Abstract
Introduction
The TNF inhibitors were the first immunobiologicals used to treat rheumatic diseases, but their use is associated with an increased risk of tuberculosis. The primary objective is to estimate the incidence of tuberculosis in patients with rheumatic diseases exposed to anti-TNF therapy. The secondary objectives are to evaluate the incidence of tuberculosis by region and subgroups of diseases, to review the presentation of tuberculosis in these patients, and to assess the time elapsed between onset of anti-TNF therapy and development of active granulomatous disease.
Methods
A systematic review of the literature was conducted in MEDLINE, the Cochrane Library, and LILACS. The primary endpoint was described as incidence and secondary outcomes, through subgroup analyses and comparisons of means.
Results
We included 52 observational studies. Among the exposed patients, 947 cases of tuberculosis were documented (62.2% pulmonary), with a cumulative incidence of 9.62 cases per 1000 patients exposed. TB incidence across different continents was distributed as follows: South America, 11.75 cases/1000 patients exposed; North America, 4.34 cases/1000 patients exposed; Europe, 6.28 cases/1000 patients exposed; and Asia, 13.47 cases/1000 patients exposed. There were no significant differences in TB incidence among the described diseases. The mean time elapsed from start of anti-TNF therapy until the endpoint was 18.05 months.
Conclusion
The incidence of TB in patients with rheumatic diseases exposed TNF inhibitor considering all countries was 9.62 cases per 1000 patients exposed. TB incidence was higher in South America and Asia compared with North America and Europe. Most cases occurred in the first XX months of use, and the pulmonary form predominated.
Key Points • Higher incidence of tuberculosis in patients exposed to anti-TNF compared with the general population. • Higher incidence of TB in countries of South America and Asia compared with North America and Europe. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tumor necrosis factor-alpha (TNF-α) is a cytokine involved in the immune response against intracellular pathogens such as Mycobacterium tuberculosis. Through its role in macrophage activation and continuous cell recruitment, it is essential for the maintenance of granuloma structure. The role of this cytokine has gained even more prominence since the advent of immunobiological therapy with TNF inhibitors or anti-TNF agents, for the treatment of rheumatic diseases [1].
Rheumatic diseases such as rheumatoid arthritis (RA), ankylosing spondylitis (AS), psoriatic arthritis (PA), and juvenile idiopathic arthritis (JIA) are systemic autoimmune diseases that share chronic joint inflammation as a core feature [2]. Several cytokines are implicated in the pathogenesis of these conditions, including TNF-α, which has a particularly prominent role in development of the systemic inflammatory process and joint damage of rheumatic arthropathies, leading to functional impairment and worsening quality of life [3, 4].
In 2017, an estimated 10 million people developed tuberculosis (TB) worldwide. Of these cases, 87% occurred in the 30 countries designated by the World Health Organization (WHO) as having a high tuberculosis burden, with two-thirds occurring in Asia and Africa. According to WHO, only 6% of cases occurred in the European region, and 3% in the Americas. The incidence of TB varies widely among countries. In 2017, it was estimated at fewer than 10 new cases per 100,000 populations in higher-income countries, versus approximately 150–400 new cases per 100,000 in most of the 30 countries with the highest absolute TB incidence [5].
The advent of biologic therapy with TNF inhibitors has greatly advanced the treatment of rheumatic diseases, allowing control of disease activity and reduction of joint damage, especially in patients who are unresponsive to conventional therapy, resulting in better clinical outcomes [6, 7]. However, there is a well-established increase in risk of active tuberculosis in individuals exposed to anti-TNF agents when compared with the general population [8,9,10]. The risk of TB in individuals exposed to anti-TNF therapy when compared with the general population is approximately 8–12 times greater in countries with a low incidence of TB, and up to 24–40 times greater in countries with a high incidence of TB [8, 11,12,13].
Previous systematic reviews and meta-analyses corroborate this increased risk of TB in users of anti-TNF therapy; however, most were restricted to randomized clinical trials, and often to a single, specific rheumatic condition [14, 15]. The present systematic review, limited to observational studies providing real-life data, aims to evaluate the actual incidence of TB in patients with rheumatic diseases exposed to anti-TNF agents. As secondary objectives, we seek to ascertain the incidence of TB across different rheumatic conditions for which anti-TNF therapy is prescribed and across subgroups of continents, as well as assess the most common forms of TB manifesting in this population and the time elapsed between first exposure to anti-TNF therapy and development of opportunistic TB infection.
Material and methods
This review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The study protocol was registered in the PROSPERO (International Prospective Register of Systematic Reviews) platform, with accession number CRD42018083323.
Inclusion and exclusion criteria
We searched for observational studies which evaluated the incidence of TB in patients with rheumatic diseases exposed to anti-TNF agents. There was no date or language limitation. Studies were selected according to inclusion criteria defined in a preexisting protocol.
Participants: patients with any related rheumatic disease (rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, juvenile idiopathic arthritis) and exposure to one of the five anti-TNF agents (infliximab, etanercept, adalimumab, golimumab, certolizumab pegol).
Exposure: exposure to an anti-TNF agent, with or without prior or concomitant standard treatment.
Outcomes: incidence of TB in users of anti-TNF agents, form of TB, time elapsed between exposure to anti-TNF agent, and development of granulomatous disease.
Comparator: patients receiving standard treatment.
Study design: observational studies.
The exclusion criteria were studies which did not report TB incidence, absence of included exposed patients, duplicate records, randomized controlled trials, meta-analyses, and case reports.
Search strategy
A systematic review of the PubMed, EMBASE, Cochrane Library, and LILACS databases was conducted. There were no limitations on start date, language, or region of publication. The database search was based on the following Medical Subject Headings (MeSH) and combinations thereof: tuberculosis, latent tuberculosis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, juvenile idiopathic arthritis, infliximab, etanercept, adalimumab, golimumab, certolizumab pegol. By way of example, the PubMed/MEDLINE search strategy is described in the supplement.
Study selection and data collection
Two independent reviewers selected articles by analysis of titles and abstracts. Potentially eligible records were retrieved for full-text review. Disagreements were resolved by consensus with a third reviewer.
Data extraction was performed independently by two reviewers, and again, any discrepancies were resolved by discussion. The quality of the eligible studies was evaluated by the Newcastle–Ottawa Scale, with a maximum score of 9 stars across different categories (selection, comparability, and exposure or outcome). The extracted data included the authors’ names, year of publication, study setting, number of patients exposed to anti-TNF agents, number of patients who developed TB, and number of patients included with each of the predefined conditions of interest, as well as time elapsed from anti-TNF exposure to TB development, mean age, gender, form of TB developed, treatment for latent TB, and TB screening test results.
Statistical analysis
All statistical analysis was carried out in the Review Manager 5.3 software environment. The primary endpoint was evaluated and expressed as the incidence (cases per 1000 exposed patients). A subgroup analysis was performed to compare incidence rates. Continuous variables were presented as means.
Considering the results of study quality assessment, the incidence of the main outcome was calculated only for those studies with lower risk of bias. Studies assigned a score of 6 stars or more were deemed to have a lower risk of bias.
Results
Search results
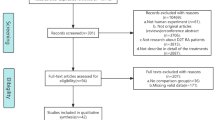
The search strategy described above identified 478 articles for systematic review: 130 records in PubMed, 170 in EMBASE and the Cochrane Library, and 8 in LILACS. After review of titles and abstracts, 270 articles were selected. The remaining 208 addressed topics irrelevant to this study and were thus excluded. Full-text reading then excluded a further 218 articles, including duplicate records. The reasons for exclusion are described in the flow diagram of study selection (Fig. 1).
Thus, 52 articles were ultimately eligible for complete data extraction (covering publications from 2003 to 2017). We evaluated the quality of these observational studies and found only three with a quality rating of < 5 stars and greater potential for bias. The quality of the included studies was thus deemed satisfactory. Table 1 illustrates the process of methodological evaluation of the quality of included studies.
Considering the 52 observational studies included (Table 2), a total of 98,483 patients were exposed to at least one anti-TNF agent. The mean age of patients on anti-TNF therapy was 42.9 years, and 62% of those exposed were females. Of the exposed patients, 947 developed TB (62.2% with the pulmonary form of the disease and 37.8% with extrapulmonary manifestations). The overall incidence of TB was 9.62 cases [CI 9.01–10.23] per 1000 exposed patients. Considering only studies with a lower risk of bias, a total of 79,172 patients were exposed to anti-TNF agents, 789 of whom developed TB, for an overall incidence of 9.97 [CI 9.27–10.66] cases per 1000 exposed patients (no significant difference vs. all studies included). The mean time elapsed from initiation of anti-TNF therapy to development of TB was 18.05 ± 9.12 months.
When assessing only the adult population exposed to anti-TNF agents, we found a total of 97,896 exposed individuals, 943 of whom developed TB. The overall incidence of TB in adults was 9.62 cases [CI 9.02–10.24] per 1000 exposed patients. Conversely, when considering only the population under 16 years of age, 576 individuals were exposed to anti-TNF therapy, 4 of whom developed TB, yielding an incidence of 6.81 cases [CI 0.16–13.47] per 1000 exposed patients. Again, this rate was not significantly different from that of the adult population exposed to anti-TNF agents.
Subgroup analysis of incidence by specific rheumatic diseases showed no statistically significant difference between the different diagnoses. A total of 73,497 patients with RA were exposed to anti-TNF agents, 677 of whom had a diagnosis of TB, for an incidence of 9.21 cases [CI 8.52–9.90] per 1000 exposed patients. Of the 12,260 patients with SA exposed to anti-TNF therapy, 138 developed TB, for an incidence of 11.25 cases [CI 9.39–10.13] per 1000 exposed patients. Of 886 exposed patients with PA, 10 developed tuberculosis, which corresponds to an incidence of 11.35 cases [CI 4.36–18.35] per 1000 exposed patients. Finally, of the 915 patients with JIA exposed to anti-TNF therapy, 6 developed treatment-emergent TB, for an incidence of 6.56 cases [CI 1.33–11.79] per 1000 exposed patients.
Another subgroup analysis was then carried out, taking into consideration the different locations of each study and their distribution by continents. In South America, a total of 2128 individuals were exposed to anti-TNF therapy, with 25 cases of TB diagnosed among those exposed, for an incidence of 11.75 cases [CI 7.17–16.33] per 1000. In North America, 15,425 individuals were prescribed at least one anti-TNF agent; 67 cases of tuberculosis developed in this population, for an incidence of 4.34 cases [CI 3.31–5.38] per 1000. In Europe, 32,629 patients were exposed to anti-TNF therapy. Of these, 205 developed TB, which corresponds to an incidence of 6.28 cases [CI 5.34–7.14] per 1000. In Asia, 48,257 individuals were exposed to anti-TNF therapy; 650 cases of tuberculosis were diagnosed, for an incidence of 13.47 cases [CI 12.44–14.49] per 1000. These data show a statistically significant difference in incidence of TB between South America and Asia in relation to Europe and North America.
Regarding latent tuberculosis approach, 14 studies included in our review evaluated the treatment of latent tuberculosis and screening prior to initiation of anti-TNF use. Considering these studies, 12,793 tuberculin tests were performed, and 5530 were positive. Treatment for complete latent tuberculosis was performed in 6414 individuals. We also found that 164 patients with PPD reagent did not undergo treatment for latent tuberculosis or had incomplete treatment. Regarding the treatments for latent tuberculosis, these were indicated in patients with tuberculin test alteration or radiographic alterations suggestive of latent disease.
Discussion
Anti-TNF therapy has proven efficacy for the control of rheumatic conditions, providing clinical improvement and modifying the natural history of the disease by targeting TNF, a cytokine involved in the pathogenesis of inflammatory arthropathies [6, 66]. Despite the clear benefits of this therapy, it is associated with and increased risk of infections, including serious infections such as TB [15, 67].
This systematic review was designed to assess the risk of developing TB in patients with rheumatic diseases who are receiving anti-TNF therapy. The decision to include only observational studies, rather than controlled trials, was made to ensure reliance on real-life data. We found a high incidence of TB among users of the various anti-TNF agents available, with an overall risk approximately 12 to 25 times greater than that of the general population [11, 13]. There was no significant difference in TB incidence among the different types of rheumatic diseases. [21, 39, 68]
This review also found a higher rate of pulmonary tuberculosis in patients taking anti-TNF agents, a finding previously reported in other studies [42, 45]. The time from onset of TNF inhibitor use to diagnosis of TB in this review was less than 24 months, which corroborates previous data on the development of TB within the first few years of anti-TNF therapy. [8, 16]
This was one of the first reviews to evaluate the incidence of TB among the different regions of the world, considering all five anti-TNF agents available at the time of writing, and in different rheumatic diseases. The incidence of TB in patients exposed to anti-TNF therapy is directly linked to the incidence of TB in the general population. Due to this impact of overall TB incidence, different incidence rates are found between groups of patients on anti-TNF therapy in different regions of the world. The present study found a higher incidence of TB among patients receiving anti-TNF agents for rheumatic diseases in regions such as Asia and South America, both of which have a significantly higher overall incidence of TB than Europe and North America [16, 42, 54, 57, 64, 69, 70].
All studies included in this review have a number of limitations, mainly attributable to their retrospective designs. Hence, data on concomitant use of disease-modifying drugs, corticosteroid therapy, and other comorbidities were incompletely recorded. In addition, many studies did not provide data on screening for and treatment of latent TB, with data often missing or otherwise unavailable. Therefore, a significant limitation of our study was our inability to assess the impact of latent TB treatment prior to exposure to anti-TNF agents. Regarding the different TNF inhibitors, the lack of data on certolizumab pegol and golimumab must be noted.
Considering that this systematic review included publications from different regions and continents, it was expected that the overall incidence found might not be reproducible in any particular population exposed to anti-TNF therapy, precisely because of the variation of TB incidence in the general population, hence, our decision to evaluate the incidence of TB in patients on anti-TNF therapy in each region separately as well, to allow better interpretation of data.
Strengths of this review include the evaluation of all articles which reported screening for latent TB, with no date or language restrictions. In addition, the results of this review confirm the previous finding of a higher incidence of TB in users of anti-TNF therapy in relation to the general population, using real-life observational data from clinical practice.
Conclusion
In summary, we found a high incidence of TB in patients receiving anti-TNF therapy for rheumatic diseases worldwide. This incidence was higher in South America and Asia than in North America and Europe. Among the several forms of TB diagnosed, pulmonary tuberculosis predominated. The time from initiation of anti-TNF therapy until development and diagnosis of tuberculosis was roughly 18 months. There was no difference in TB incidence across the different rheumatic diseases for which patients were receiving anti-TNF agents.
References
Wallis RS, Ehlers S (2005) Tumor necrosis factor and granuloma biology: explaining the differential infection risk of etanercept and infliximab. Semin Arthritis Rheum 34(5 SUPPL):34–38. https://doi.org/10.1016/j.semarthrit.2005.01.009
Faleiro LR, Araujo LHR, Varavallo MA (2011) A terapia anti-TNF-a na artrite reumatóide. Semina: Ciênc Biol Saúde 32(1):77–94. https://doi.org/10.5433/1679-0367.2011v32n1p77
Firestein GS, Budd RC, Gabriel SE, McInnes IB, O’Dell J (2013) Kelley’s textbook of rheumatology. Vol Ninth. (Elsevier, ed.).Philadelphia
Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman M (2015) Rheumatology Vol Sixth. (Elsevier, ed.). Philadelphia
World Health Organization (2018) Global tuberculosis report 2018 [Internet]. Geneva: World Health Organization. Available from: Available from: http://apps.who.int/medicinedocs/en/m/abstract/Js23553en/
Maini R, St Clair EW, Breedveld F et al (1999) Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 354(9194):1932–1939. https://doi.org/10.1016/S0140-6736(99)05246-0
Van Der Heijde D, Dijkmans B, Geusens P et al (2005) Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 52(2):582–591. https://doi.org/10.1002/art.20852
Dixon WG, Hyrich KL, Watson KD, Lunt M, Galloway J, Ustianowski A, B S R B R Control Centre Consortium, Symmons DP, BSR Biologics Register (2010) Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR). Ann Rheum Dis 69(3):522–528. https://doi.org/10.1136/ard.2009.118935
Scrivo R, Armignacco O (2014) Tuberculosis risk and anti-tumour necrosis factor agents in rheumatoid arthritis: a critical appraisal of national registry data. Int J Rheum Dis 17(7):714–722. https://doi.org/10.1111/1756-185X.12375
Carmona L, Gómez-Reino JJ, Rodríguez-Valverde V, Montero D, Pascual-Gómez E, Mola EM, Carreño L, Figueroa M, BIOBADASER Group (2005) Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum 52(6):1766–1772. https://doi.org/10.1002/art.21043
Tubach F, Salmon D, Ravaud P, Allanore Y, Goupille P, Bréban M, Pallot-Prades B, Pouplin S, Sacchi A, Chichemanian RM, Bretagne S, Emilie D, Lemann M, Lortholary O, Mariette X, Research Axed on Tolerance of Biotherapies Group (2009) Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French research axed on tolerance of biotherapies registry. Arthritis Rheum 60(7):1884–1894. https://doi.org/10.1002/art.24632
Seong S-S, Choi C-B, Woo J-H, Bae KW, Joung CL, Uhm WS, Kim TH, Jun JB, Yoo DH, Lee JT, Bae SC (2007) Incidence of tuberculosis in Korean patients with rheumatoid arthritis (RA): effects of RA itself and of tumor necrosis factor blockers. J Rheumatol 34(4):706–711 http://www.scopus.com/inward/record.url?eid=2-s2.0-34247332718&partnerID=40&md5=37282bf3735840802a493e16fce30fa9. Accessed Aug 2018
Cagatay T, Bingol Z, Kıyan E, Yegin Z, Okumus G, Arseven O, Erkan F, Gulbaran Z, Erelel M, Ece T, Cagatay P, Kılıçaslan Z (2018) Follow-up of 1887 patients receiving tumor necrosis-alpha antagonists: tuberculin skin test conversion and tuberculosis risk. Clin Respir J 12(4):1668–1675. https://doi.org/10.1111/crj.12726
Ai J-W, Zhang S, Ruan Q-L, Yu YQ, Zhang BY, Liu QH, Zhang WH (2015) The risk of tuberculosis in patients with rheumatoid arthritis treated with tumor necrosis factor- antagonist: a metaanalysis of both randomized controlled trials and registry/cohort studies. J Rheumatol 42(12):2229–2237. https://doi.org/10.3899/jrheum.150057
Zhang Z, Fan W, Yang G et al (2017) Risk of tuberculosis in patients treated with TNF- α antagonists : a systematic review and meta-analysis of randomised controlled trials. https://doi.org/10.1136/bmjopen-2016-012567
Yonekura CL, Oliveira RDR, Titton DC et al (2017) Incidência de tuberculose em pacientes com artrite reumatoide em uso de bloqueadores do TNF no Brasil: dados do Registro Brasileiro de Monitoração de Terapias Biológicas BiobadaBrasil. Rev Bras Reumatol 57(S 2):477–483. https://doi.org/10.1016/j.rbr.2017.05.003
Lim CH, Chen H-H, Chen Y-H, Chen DY, Huang WN, Tsai JJ, Hsieh TY, Hsieh CW, Hung WT, Lin CT, Lai KL, Tang KT, Tseng CW, Chen YM (2017) The risk of tuberculosis disease in rheumatoid arthritis patients on biologics and targeted therapy: a 15-year real world experience in Taiwan. PLoS One 12(6):e0178035. https://doi.org/10.1371/journal.pone.0178035
Garziera G, Morsch ALB, Otesbelgue F, Staub FL, Palominos PE, Brenol CV, Silva DR (2017) Latent tuberculosis infection and tuberculosis in patients with rheumatic diseases treated with anti-tumor necrosis factor agents. Clin Rheumatol 36(8):1891–1896. https://doi.org/10.1007/s10067-017-3714-6
Lim C-H, Lin C-H, Chen D-Y, Chen YM, Chao WC, Liao TL, Chen HH (2016) One-year tuberculosis risk in rheumatoid arthritis patients starting their first tumor necrosis factor inhibitor therapy from 2008 to 2012 in Taiwan: a nationwide population-based cohort study. PLoS One 11(11):e0166339. https://doi.org/10.1371/journal.pone.0166339
Liao TL, Lin CH, Chen YM, Chang CL, Chen HH, Chen DY (2016) Different risk of tuberculosis and efficacy of isoniazid prophylaxis in rheumatoid arthritis patients with biologic therapy: a nationwide retrospective cohort study in Taiwan. PLoS One 11(4):1–14. https://doi.org/10.1371/journal.pone.0153217
Kisacik B, Pamuk ON, Onat AM, Erer SB, Hatemi G, Ozguler Y, Pehlivan Y, Kilic L, Ertenli I, Can M, Direskeneli H, Keser G, Oksel F, Dalkilic E, Yilmaz S, Pay S, Balkarli A, Cobankara V, Cetin GY, Sayarlioglu M, Cefle A, Yazici A, Avci AB, Terzioglu E, Ozbek S, Akar S, Gul A (2016) Characteristics predicting tuberculosis risk under tumor necrosis factor-α inhibitors: report from a large multicenter cohort with high background prevalence. J Rheumatol 43(3):524–529. https://doi.org/10.3899/jrheum.150177
Watanabe A, Matsumoto T, Igari H, Sawa J, Yamaguchi Y, Sakatani M (2016) Risk of developing active tuberculosis in rheumatoid arthritis patients on adalimumab in Japan. Int J Tuberc Lung Dis 20(1):101–108. https://doi.org/10.5588/ijtld.15.0283
Rotar Ž, Tomsic M (2016) Incidence rate of tuberculosis in rheumatoid arthritis patients treated with TNF-α inhibitors-data from the slovenian national BioRx.si registry, Ann Rheum Dis. 75:1016–1017. https://doi.org/10.1136/annrheumdis-2016-eular.1903
Rahman P, Choquette D, Bensen WG, Khraishi M, Chow A, Zummer M, Shaikh S, Sheriff M, Dixit S, Sholter D, Psaradellis E, Sampalis JS, Letourneau V, Lehman AJ, Nantel F, Rampakakis E, Otawa S, Shawi M (2016) Biologic treatment registry across Canada (BioTRAC): a multicentre, prospective, observational study of patients treated with infliximab for ankylosing spondylitis. BMJ Open 6(4):1–10. https://doi.org/10.1136/bmjopen-2015-009661
Mourão AF, Santos MJ, Melo Gomes JA, Martins FM, Mendonça SC, Oliveira Ramos F, Fernandes S, Salgado M, Guedes M, Carvalho S, Costa JA, Brito I, Duarte C, Furtado C, Lopes A, Rodrigues A, Sequeira G, Branco JC, Fonseca JE, Canhão H (2016) Effectiveness and long-term retention of anti-tumour necrosis factor treatment in juvenile and adult patients with juvenile idiopathic arthritis: data from Reuma.pt. Pediatr Rheumatol Online J 55(4):697–703. https://doi.org/10.1093/rheumatology/kev398
Calzada-Hernández J, Anton-López J, Bou-Torrent R, Iglesias-Jiménez E, Ricart-Campos S, Martín de Carpi J, Carmen García de Vicuña Muñoz de la Nava, Torrente-Segarra V, Sánchez-Manubens J, Giménez-Roca C, Rozas-Quesada L, Juncosa-Morros MT, Fortuny C, Noguera-Julian A (2015) Tuberculosis in pediatric patients treated with anti-TNFα drugs: a cohort study. Pediatr Rheumatol Online J 13(1):54. https://doi.org/10.1186/s12969-015-0054-4
Hsin Y-C, Zhuang L-Z, Yeh K-W, Chang C-W, Horng J-T, Huang J-L (2015) Risk of tuberculosis in children with juvenile idiopathic arthritis: a nationwide population-based study in Taiwan. PLoS One 10(6):e0128768. https://doi.org/10.1371/journal.pone.0128768
Jung SM, Ju JH, Park M-S, Kwok SK, Park KS, Kim HY, Yim HW, Park SH (2015) Risk of tuberculosis in patients treated with anti-tumor necrosis factor therapy: a nationwide study in South Korea, a country with an intermediate tuberculosis burden. Int J Rheum Dis 18(3):323–330. https://doi.org/10.1111/1756-185X.12530
Borekci S, Atahan E, Demir Yilmaz D, Mazıcan N, Duman B et al (2015) Factors affecting the tuberculosis risk in patients receiving anti-tumor necrosis factor-α treatment. Respiration 90(3):191–198. https://doi.org/10.1159/000434684
Gomes CMF, Terreri MT, de Moraes-Pinto MI et al (2015) Incidence of active mycobacterial infections in Brazilian patients with chronic inflammatory arthritis and negative evaluation for latent tuberculosis infection at baseline-a longitudinal analysis after using TNFα blockers. Mem Inst Oswaldo Cruz 110(7):921–928. https://doi.org/10.1590/0074-02760150235
Chiu YM, Lang HC, Lin HY et al (2014) Risk of tuberculosis, serious infection and lymphoma with disease-modifying biologic drugs in rheumatoid arthritis patients in Taiwan. Int J Rheum Dis 17(s3):9–19. https://doi.org/10.1111/1756-185X.12539
Mok C, Chan K, Lee K, Tam L, Lee K (2014) Factors associated with withdrawal of the anti-TNFa biologics in the treatment of rheumatic disease: data from the Hong Kong Biologics Registry. Int J Rheum Dis 17(Suppl. 3):1–8. https://doi.org/10.1111/1756-185X.12264
Yoo JW, Jo KW, Kang BH, Kim MY, Yoo B et al (2014) Mycobacterial diseases developed during anti-tumour necrosis factor-a therapy. Eur Respir J 44(5):1289–1295. https://doi.org/10.1183/09031936.00063514
Ke W-M, Chen L-S, Parng I-M, Chen W-W, On AWF (2013) Risk of tuberculosis in rheumatoid arthritis patients on tumour necrosis factor-alpha inhibitor treatment in Taiwan. Int J Tuberc Lung Dis 17(12):1590–1595. https://doi.org/10.5588/ijtld.13.0368
Lee SK, Kim SY, Kim EY, Jung JY, Park MS, Kim YS, Kim SK, Chang J, Kang YA (2013) Mycobacterial infections in patients treated with tumor necrosis factor antagonists in South Korea. Lung 191(5):565–571. https://doi.org/10.1007/s00408-013-9481-5
Sarychev A, Shovkun L (2013) PReS-FINAL-2019: tuberculosis infection observed in patients receiving biological DMARDs for treatment of juvenile idiopathic arthritis. Pediatr Rheumatol 11. https://doi.org/10.1186/1546-0096-11-S2-P32
Žlnay M, Žlnay D, Salkova M, Oetterová M, Mičeková D, Barancikova K, Rovenský J, Rybár I (2013) The risk of tuberculosis in patients with ankylosing spondylitis during anti-TNF therapy: data from national database in Slovakia. Ann Rheum Dis 72. https://doi.org/10.1136/annrheumdis-2013-eular.1541
He D, Bai F, Zhang S, Jiang T, Shen J et al (2013) High incidence of tuberculosis infection in rheumatic diseases and impact for chemoprophylactic prevention of tuberculosis activation during biologics therapy. Clin Vaccine Immunol 20(6):892–899. https://doi.org/10.1128/CVI.00049-13
Kim HW, Park JK, Yang JA, Yoon YI, Lee EY, Song YW, Kim HR, Lee EB (2014) Comparison of tuberculosis incidence in ankylosing spondylitis and rheumatoid arthritis during tumor necrosis factor inhibitor treatment in an intermediate burden area. Clin Rheumatol 33(9):1307–1312. https://doi.org/10.1007/s10067-013-2387-z
Jo KW, Hong Y, Jung YJ, Yoo B, Lee CK et al (2013) Incidence of tuberculosis among anti-tumor necrosis factor users in patients with a previous history of tuberculosis. Respir Med 107(11):1797–1802. https://doi.org/10.1016/j.rmed.2013.08.011
Calzada-Hernández J, Julian AN, Campos SR, Torrent RB, Jiménez EI et al (2013) PReS-FINAL-2265: tuberculosis in pediatric patients who are receiving anti-TNF agents. Pediatr Rheumatol 11. https://doi.org/10.1186/1546-0096-11-S2-P255
Winthrop KL, Baxter R, Liu L, Varley CD, Curtis JR, Baddley JW, McFarland B, Austin D, Radcliffe L, Suhler E, Choi D, Rosenbaum JT, Herrinton LJ (2013) Mycobacterial diseases and antitumour necrosis factor therapy in USA. Ann Rheum Dis 72(1):37–42. https://doi.org/10.1136/annrheumdis-2011-200690
Bracaglia C, Buonuomo PS, Tozzi AE, Pardeo M, Nicolai R, Campana A, Insalaco A, Cortis E, de Benedetti F (2012) Safety and efficacy of etanercept in a cohort of patients with juvenile idiopathic arthritis under 4 years of age. J Rheumatol 39(6):1287–1290. https://doi.org/10.3899/jrheum.111555
Tomsic M, Rotar Z (2012) The frequency of tuberculosis chemoprophylaxis prior to TNF-α inhibitor treatment, and the incidence tuberculosis infection using a two-step screening algorithm for latent tuberculosis infection: data from the BioRx.si registry. Ann Rheum Dis 71(11):1909–1911. https://doi.org/10.1136/annrheumdis-2012-201913
Kim E-M, Uhm W-S, Bae S-C, Yoo D-H, Kim T-H (2011) Incidence of tuberculosis among Korean patients with ankylosing spondylitis who are taking tumor necrosis factor blockers. J Rheumatol 38(10):2218–2223. https://doi.org/10.3899/jrheum.110373
Nobre CA, Callado MRM, Lima JRC, Gomes KWP (2012) Tuberculosis infection in rheumatic patients with infliximab therapy: experience with 157 patients. Rheumatol Int 32(9):2769–2775. https://doi.org/10.1007/s00296-011-2017-5
Kilic O, Kasapcopur O, Camcioglu Y, Cokugras H, Arisoy N, Akcakaya N (2012) Is it safe to use anti-TNF-alpha agents for tuberculosis in children suffering with chronic rheumatic disease? Rheumatol Int 32(9):2675–2679. https://doi.org/10.1007/s00296-011-2030-8
Titton DC, Silveira IG, Louzada-junior P et al (2011) Registro Brasileiro de Biológicos : preliminares do BiobadaBrasil. Rev Bras Reumatol 51(2):145–160
Pérez-Sola MJ, Torre-Cisneros J, Pérez-Zafrilla B, Carmona L, Descalzo MA, Gómez-Reino JJ (2011) Infections in patients treated with tumor necrosis factor antagonists: incidence, etiology and mortality in the BIOBADASER registry. Med Clin (Barc) 137(12):533–540. https://doi.org/10.1016/j.medcli.2010.11.032
Liza MI, Lim AL, H H, G SC (2010) Anti-TNFa therapy and tuberculosis risk : a two-centre experience. Int J Rheum Dis 13:90. https://doi.org/10.1111/j.1756-185X.2010.01498.x
Laas K, Savi T, O K (2010) Active tuberculosis among patients with inflammatory rheumatic diseases treated with TNF-α inhibitors in a rheumatology center in Estonia. Scand J Rheumatol 39:32. https://doi.org/10.3109/03009742.2010.530485
Cagatay T, Aydin M, Sunmez S, Cagatay P, Gulbaran Z, Gul A, Artim B, Kilicaslan Z (2010) Follow-up results of 702 patients receiving tumor necrosis factor-alpha antagonists and evaluation of risk of tuberculosis. Rheumatol Int 30(11):1459–1463. https://doi.org/10.1007/s00296-009-1170-6
Elbek O, Uyar M, Aydin N et al (2009) Increased risk of tuberculosis in patients treated with antitumor necrosis factor alpha. Clin Rheumatol 28(4):421–426. https://doi.org/10.1007/s10067-008-1067-x
Favalli EG, Desiati F, Atzeni F, Sarzi-Puttini P, Caporali R, Pallavicini FB, Gorla R, Filippini M, Marchesoni A (2009) Serious infections during anti-TNF?? Treatment in rheumatoid arthritis patients. Autoimmun Rev 8(3):266–273. https://doi.org/10.1016/j.autrev.2008.11.002
Rybar I, Rozborilova E, Zanova E, Micekova D, Solovic I, Rovensky J (2008) The effectiveness for prevention of tuberculosis in patients with inflammatory rheumatic diseases treated with TNF inhibitors. Bratisl Lek Listy 109(4):164–167 http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L352509877. Accessed Aug 2018
Garcia-Vidal C, Rodríguez-Fernández S, Teijón S, Esteve M, Rodríguez-Carballeira M, Lacasa JM, Salvador G, Garau J (2009) Risk factors for opportunistic infections in infliximab-treated patients: the importance of screening in prevention. Eur J Clin Microbiol Infect Dis 28(4):331–337. https://doi.org/10.1007/s10096-008-0628-x
Gomez-Reino JJ, Carmona L, Angel Descalzo M (2007) Risk of tuberculosis in patients treated with tumor necrosis factor antagonists due to incomplete prevention of reactivation of latent infection. Arthritis Rheum 57(5):756–761. https://doi.org/10.1002/art.22768
Narayanan K, Anand KP (2007) Long-term follow up of infliximab therapy in inflammatory arthritis. Indian J Rheumatol 2(1):8–10 http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L351266264. Accessed Aug 2018
Takeuchi T, Tatsuki Y, Nogami Y, Ishiguro N, Tanaka Y, Yamanaka H, Kamatani N, Harigai M, Ryu J, Inoue K, Kondo H, Inokuma S, Ochi T, Koike T (2008) Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis 67(2):189–194. https://doi.org/10.1136/ard.2007.072967
Sichletidis L, Settas L, Spyratos D, Chloros D, Patakas D (2006) Tuberculosis in patients receiving anti-TNF agents despite chemoprophylaxis. Int J Tuberc Lung Dis 10(10):1127–1132
Brassard P, Kezouh A, Suissa S (2006) Antirheumatic drugs and the risk of tuberculosis. Clin Infect Dis 43(6):717–722. https://doi.org/10.1086/506935
Askling J, Fored CM, Brandt L, Baecklund E, Bertilsson L, Cöster L, Geborek P, Jacobsson LT, Lindblad S, Lysholm J, Rantapää-Dahlqvist S, Saxne T, Romanus V, Klareskog L, Feltelius N (2005) Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum 52(7):1986–1992. https://doi.org/10.1002/art.21137
Strusberg I et al (2000) Uso de infliximab en pacientes de un centro reumatologico/use of infliximab in patients of a rheumatologic center. Med (Buenos Aires) 60(suppl 1):561–564. https://doi.org/10.1016/j.aprim.2009.04.011
Wolfe F, Michaud K, Anderson J, Urbansky K (2004) Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum 50(2):372–379. https://doi.org/10.1002/art.20009
Gómez-Reino JJ, Carmona L, Rodríguez Valverde V, Mola EM, Montero MD (2003) Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum 48(8):2122–2127. https://doi.org/10.1002/art.11137
Smolen JS, Schöls M, Braun J, Dougados M, FitzGerald O, Gladman DD, Kavanaugh A, Landewé R, Mease P, Sieper J, Stamm T, Wit M, Aletaha D, Baraliakos X, Betteridge N, Bosch FVD, Coates LC, Emery P, Gensler LS, Gossec L, Helliwell P, Jongkees M, Kvien TK, Inman RD, McInnes I, Maccarone M, Machado PM, Molto A, Ogdie A, Poddubnyy D, Ritchlin C, Rudwaleit M, Tanew A, Thio B, Veale D, Vlam K, van der Heijde D (2018) Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis 77(1):3–17. https://doi.org/10.1136/annrheumdis-2017-211734
Haroon N, Inman RD (2009) Infectious complications of biological therapy. Curr Opin Rheumatol 21(4):397–403. https://doi.org/10.1097/BOR.0b013e32832c792d
Chiu YM, Tang CH, Hung ST, Yang YW, Fang CH, Lin HY (2017) A real-world risk analysis of biological treatment (adalimumab and etanercept) in a country with a high prevalence of tuberculosis and chronic liver disease: a nationwide population-based study. Scand J Rheumatol 46(3):236–240. https://doi.org/10.1080/03009742.2016.1202318
Navarra SV, Tang B, Lin H-Y et al (2014) Risk of tuberculosis with anti-tumor necrosis factor-a therapy: substantially higher number of patients at risk in Asia. Int J Rheum Dis 17:291–298. https://doi.org/10.1111/1756-185X.12188
Dixon WG, Symmons DPM, Lunt M, Watson KD, Hyrich KL, Silman AJ (2007) Serious infection following anti–tumor necrosis factor α therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies. Arthritis Rheum 56(9):2896–2904. https://doi.org/10.1002/art.22808
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Sartori, N.S., de Andrade, N.P.B. & da Silva Chakr, R.M. Incidence of tuberculosis in patients receiving anti-TNF therapy for rheumatic diseases: a systematic review. Clin Rheumatol 39, 1439–1447 (2020). https://doi.org/10.1007/s10067-019-04866-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04866-x