middle meningeal artery branches, and the choroid plexus [41]. Additionally, there is more lymphatic vessels concentration along the superior sagittal, straight sinuses, and middle meningeal arteries [42]. This spatial location and density of meningeal lymphatics might associate with the distribution of enhancement present in RM.
The induction therapy for RM included oral/ intravenous steroids as initial therapy in 97% of the cohort. The only exception was 1 case on MTX. Eighty percent received an intravenous steroid pulse as initial treatment, followed by a prednisone taper in 67%. Disease-modifying-anti-rheumatic drugs (DMARDs) alone or combined were used in 40% (Table 1). RTX was the most used biologic (8 cases), followed by tocilizumab (2 cases), and infliximab (1 case). Our review describes a higher rate of RTX in RM induction therapy [4]. The recent publication of our cases (2018, 2019) and the increased use of RTX to treat extra-articular RA complications might explain this predominance. Biologic therapy was used in 37% of the sample, and immunosuppressive therapy in 20% (cyclophosphamide 5 cases, azathioprine 1 case). The most common combination therapy for RM induction was steroids, plus biologics, and/or immunosuppressants (53.3%), followed by steroids/DMARDs in 16.6%. Our case was the only receiving intravenous immunoglobulin. Overall, improvement in the 31 cases (Table 1) was defined by a resolution of symptoms and imaging findings.
Are active/latent infections associated with RM onset?
Our case highlights the puzzling simultaneous development of RM and infection in a patient without RA history, so we decided to further explore this association. We assess the presence of infections in 69-RM reported cases (2000–2020) (Supplemental data). Out of 69, 3 had an active/latent infection at the time of RM diagnosis (4.3%). Two of the 3 cases were QuantiFERON Gold-positive. One was treated as active TB for 12 months [7]. The second case received coverage for latent TB [8]. The third was our case (supplemental data). The patients were male without a previous RA history. The remaining 66 patients without infections did have a prior history or RA. We decided to stratify the 31 cases from 2017 to 2020 in 2 groups: a group without a previous history of RA (without-RA-group), and a group with previous history (previous-RA-group). Each group had 10 and 21 patients, respectively. Our purpose is to describe, if present, a trend of RM features in each group. We compared frequencies using chi-square, we only mention significant p values.
The without-RA-group had more active/latent infection (30% vs 0%, Pearson chi-square = 0.008), in comparison with the previous-RA-group. Both groups had similar frequencies of cranial nerve involvement (20% vs 19%), and headache (60% vs 57.1%). The without-RA-group presented more with synovitis (30% vs 19%), seizures (50% vs 38%), and focal weakness (90% vs 71%). The previous-RA-group tended to present with RMSA (76.2% vs 50%), lung involvement (19% vs 0%), and recurred more (71.4% vs 50%) in comparison with the without-RA group. The latter group had more frequently RF > 50 IU/mL (70% vs 61.9%) and ACPA > 125 u/mL (80% vs 66.7%) in comparison with the previous-RA-group. In CSF, the WBC was similar (50% vs 48%). CSF-protein was higher in the previous-RA-group (76% vs 30%, Spearman chi-square = 0.027), supported by a predominant OB presence (56% vs 43%). Bilateral contrast enhancement in meningeal layers is more frequent in the previous-RA-group (62% vs 40%), as well as PE (43% vs 30%). LE alone predominates in the without-RA-group (60% vs 40%). Meningeal inflammation is present in all the cases except for 1 inconclusive result. Rheumatoid nodules tended to present similarly (38.46% vs 33.3%), whereas vasculitis is more frequent in the without-RA-group (22.2% vs 7.69%).
In the cohort, routinely ordered infectious workup (supplemental data) in blood and CSF included testing for lyme, syphilis, HIV, hepatitis B/C, blood cultures, TB, viral (HSV, VZV and Epstein-Barr), and bacterial infections. Testing for fungi was performed less frequently. Only our patient was studied for free-living amoeba. Brain biopsies were consistently examined for microorganisms [7, 22].
In summary, the 31 cases present with common symptoms, signs, imaging, and pathology findings already described [3, 4]. When searching for different clinical trends between both groups, the without-RA group tends to present more with active infections (p < 0.01), headaches, focal weakness, seizures, synovitis, RF > 50/ACPA > 125 values, unilateral meningeal enhancement, LE alone, and vasculitis in the brain biopsy. Instead the previous-RA-group, in comparison with the without-RA-Group, tends to present more with RMSA, lung involvement, higher CSF protein (p < 0.05), oligoclonal bands, PE, and bilateral meningeal enhancement. The 3 active/latent infections reported were TB and NF, which are acquired through the respiratory system. Two of the patients received infection-treatment while our patient refused. It is unknown whether antimicrobial coverage had an impact on disease morbidity.
How did our patient survive NF?
NF is responsible for primary amebic meningoencephalitis (PAM). This free-living amoeba is present not only in natural water reservoirs, but also in domestic water, and neti pot nasal irrigation equipment [43]. NF enters the nasal cavity, crosses the cribriform plate, and through the olfactory nerve penetrates CNS causing meningoencephalitis. PAM has a 90% mortality rate. As per CDC, PAM and NF infection can be diagnosed by detecting (a) NF organisms, or (b) nucleic acid, or (c), or antigen; in CSF, biopsy, or tissue specimens [44]. Our patient met CDC criteria for NF with a 99% match for NF in PCR (Fig. 3). The results were positive in 2 different samples, taken in 2 different hospital admissions.
Why did our patient survive the “brain eater” without treatment? As per today, it is unknown whether virulence can differ among NF strains [45]. We can speculate that our patient was exposed to a less virulent NF strain. A hyper-immune response to NF, able to neutralize the infection but, favoring an autoimmune disease by bystander effect is also possible. Subjects carrying different toll-like-receptor 4 (TLR4) single nucleotide polymorphisms (SNP) can unleash a broad spectrum of systemic inflammatory response intensity [46]. We did not determine TLR4-SNPs, but our patient could have carried a TLR4-SNP able to trigger an enhanced TLR4-pathway activation, aiding RA by subsequent production of cytokines [47].
Association between RA and free-living amoeba
Many microorganisms, including Mycobacterium tuberculosis, and Acanthamoeba are associated with RA [48]. Acanthamoeba and NF are free-living amoeba that cause a fatal infection in the CNS by reaching the neuro-olfactory epithelium [49]. Therefore, they might share structural features. Higher IgG and IgM antibody titers against Acanthamoeba have been described in RA patients vs. healthy controls (57.8% vs 41.2%) [50]. Jeansson et al. describe higher titers of IgM (64% vs 47%), and lower IgG (57% vs 71%) [51]. Additionally, synovial membranes from RA patients immuno-react strongly to Acanthamoeba-antibodies [51]. The authors identify a cross-reactive epitope between RA-synovium and Acanthamoeba, proposing this shared epitope as a potential cause of RA [52]. We did not find shared epitopes between Acanthamoeba and NF in the literature; however, TLRs are known to be stimulated by both amoebae. TLRs recognize structural molecules shared by microbes. Mice infected with Acanthamoeba have increased expression of TLR2 and TLR4 in the neocortex [53], and NF is recognized by TLR2 and TLR4 in cultures of mucoepithelial human cells [47]. This may support shared structures by Acanthamoeba and NF. We did not find studies describing NF antibodies in RA.
We can only describe the association between NF DNA and RM onset in our patient. Whether NF induced RM is unknown, but some of the potential mechanism that could explain such causative relation include molecular mimicry, superantigens, and bystander activation [48].
Conclusions
This review supports that RM can precede and be the first presentation of RA. Additionally, we find that the infection rate in patients with de novo RM is higher in those patients without a prior history of RA in comparison with those with previous RA history. Exhaustive infectious workup and anamnesis (e.g., neti pot irrigation) might benefit patients with RM without RA history. Our sample was small, and more studies could confirm characteristic clinical features in each studied group and whether different management might lead to better outcomes. Given the association between Acanthamoeba and RA, it is compelling to investigate Naegleria fowleri antibodies in patients with RA.
References
Deane KD, Demoruelle MK, Kelmenson LB, Kuhn KA, Norris JM, Holers VM (2017) Genetic and environmental risk factors for rheumatoid arthritis. Best Pract Res Clin Rheumatol 31(1):3–18. https://doi.org/10.1016/j.berh.2017.08.003
Marcucci E, Bartoloni E, Alunno A, Leone MC, Cafaro G, Luccioli F, Valentini V, Valentini E, La Paglia GMC, Bonifacio AF, Gerli R (2018) Extra-articular rheumatoid arthritis. Reumatismo 70(4):212–224. https://doi.org/10.4081/reumatismo.2018.1106
Kato T, Hoshi K, Sekijima Y, Matsuda M, Hashimoto T, Otani M, Suzuki A, Ikeda S (2003) Rheumatoid meningitis: an autopsy report and review of the literature. Clin Rheumatol 22(6):475–480. https://doi.org/10.1007/s10067-003-0788-0
Qin Z, Kim J, Valencia D, Hamoodi L, Neltner J, Sizemore T, Lightfoot R Jr (2020) Rheumatoid meningitis: a case report and review of the literature. Neurol Clin Pract 10(1):73–83. https://doi.org/10.1212/cpj.0000000000000678
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, Hawker G (2010) 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62 (9):2569–2581. https://doi.org/10.1002/art.27584
Qvarnstrom Y, Visvesvara GS, Sriram R, da Silva AJ (2006) Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J Clin Microbiol 44(10):3589–3595. https://doi.org/10.1128/jcm.00875-06
Lubomski M, Sy J, Buckland M, Lee AS, Richards B, Thompson E, Fulham M, Breen N, Morris K, Halmagyi GM (2019) Rheumatoid leptomeningitis presenting with an acute neuropsychiatric disorder. Pract Neurol 19(1):68–71. https://doi.org/10.1136/practneurol-2018-001978
McKenna MC, Vaughan D, Bermingham N, Cronin S (2019) Rheumatoid arthritis presenting as rheumatoid meningitis. BMJ Case Rep 12(1). https://doi.org/10.1136/bcr-2018-226649
Scheitel M, Ives ST, Nasr R, Nolan MW (2019) When the plot thickens: a rare complication of rheumatoid arthritis. J Community Hosp Intern Med Perspect 9(2):143–146. https://doi.org/10.1080/20009666.2019.1593780
Pellerin D, Wodkowski M, Guiot MC, AlDhukair H, Blotsky A, Karamchandani J, Vinet E, Lafontaine AL, Lubarsky S (2019) Rheumatoid meningitis presenting with acute parkinsonism and protracted non-convulsive seizures: an unusual case presentation and review of treatment strategies. Front Neurol 10:163. https://doi.org/10.3389/fneur.2019.00163
Abdullah HMA, Omar M, Jbeli A, Fanciullo J (2019) Meningeal rheumatoid nodules in a 55-year-old man presenting with chronic headaches and oculomotor nerve palsy: an uncommon extra-articular manifestation of rheumatoid arthritis. BMJ Case Rep 12(12). https://doi.org/10.1136/bcr-2019-231474
Nissen MS, Nilsson AC, Forsberg J, Milthers J, Wirenfeldt M, Bonde C, Byg KE, Ellingsen T, Blaabjerg M (2019) Use of cerebrospinal fluid biomarkers in diagnosis and monitoring of rheumatoid meningitis. Front Neurol 10:666. https://doi.org/10.3389/fneur.2019.00666
Yagita K, Shinde A, Suenaga T (2019) Rheumatoid meningitis can present MRI findings that mimic chronic subdural haematoma. BMJ Case Rep 12(8):e229642. https://doi.org/10.1136/bcr-2019-229642
Grose D, Linger M, Tinni S, Sahathevan R (2019) Rheumatoid meningitis: a rare cause of unilateral pachymeningitis. BMJ Case Rep 12(4). https://doi.org/10.1136/bcr-2018-227905
Finkelshtein V, Lampl Y, Lorberboym M, Kanner A, Ben-Ami Raichman D, Dabby R, Tanay A (2018) Self-limited rheumatoid meningitis as a presenting symptom of rheumatoid arthritis. Isr Med Assoc J 20(4):262–264
Parsons AM, Zuniga LA, Hoxworth JM, Lyons M, Aslam F, Goodman BP (2018) Rheumatoid meningitis: a case review. Neurologist 23(3):83–85. https://doi.org/10.1097/nrl.0000000000000158
Gherghel N, Stan A, Stan H (2018) Pearls & Oy-sters: rheumatoid meningitis occurring during treatment with etanercept. Neurology 91(17):806–808. https://doi.org/10.1212/wnl.0000000000006397
Tiniakou E, Kontzialis M, Petri M (2018) Rheumatoid Pachymeningitis: a rare complication of rheumatoid arthritis. J Rheumatol 45(9):1325–1326. https://doi.org/10.3899/jrheum.171074
Oono M, Fujita Y, Uchida N, Kawai U, Fujita-Nakata M, Nakanishi M, Sanada M, Nagayama S, Matsui M (2018) Rheumatoid meningitis developed in patient with stable rheumatoid arthritis and myasthenia gravis-detailed analysis of intracranial inflammation using flow cytometry. J Neuroinflammation 15(1):151. https://doi.org/10.1186/s12974-018-1196-3
Harrison NS, Kishore S, Majithia V (2018) Rheumatoid meningitis: successful remission with rituximab. BMJ Case Rep 11(1):e226642. https://doi.org/10.1136/bcr-2018-226642
Akamatsu M, Maki F, Akiyama H, Hara D, Hoshino M, Hasegawa Y (2018) Rheumatoid meningitis presenting with a stroke-like attack treated with recombinant tissue plasminogen activator: a case presentation. BMC Neurol 18(1):139. https://doi.org/10.1186/s12883-018-1143-z
Alexander SK, Di Cicco M, Pohl U (2018) Cifelli A (2018) rheumatoid disease: an unusual cause of relapsing meningoencephalitis. BMJ Case Rep. https://doi.org/10.1136/bcr-2017-222587
Schuster S, Braass H, Iking-Konert C, Schnoor U, Matschke J, Gerloff C, Thomalla G, Magnus T (2018) Rheumatoid meningitis: a rare cause of aseptic meningitis with frequently stroke-like episodes. Neurol Clin Pract 8(5):451–455. https://doi.org/10.1212/cpj.0000000000000504
Jessee RC (2017) Keenan RT rheumatoid arthritis presenting as rheumatoid meningitis: a case report. In
Degboe Y, Fajadet B, Laurent C, Cantagrel A, Constantin A, Ruyssen-Witrand A (2017) A rare case of rheumatoid pachyleptomeningitis successfully treated with rituximab. Rheumatology (Oxford) 56(7):1238–1240. https://doi.org/10.1093/rheumatology/kex059
Moeyersoons A, Verschueren P, Tousseyn T, De Langhe E (2018) Rheumatoid granulomatous disease and pachymeningitis successfully treated with rituximab. Acta Clin Belg 73(4):307–312. https://doi.org/10.1080/17843286.2017.1375193
Lee Ching C, Kenyon L, Berk M, Park C (2019) Rheumatoid meningitis sine arthritis. J Neuroimmunol 328:73–75. https://doi.org/10.1016/j.jneuroim.2018.12.001
Matsuda S, Yoshida S, Takeuchi T, Fujiki Y, Yoshikawa A, Makino S (2019) Asymptomatic rheumatoid meningitis revealed by magnetic resonance imaging, followed by systemic rheumatic vasculitis: a case report and a review of the literature. Mod Rheumatol 29(2):370–376. https://doi.org/10.1080/14397595.2016.1232333
Lattanzi S, Cagnetti C, Di Bella P, Scarpelli M, Silvestrini M, Provinciali L (2014) Leptomeningeal inflammation in rheumatoid arthritis. Neurol Neuroimmunol Neuroinflamm 1(4):e43–e43. https://doi.org/10.1212/NXI.0000000000000043
Servioli MJ, Chugh C, Lee JM, Biller J (2011) Rheumatoid meningitis. Front Neurol 2:84–84. https://doi.org/10.3389/fneur.2011.00084
Jones SE, Belsley NA, McLoud TC, Mullins ME (2006) Rheumatoid meningitis: radiologic and pathologic correlation. AJR Am J Roentgenol 186(4):1181–1183. https://doi.org/10.2214/ajr.05.0859
Nihat A, Chinthapalli K, Bridges L, Johns P, Sofat N, Moynihan B (2016) Rheumatoid meningitis. Pract Neurol 16(4):312–314. https://doi.org/10.1136/practneurol-2015-001306
Walsh DA, Catravas J, Wharton J (2000) Angiotensin converting enzyme in human synovium: increased stromal [(125)I]351A binding in rheumatoid arthritis. Ann Rheum Dis 59(2):125–131. https://doi.org/10.1136/ard.59.2.125
Timmermans WM, van Laar JA, van Hagen PM, van Zelm MC (2016) Immunopathogenesis of granulomas in chronic autoinflammatory diseases. Clin Transl Immunology 5(12):e118. https://doi.org/10.1038/cti.2016.75
Notman DD, Kurata N, Tan EM (1975) Profiles of antinuclear antibodies in systemic rheumatic diseases. Ann Intern Med 83(4):464–469. https://doi.org/10.7326/0003-4819-83-4-464
Takase K, Horton SC, Ganesha A, Das S, McHugh A, Emery P, Savic S, Buch MH (2014) What is the utility of routine ANA testing in predicting development of biological DMARD-induced lupus and vasculitis in patients with rheumatoid arthritis? Data from a single-centre cohort. Ann Rheum Dis 73(9):1695–1699. https://doi.org/10.1136/annrheumdis-2014-205318
Smirniotopoulos JG, Murphy FM, Rushing EJ, Rees JH, Schroeder JW (2007) Patterns of contrast enhancement in the brain and meninges. RadioGraphics 27(2):525–551. https://doi.org/10.1148/rg.272065155
Berndt D, Millward JM, Schnorr J, Taupitz M, Stangl V, Paul F, Wagner S, Wuerfel JT, Sack I, Ludwig A, Infante-Duarte C (2017) Inflammation-induced brain endothelial activation leads to uptake of electrostatically stabilized iron oxide nanoparticles via sulfated glycosaminoglycans. Nanomedicine 13(4):1411–1421. https://doi.org/10.1016/j.nano.2017.01.010
Wang S, Hesse B, Roman M, Stier D, Castillo-Michel H, Cotte M, Suuronen JP, Lagrange A, Radbruch H, Paul F, Taupitz M, Schellenberger E, Sack I, Infante-Duarte C (2019) Increased retention of gadolinium in the inflamed brain after repeated administration of gadopentetate dimeglumine: a proof-of-concept study in mice combining ICP-MS and micro- and nano-SR-XRF. Investig Radiol 54(10):617–626. https://doi.org/10.1097/rli.0000000000000571
Wang JY, Roehrl MH (2002) Glycosaminoglycans are a potential cause of rheumatoid arthritis. Proc Natl Acad Sci U S A 99(22):14362–14367. https://doi.org/10.1073/pnas.222536599
Absinta M, Ha SK, Nair G, Sati P, Luciano NJ, Palisoc M, Louveau A, Zaghloul KA, Pittaluga S, Kipnis J, Reich DS (2017) Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI Elife 6:6. https://doi.org/10.7554/eLife.29738
Hershenhouse KS, Shauly O, Gould DJ, Patel KM (2019) Meningeal lymphatics: a review and future directions from a clinical perspective. Neuroscience Insights 14:1179069519889027. https://doi.org/10.1177/1179069519889027
Yoder JS, Straif-Bourgeois S, Roy SL, Moore TA, Visvesvara GS, Ratard RC, Hill VR, Wilson JD, Linscott AJ, Crager R, Kozak NA, Sriram R, Narayanan J, Mull B, Kahler AM, Schneeberger C, da Silva AJ, Poudel M, Baumgarten KL, Xiao L, Beach MJ (2012) Primary amebic meningoencephalitis deaths associated with sinus irrigation using contaminated tap water. Clin Infect Dis 55(9):e79–e85. https://doi.org/10.1093/cid/cis626
CfDCa P (2017) Diagnosis & detection. Centers for Disease Control and Prevention, Centers for Disease Control and Prevention
Coupat-Goutaland B, Régoudis E, Besseyrias M, Mularoni A, Binet M, Herbelin P, Pélandakis M (2016) Population structure in Naegleria fowleri as revealed by microsatellite markers. PLoS One 11(4):e0152434–e0152434. https://doi.org/10.1371/journal.pone.0152434
Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E, Willeit J, Schwartz DA (2002) Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med 347(3):185–192. https://doi.org/10.1056/NEJMoa012673
Martinez-Castillo M, Santos-Argumedo L, Galvan-Moroyoqui JM, Serrano-Luna J, Shibayama M (2018) Toll-like receptors participate in Naegleria fowleri recognition. Parasitol Res 117(1):75–87. https://doi.org/10.1007/s00436-017-5666-9
Arleevskaya MI, Kravtsova OA, Lemerle J, Renaudineau Y, Tsibulkin AP (2016) How rheumatoid arthritis can result from provocation of the immune system by microorganisms and viruses. Front Microbiol 7:1296. https://doi.org/10.3389/fmicb.2016.01296
Martinez AJ (1996) Free-living amebas: Naegleria, Acanthamoeba and Balamuthia. In: th, Baron S (eds) Medical Microbiology. University of Texas Medical Branch at Galveston The University of Texas Medical Branch at Galveston., Galveston (TX),
Eftekhar M, Athari A, Haghighi A, Mosaffa N, Shahram F, Abadi A (2010) Seroprevalence of Acanthamoeba antibodies in rheumatoid arthritis patients by IFAT, Tehran, Iran 2007. Iran J Parasitol 5(1):35–40
Jeansson S, Kvien TK (2001) Acanthamoeba polyphaga in rheumatoid arthritis: possibility for a chronic infection. Scand J Immunol 53(6):610–614. https://doi.org/10.1046/j.1365-3083.2001.00926.x
Jeansson S, Klingen T, Roald B, Rud E, Kvien T (2001) THU0093 rheumatoid synovia and acanthamoeba polyphaga: immunohistochemical evidence of crossreactivity. Ann Rheum Dis 60(Suppl 1):A382–A383. https://doi.org/10.1136/annrheumdis-2001.970
Kot K, Kosik-Bogacka D, Lanocha-Arendarczyk N, Wojtkowiak-Giera A, Kolasa-Wolosiuk A (2019) Expression of toll-like receptors (TLR2 and TLR4) in the eyes of mice with disseminated Acanthamoebiasis. Biomed Res Int 2019:1401894–1401898. https://doi.org/10.1155/2019/1401894
Acknowledgments
We are thankful to Lisa E. Thompson, M.D. for comments that improved the manuscript. We also thank Kimberly Mergen, M.S., and Susan Madison-Antenucci, Ph.D. at Wadsworth Center, New York State Department of Health, for assistance in technique support and PCR interpretation.
Funding
No targeted funding reported.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Statement of informed consent
Informed consent has been obtained from the patient to access and collect data from medical records with the purpose of scientific publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 165 kb).
Rights and permissions
About this article
Cite this article
Rodriguez Alvarez, M., Rodríguez Valencia, L.M., Seidman, R. et al. Rheumatoid meningitis and infection in absence of rheumatoid arthritis history: review of 31 cases. Clin Rheumatol 39, 3833–3845 (2020). https://doi.org/10.1007/s10067-020-05221-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-05221-1






