Abstract
The aim of this study was to determine whether 11 polymorphisms of endoplasmic reticulum aminopeptidase 1 (ERAP1) confer susceptibility to ankylosing spondylitis (AS). The authors conducted meta-analyses on associations between ERAP1 polymorphisms and AS susceptibility by using fixed and random effects models. A total of 19 articles were included in this meta-analysis, which comprised a total of 19,902 AS patients and 39,750 controls. The meta-analysis revealed a significant association between AS and the minor alleles of the rs30187 polymorphism in all study subjects (OR = 1.255, 95 % CI = 1.147–1.373, P = 8.0 × 10−8). Stratification by ethnicity led to the identification of a significant association between this polymorphism and AS in European patients (OR = 1.283, 95 % CI = 1.237–1.331, P < 1.0 × 10−9). Meta-analyses of the results for the rs27044, rs10050860, rs2287987, rs17482078, and rs26653 polymorphisms showed the same pattern that was found for rs30187. Interestingly, the rs27037 polymorphism was significantly associated with AS susceptibility in both European and Asian patients. Meta-analysis showed a significant association between AS and the minor alleles of the rs27980 and rs27582 polymorphisms in the East Asian patients (OR = 0.904, 95 % CI = 0.818–0.999, P = 0.047; OR = 0.871, 95 % CI = 0.772–0.982, P = 0.024, respectively) (Table 2). However, these polymorphisms have not been studied in Europeans. This meta-analysis shows that the ERAP1 polymorphisms are associated with the development of AS in Europeans and East Asians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disorder characterized by the inflammation of the spinal and sacroiliac joints, which initially causes bone and joint erosion and leads to ankylosis [1]. Strong genetic factors have been implicated in the etiology of AS. Human leukocyte antigen (HLA) B27 was the first genetic factor to be associated with AS, and it confers the greatest susceptibility to this disease. However, increasing evidence indicates that genes other than HLA-B27 also contribute to AS susceptibility [2].
Genome-wide association studies suggest that endoplasmic reticulum aminopeptidase 1 (ERAP1, a known aminopeptidase regulator of tumor necrosis factor receptor 1 [TNFR1] shedding), also known as ARTS1, is a potential candidate (for contributing to AS susceptibility) that is not an HLA gene [3]. ERAP1, which is located on chromosome 5q15, trims peptides for class I HLA presentation and cleaves cell-surface cytokine receptors. ERAP1 was recently found to be a key determinant of the repertoire of peptides that are presented by class I HLA molecules and initiate T-cell responses [4]. A genome-wide association study revealed that two single-nucleotide polymorphisms (SNPs) (rs27044, rs30187) of ERAP1 were significantly associated with AS, and demonstrated that rs17482078, rs10050860, and rs2287987 polymorphisms also trended toward association with AS [3]. Although some studies have demonstrated that SNPs of ERAP1 are associated with AS susceptibility, others disagree [5–23]. It would appear that allelic frequencies often differ substantially between populations, and ethnicity-specific association studies are needed to confirm genetic associations in different populations. Meta-analysis provides a powerful means of summarizing the results produced by different studies [24–26], and in the present study, we performed a meta-analysis to investigate whether ERAP1 polymorphisms are associated with susceptibility to AS in different ethnic populations.
Methods
Identification of eligible studies and data extraction
A search was performed using MEDLINE and EMBASE for studies that examined associations between the polymorphisms of ERAP1 and AS to identify articles in which the ERAP1 polymorphisms were determined in AS patients and controls (up to June 2014). Combinations of keywords such as “endoplasmic reticulum aminopeptidase 1,” “ERAP1,” “polymorphism,” “ankylosing spondylitis,” and “AS” were used as Medical Subject Heading (MeSH) and text words. References cited in the resulting studies were also investigated to identify additional studies not indexed by MEDLINE and EMBASE. Genetic association studies that determined the distributions of ERAP1 alleles in AS patients and controls were eligible for inclusion. Studies were included in the analysis if (1) they were case control studies, (2) contained original data, and (3) had sufficient data to calculate odds ratios (ORs). Studies were excluded if (1) they contained data obtained from other studies, (2) the number of alleles could not be ascertained, or (3) family members were included in the study, because such analyses are based on linkage considerations. The following information was extracted from each study: first author, year of publication, ethnic make-up of the study population, numbers of cases and controls, and the allele frequencies of the ERAP1 polymorphisms.
Evaluation of statistical associations
The allelic frequencies of the ERAP1 polymorphisms from the respective studies were determined using the allele counting method. We examined the difference between the effect of the minor allele, designated as 2, and that of the common allele, designated as 1, of each ERAP1 polymorphism. Point estimates of risk, ORs, and 95 % confidence intervals (CIs) were estimated for each study. Cochran’s Q statistic was used to assess variations or heterogeneities within each study and between studies [21]. This heterogeneity test was conducted with the null hypothesis that all the studies were analyzing the same effect. In addition, we quantified the effect of heterogeneity using I 2 = 100 % × (Q − df) / Q [22]. I 2 ranges between 0 and 100 % and represents the proportion of variability attributable to heterogeneity rather than chance between the studies. I 2 values of 25, 50, and 75 % were assigned as low, moderate, and high estimates, respectively. A fixed effects model assumes that the effects of individual genetic factors on AS susceptibility are similar for all the included studies and that observed variations between studies are caused by chance alone [23]. The random effects model assumes that different studies show substantial diversity and assesses both within-study sampling errors and between-study variances [24]. If study groups show no heterogeneity, the fixed and random effects models produce similar results, and if not, the random effects model usually produces wider CIs than does the fixed effects model. Accordingly, the random effects model was used to evaluate polymorphisms that had significant between-study heterogeneity. Statistical manipulations were performed using a Comprehensive Meta-Analysis computer program (Biostatics, Englewood, NJ, USA).
Results
Studies included in the meta-analysis
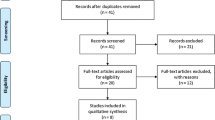
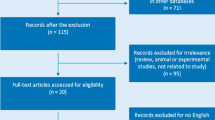
Electronic and manual literature searches resulted in the identification of 87 studies, and 22 of these studies were selected for a full-text review based on their titles and abstracts [9–16, 25–28]. Two studies were excluded because one used the data analyzed in another study and another did not have extractable allele data. Thus, 20 studies met the inclusion criteria (Table 1) [5–23, 27]. However, one of these studies contained data on three groups [13] and two studies had data on two different groups [17]. These groups were treated independently. Thus, for this meta-analysis, we considered 22 separate comparisons, which comprised, in total, 19,902 AS patients and 39,750 controls. Due to the limited number of studies on polymorphisms, 11 types of meta-analyses were performed (Table 2). Selected characteristics of the 21 studies are summarized in Table 1.
Meta-analysis of the association between rs30187, rs27044, rs10050860, rs2287987, rs17482078, and rs26653 polymorphisms and AS susceptibility
The meta-analysis identified a significant association between AS and the minor alleles of the rs30187 polymorphism in the overall population (OR = 1.255, 95 % CI = 1.147–1.373, P = 8.0 × 10−8) (Table 2, Fig. 1). Stratification by ethnicity led to the identification of a significant association between this polymorphism and AS in Europeans (OR = 1.283, 95 % CI = 1.237–1.331, P < 1.0 × 10−9), but not in East Asians and Middle Easterners (Table 2, Fig. 1). Meta-analyses of results of the studies on rs27044, rs10050860, rs2287987, rs17482078, and rs26653 polymorphisms showed that these polymorphisms had the same pattern as rs30187 in the overall population and in the European group. All of these polymorphisms were associated with AS susceptibility in Europeans (Table 2).
Meta-analysis of the relationship between the rs27434, rs27037, rs27980, and rs27582 polymorphisms and AS susceptibility
Meta-analysis revealed a significant association between the minor alleles of the rs27434 and rs27037 polymorphisms and the susceptibility to AS in Europeans (OR = 1.214, 95 % CI = 1.131–1.304, P < 1.0 × 10−9; OR = 1.238, 95 % CI = 1.159–1.323, P < 1.0 × 10−9, respectively) (Table 2, Fig. 2). The rs27037 polymorphism was significantly associated with AS susceptibility in both European and Asian patients (Table 2). Meta-analysis showed a significant association between susceptibility to AS and the minor alleles of the rs27980 and rs27582 polymorphisms in East Asians (OR = 0.904, 95 % CI = 0.818–0.999, P = 0.047; OR = 0.871, 95 % CI = 0.772–0.982, P = 0.024, respectively) (Table 2). However, these polymorphisms had not been studied in Europeans.
Meta-analysis of the relationship between rs27529 and AS susceptibility
No association was found between the rs27529 polymorphism and AS susceptibility by meta-analysis (OR = 1.007, 95 % CI = 0.644–1.573, P = 0.977) in the overall population (Table 2). Due to the limited number of available studies, stratification by ethnicity was not performed for this meta-analysis.
Heterogeneity and publication bias
Heterogeneity was found between the studies during the meta-analyses for the association between AS susceptibility and some ERAP1 polymorphisms. However, heterogeneity was not found during the meta-analyses of the rs10050860, rs27037, rs17482078, rs27980, and rs27582 polymorphisms. Publication bias causes a disproportionate number of positive studies and poses a problem for meta-analyses. However, we found no evidence of publication bias for all study subjects (Egger’s regression test P values >0.1).
Discussion
AS is strongly associated with HLA-B27, but only 1–5 % of HLA-B27-positive individuals develop AS, which suggests that other genes contribute to the risk of AS development [2]. A previous genome-wide association study revealed that ERAP1 polymorphisms are associated with AS at a high level of statistical significance [3], but more recent studies, in different ethnic groups, have produced mixed results [5–23].
In our previous meta-analysis, we found that the rs27044, rs17482078, rs10050860, rs30187, and rs2287987 polymorphisms of ERAP1 were associated with the development of AS in Europeans [28]. In the present study, we updated our meta-analysis on the association between ERAP1 polymorphisms and AS. We undertook an ethnicity-specific meta-analysis of the associations between the ERAP1 polymorphisms and AS susceptibility in European, East Asian, and Middle Eastern populations. Our meta-analysis revealed a significant association between AS and the rs30187 polymorphism in the group containing all study subjects and in the group containing Europeans. Meta-analyses of results for the rs27044, rs10050860, rs2287987, rs17482078, and rs26653 polymorphisms showed the same pattern as rs30187. Our findings support associations between AS susceptibility in East Asians and the ERAP1 polymorphisms rs27037, rs27980, and rs27582, but not the rs30187, rs27434, and rs27044 polymorphisms. The rs27037 polymorphisms were significantly associated with AS susceptibility in both Europeans and Asians. In addition, meta-analysis showed a significant association between AS and the rs27980 and rs27582 polymorphisms in East Asians. However, these polymorphisms had not been studied in Europeans. The minor alleles of the rs27980 and rs27582 polymorphisms in East Asians were protective for developing AS unlike other ERAP1 polymorphisms. The reason for the difference remains unclear but may be in part explained by different linkage disequilibrium with causative polymorphisms or gene of AS. These findings suggest that ERAP1 polymorphisms are associated with the development of AS in Europeans as well as Asians. The allelic frequencies of genes may often differ substantially between populations, and an association between the polymorphisms and a particular disease may depend on ethnicity. Therefore, ethnicity-specific association studies are required to confirm genetic associations in different populations. The ORs for all associations were a little above 1 and small, while the OR for HLA B27 and the susceptibility of AS has been known as very high. However, most associations of polymorphisms in complex diseases refer to small odds ratios [29]. Our finding suggests that ERAP1 is one of the associated genes with AS susceptibility, although the associations had small effect and were not strong as much as HLA-B27.
The role of ERAP1 in the pathogenesis of AS has yet to be fully understood. ERAP1 is known to be an important determinant of the repertoire of peptides that are presented by class I HLA molecules and, consequently, initiate T cell responses. ERAP1 encodes an endoplasmic reticulum aminopeptidase that is involved in trimming peptides to optimal lengths for class I HLA presentation [4]. Thus, ERAP1 variants that may affect the aminopeptidase function could perturb peptide presentation. AS is primarily an HLA class I-mediated autoimmune disease, and more than 90 % of patients carry the HLA-B27 gene. The association between ERAP1 and AS supports the arthritogenic peptide hypothesis, which suggests that the disease is triggered by the presentation of a peptidase, on HLA-B27 molecules at the surface of antigen-presenting cells, to cytotoxic CD8-positive cells. ERAP1 cleaves cell surface receptors for pro-inflammatory cytokines, which downregulates their signaling [30]. Thus, genetic variants that alter this function of ERAP1 could have proinflammatory effects. Furthermore, ERAP1 binds directly to the extracellular domain of TNFR1 and promotes the IL-1β-mediated cleavage of its ectodomain, to generate soluble TNFR1. This ERAP1-assisted generation of extracellular TNFR1 could be crucial for regulating the bioactivity of TNF, which plays a key role in the regulation of inflammation [30]. However, it is not known whether ERAP1 polymorphisms affect the clinical manifestations of AS. Further studies are required to determine the natures of the associations between ERAP1 polymorphisms and disease severity.
The present study has some limitations that should be considered. First, publication bias, heterogeneity, and confounding factors may have distorted the meta-analysis. Second, our ethnicity-specific meta-analysis included data mainly from European and East Asian populations, and thus, our results are applicable only to these ethnic groups. Third, the majority of these studies were performed on populations of European descent, and only two to four studies were conducted on East Asians, which might mean that our investigations using the East Asian group were underpowered. Fourth, the possibility of contributions to AS susceptibility by ERAP1 haplotypes also needs to be examined by meta-analysis. Unfortunately, in the present study, we could not conduct meta-analysis on AS susceptibility and ERAP1 haplotypes due to limited data. Fifth, it would have been interesting to determine if an association exists between ERAP1 polymorphisms and HLA-B27 status, HLA-B27 activity, or the clinical features of AS, but this was not possible because the available data was limited.
In conclusion, this meta-analysis of published data confirms that the ERAP1 polymorphisms are associated with AS susceptibility in Europeans and East Asians. The allelic frequencies of genes often differ substantially between populations, and thus, further ethnicity-specific association studies are required to confirm genetic associations with AS susceptibility in different populations. This meta-analysis demonstrates the need for further studies to determine whether ERAP1 polymorphisms contribute to AS susceptibility in various ethnic groups.
References
Brown MA, Wordsworth BP, Reveille JD (2002) Genetics of ankylosing spondylitis. Clin Exp Rheumatol 20(6 Suppl 28):S43–9
Lee YH, Rho YH, Choi SJ, Ji JD, Song GG (2005) Ankylosing spondylitis susceptibility loci defined by genome-search meta-analysis. J Hum Genet 50(9):453–9
Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A et al (2007) Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet 39(11):1329–37
Chang SC, Momburg F, Bhutani N, Goldberg AL (2005) The ER aminopeptidase, ERAP1, trims precursors to lengths of MHC class I peptides by a “molecular ruler” mechanism. Proc Natl Acad Sci U S A 102(47):17107–12
Zhang Z, Dai D, Yu K, Yuan F, Jin J, Ding L et al (2014) Association of HLA-B27 and ERAP1 with ankylosing spondylitis susceptibility in Beijing Han Chinese. Tissue Antigens 83(5):324–9
Bettencourt BF, Rocha FL, Alves H, Amorim R, Caetano-Lopes J, Vieira-Sousa E et al (2013) Protective effect of an ERAP1 haplotype in ankylosing spondylitis: investigating non-MHC genes in HLA-B27-positive individuals. Rheumatology (Oxford) 52(12):2168–76
Cinar M, Akar H, Yilmaz S, Simsek I, Karkucak M, Sagkan RI et al (2013) A polymorphism in ERAP1 is associated with susceptibility to ankylosing spondylitis in a Turkish population. Rheumatol Int 33(11):2851–8
Cherciu M, Popa LO, Bojinca M, Dutescu MI, Bojinca V, Bara C et al (2013) Functional variants of ERAP1 gene are associated with HLA-B27 positive spondyloarthritis. Tissue Antigens 82(3):192–6
Mahmoudi M, Jamshidi AR, Amirzargar AA, Farhadi E, Nourijelyani K, Fallahi S et al (2012) Association between endoplasmic reticulum aminopeptidase-1 (ERAP-1) and susceptibility to ankylosing spondylitis in Iran. Iran J Allergy Asthma Immunol 11(4):294–300
Wang CM, Ho HH, Chang SW, Wu YJ, Lin JC, Chang PY et al (2012) ERAP1 genetic variations associated with HLA-B27 interaction and disease severity of syndesmophytes formation in Taiwanese ankylosing spondylitis. Arthritis Res Ther 14(3):R125
Wu W, Ding Y, Chen Y, Hua Z, Liu H, Wang H et al (2012) Susceptibility to ankylosing spondylitis: evidence for the role of ERAP1, TGFb1 and TLR9 gene polymorphisms. Rheumatol Int 32(8):2517–21
Szczypiorska M, Sanchez A, Bartolome N, Arteta D, Sanz J, Brito E et al (2011) ERAP1 polymorphisms and haplotypes are associated with ankylosing spondylitis susceptibility and functional severity in a Spanish population. Rheumatology (Oxford) 50(11):1969–75
Evans DM, Spencer CC, Pointon JJ, Su Z, Harvey D, Kochan G et al (2011) Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet 43(8):761–7
Li C, Lin Z, Xie Y, Guo Z, Huang J, Wei Q et al (2011) ERAP1 is associated with ankylosing spondylitis in Han Chinese. J Rheumatol 38(2):317–21
Bang SY, Kim TH, Lee B, Kwon E, Choi SH, Lee KS et al (2011) Genetic studies of ankylosing spondylitis in Koreans confirm associations with ERAP1 and 2p15 reported in white patients. J Rheumatol 38(2):322–4
Zvyagin IV, Dorodnykh VY, Mamedov IZ, Staroverov DB, Bochkova AG, Rebrikov DV et al (2010) Association of ERAP1 allelic variants with risk of ankylosing spondylitis. Acta Nat 2(3):72–7
Australo-Anglo-American Spondyloarthritis C, Reveille JD, Sims AM, Danoy P, Evans DM, Leo P et al (2010) Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet 42(2):123–7
Pazar B, Safrany E, Gergely P, Szanto S, Szekanecz Z, Poor G (2010) Association of ARTS1 gene polymorphisms with ankylosing spondylitis in the Hungarian population: the rs27044 variant is associated with HLA-B*2705 subtype in Hungarian patients with ankylosing spondylitis. J Rheumatol 37(2):379–84
Choi CB, Kim TH, Jun JB, Lee HS, Shim SC, Lee B et al (2010) ARTS1 polymorphisms are associated with ankylosing spondylitis in Koreans. Ann Rheum Dis 69(3):582–4
Davidson SI, Wu X, Liu Y, Wei M, Danoy PA, Thomas G et al (2009) Association of ERAP1, but not IL23R, with ankylosing spondylitis in a Han Chinese population. Arthritis Rheum 60(11):3263–8
Harvey D, Pointon JJ, Evans DM, Karaderi T, Farrar C, Appleton LH et al (2009) Investigating the genetic association between ERAP1 and ankylosing spondylitis. Hum Mol Genet 18(21):4204–12
Maksymowych WP, Inman RD, Gladman DD, Reeve JP, Pope A, Rahman P (2009) Association of a specific ERAP1/ARTS1 haplotype with disease susceptibility in ankylosing spondylitis. Arthritis Rheum 60(5):1317–23
Wellcome Trust Case Control C, Australo-Anglo-American Spondylitis C, Burton PR, Clayton DG, Cardon LR, Craddock N et al (2007) Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet 39(11):1329–37
Lee YH, Bae SC, Choi SJ, Ji JD, Song GG (2012) The association between the PTPN22 C1858T polymorphism and rheumatoid arthritis: a meta-analysis update. Mol Biol Rep 39(4):3453–60
Lee YH, Rho YH, Choi SJ, Ji JD, Song GG (2007) PADI4 polymorphisms and rheumatoid arthritis susceptibility: a meta-analysis. Rheumatol Int 27(9):827–33
Lee YH, Choi SJ, Ji JD, Song GG (2012) Vitamin D receptor ApaI, TaqI, BsmI, and FokI polymorphisms and psoriasis susceptibility: a meta-analysis. Mol Biol Rep 39(6):6471–8
Wen Y-F, Wei JC-C, Hsu Y-W, Chiou H-Y, Wong HS-C, Wong R-H et al (2014) rs10865331 associated with susceptibility and disease severity of ankylosing spondylitis in a Taiwanese population. PLoS One 9(9):e104525
Lee YH, Choi SJ, Ji JD, Song GG (2011) Associations between ERAP1 polymorphisms and ankylosing spondylitis susceptibility: a meta-analysis. Inflamm Res 60(11):999–1003
Ioannidis JP, Trikalinos TA, Ntzani EE, Contopoulos-Ioannidis DG (2003) Genetic associations in large versus small studies: an empirical assessment. Lancet 361(9357):567–71
Cui X, Hawari F, Alsaaty S, Lawrence M, Combs CA, Geng W et al (2002) Identification of ARTS-1 as a novel TNFR1-binding protein that promotes TNFR1 ectodomain shedding. J Clin Invest 110(4):515–26
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or non-financial conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Lee, Y.H., Song, G.G. Associations between ERAP1 polymorphisms and susceptibility to ankylosing spondylitis: a meta-analysis. Clin Rheumatol 35, 2009–2015 (2016). https://doi.org/10.1007/s10067-016-3287-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-016-3287-9