Abstract
Chronic nonspecific musculoskeletal pain (CNMP) is an idiopathic condition often seen in general practice and rheumatology clinics, the aetiology of which may include vitamin D deficiency. The objective of the present study is to evaluate the effectiveness of vitamin D supplementation in the management of CNMP through a systematic review and meta-analysis. According to PRISMA guidelines, PubMed, Embase, Web of Science, Cochrane and Scopus electronic databases were searched for randomised controlled trials comparing vitamin D supplementation to a control or placebo in CNMP patients; the search was not limited by language or date. Meta-analysis was performed using the mean and standardised mean difference which was computed with 95 % confidence intervals, and overall effect size was calculated. Both fixed and random effects models were used in meta-analysis to account for heterogeneity in the studies. The initial search identified 107 studies, of which 10 were potentially relevant, with 7 studies excluded because they did not meet selection criteria. Three studies were included in the meta-analysis. We found no effect of vitamin D supplementation (standardised mean difference (SMD) 0.004; 95 % confidence interval (CI) −0.248 to 0.256) on pain in CNMP patients. Forest plot is used to present the results from meta-analysis. Contrary to a widespread clinical view, there is a moderate level of evidence that vitamin D supplementation is not helpful for treating CNMP patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic nonspecific musculoskeletal pain (CNMP) is an idiopathic condition which is a common presentation to rheumatology clinics [1, 2]. CNMP is associated with decreased physical health, mental well-being, social life [3, 4], work ability [5] and disability [6]. Many sufferers become stuck on a descending spiral of economic, social, emotional and physical disadvantage [7, 8]. CNMP is a significant burden to the economy [9]. The aetiology of CNMP is not well understood, and although many potential contributors have been identified [10], a clear nociceptive source has not, and empirical data concerning other contributors are lacking. As a result, CNMP is difficult to diagnose, prevent or treat. One potential contributor that receives substantial attention clinically and has been investigated in a range of clinical studies is vitamin D deficiency [11, 12].
Vitamin D is involved in many regulatory biological processes. In addition to calcium homeostasis, vitamin D is thought to have anti-inflammatory, anti-apoptotic and anti-fibrotic effects; it is thought to play a role in regulating blood pressure and in innate and adaptive immune system function [13, 14]. This range of biological effects highlights the potential role of vitamin D deficiency in the development of symptoms associated with acute and chronic rheumatic diseases, and it is biologically plausible that vitamin D deficiency contributes to the development and maintenance of CNMP. That people with vitamin D deficiency can present with nonspecific muscular pain and bone pain has been reported [15], and several studies have suggested a causative role [16–18]. However, a recent Cochrane review [19] investigated vitamin D supplementation for the treatment of a range of chronic painful conditions and concluded no substantial effect. Given the different pathophysiological origins of the condition included, such as osteoarthritis, rheumatoid arthritis and fibromyalgia, such a finding was perhaps not surprising. The authors of that review suggested that specific conditions should be investigated individually. We contend that another aspect of that review may have contributed to its null findings: there was no attempt to confine source literature to direct comparisons between vitamin D and a control or placebo. This is important in this field because it is arguably very difficult to isolate the treatment effect attributable to vitamin D supplementation when it is instigated as a part of multimodal intervention.
Despite the clinically topical nature of the issue and the substantial literature, no attempt has been made to conduct meta-analyses. Meta-analyses provide the obvious advantage of increasing power and estimating effect sizes, which can then be re-tested in subsequent studies [20]. We aimed to fill these substantial gaps in the literature by using gold standard systematic review and meta-analysis methodology to evaluate the evidence concerning the effect of vitamin D supplementation, when compared in a randomised controlled trial to a placebo, on pain in people with CNMP.
Methods
Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement [21] was followed for this review.
Inclusion criteria
Only randomised controlled trial (RCT) or randomised double-blind control study designs were eligible. For inclusion, RCTs had to compare vitamin D supplementation to a control or placebo and measure the pain outcome using Visual Analogue Scale (VAS). No restrictions were applied for language but restricted the studies to those conducted on “humans”.
Exclusion criteria
Studies including patients previously diagnosed with an inflammatory joint disease, postsurgical patients or patients with experimentally induced pain were excluded.
Search strategy
An electronic search was performed on five databases—PubMed, Embase, Web of Science, Cochrane and Scopus. The search period was set from the time of commencement of these databases up to 3 November 2015. The search strategies for each database are listed in Table 1. MG and SV independently searched for potentially eligible studies based on the study title and then read the abstracts and selected potentially relevant studies, from which studies not matching the selection criteria were excluded. Full articles of the remaining studies were reviewed for inclusion. The reference lists of selected studies were manually searched to find additional potential papers.
Data extraction
The final selection of the studies was collectively made by the group. Data extraction was performed by MG and MM using a standardised data extraction form similar to Table 2 highlighting the characteristics of selected studies. Data was extracted on sample size, characteristics of participants, intervention type and control group, main outcome and adverse events. The review team was never blinded to authors’ names or institutions, journal of publication and study results. MM provided the statistical support and help in performing the analysis. The manuscript was collectively written by the team, and all authors approved the final draft.
Quality assessment of selected studies
The five-point Jadad score was used to assess the methodological quality of studies. Following, questionnaire formed the basis of scoring [22]
-
1.
Was the study described as randomised?
-
2.
Was the study described as double blind?
-
3.
Was there a description of withdrawals and dropouts?
Each question is answered either yes or no; with each yes, the study is scored 1 point and no scored 0 points. For well-described method of randomisation and blinding, additional points are given respectively. However, 1 point each was deducted if the described method of randomisation and blinding was incorrect. Clinical trials scoring more than 3 are considered as high quality (refer Table 3).
Data analysis
Meta-analysis of the standardised mean differences (SMDs), and their standard errors, in VAS scores was performed between vitamin D-treated and placebo-treated groups. SMD has several versions such as Cohen’s d [23], Glass’s ∆ [24] and Hedges’ g [25]; however, we have used the simple SMD which is the ratio of the mean difference and the standard deviations. The value SMD less than 0.5 is considered to be small effect, from 0.5 to 0.8 medium effect and greater than 0.8 large effect [26]. Summary effect estimates were calculated with the fixed effects models. Analysis was performed in Stata, version 12.1, software (StataCorp LP, College Station, TX) using the metan commands [27]. The heterogeneity between studies was assessed by computing the I2 statistics. A value of 0 inferred no heterogeneity, and value above 50 % is recognised as substantial heterogeneity [26]. Following Bailey [28], we used fixed effects model, as the objective of this study is to test whether the intervention has produced an effect in a set of homogenous studies. In the fixed effects model, we weighted the data by the amount of information (inverse of the variance of the study) that is captured by the study.
Results
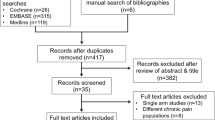
The initial search located 101 studies from the databases (PubMed 14, Embase 20, Web of Science 19, Cochrane Library 45 and Scopus 9). After reviewing the title, the abstract and removal of the duplicates, ten studies were identified for potential inclusion (Fig. 1). Full text of these articles was reviewed and assessed. Of these ten articles, seven were excluded because they did not meet our selection criteria of study design, thus leaving us with only three studies, for conducting the systematic review [29–31]. These three studies used a RCT study design to investigate the effect of vitamin D supplementation (treatment group) compared to a placebo (control group) in CNMP patients.
The characteristics of the selected studies are described in Table 2. The three studies included in the meta-analysis evaluated 492 participants in total. The participants were generally adults with their mean ages ranging from 41 to 76 years. The majority of participants in all studies were females. All studies measured pain, and one study also measured functional mobility and quality of life. Study sample sizes ranged from 84 to 288 subjects. All three studies used VAS to measure changes in pain (outcome); in addition, studies also used the timed up and go test (TUG) [29], functional pain scores (FPS) [31] and Likert scales [30]. All studies used oral route for administering vitamin D except Sakalli et al. study which, in addition. also used intramuscular route for administering vitamin D. This is reflected in the Fig. 2 which shows four studies namely study A, Schreuder et al. study; study B1, Sakalli et al. oral vitamin D supplementation group; study B2, Sakalli et al. intramuscular vitamin D supplementation group; and study C, Warner et al. study. We did this to test if the mode of administration influenced the strength of the clinical effect. The trial period of selected studies was 4 weeks [29] and 12 weeks [32, 33]. Of the three included studies, only one study reported that none of the participants experienced adverse events during the trial or in the follow-up period [30]. In general, all studies scored highly on methodological quality with two studies scoring 5 and one study scoring 4 (Table 3).
Out of the three selected studies, two showed reduction in pain, following treatment with single mega-dose vitamin D supplementation [29, 30], and one showed no effect on pain following vitamin D supplementation [31].
Meta-analysis result
The results from meta-analysis are presented in the forest plot (Fig. 2). The horizontal lines, depicting the length of confidence intervals, for study A and study B1 are on treatment side indicating a modest effect of the intervention on pain in CNMP patients, while for study B2 and study C, the lines are on control side representing no effect of intervention. The overall effect (represented by black diamond in Fig. 2) lies on the line of no effect, indicating that the average effect size of the pooled analysis is 0. The I2 statistic is 62.4 %, indicating a moderate level of heterogeneity in the pooled analysis, which confirms that the variation is not due to chance. The overall pooled SMD was 0 with confidence interval (CI) ranging between −0.25 and 0.26, p value = 0.97, indicating that the intervention has no clinical effect on the CNMP (Fig. 2). The test for overall effect is not statistically significant.
To split the variance as within- and between-study variance, we also analysed data using random effects model (Fig. 3). The overall pooled SMD was 0.05 with CI ranging from −0.37 to 0.46, I2 = 61.4 and p value = 0.05 reiterating no statistical significance.
Discussion
We aimed to evaluate the evidence concerning the effect of vitamin D supplementation, when compared in a RCT to a placebo, on pain in people with CNMP. Our results are in contrast to the prevailing clinical opinion [1, 17, 32] insofar as they suggest that vitamin D supplementation does not decrease pain in CNMP, when compared to a placebo. Our results also show, however, that robust RCT data are perhaps more limited than would be assumed: despite a comprehensive search strategy, only three RCTs, with total of 492 participants, satisfied our a priori criteria. The included trials composed of participants aged between 41 and 76 years with vitamin D levels of 20 nmol/L or less. A range of doses of vitamin D were administered in each included trial, but there was no evidence of a dose-response relationship.
The current study raises new questions for the investigation of CNMP. Our results clearly suggest that vitamin D supplementation is not helpful for CNMP. According to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) [33], we suggest that there is low to moderate evidence (one high-quality study or several studies with some limitations but consistent results) [33] that vitamin D supplementation is not helpful for people with CNMP.
It is notable that the proposed mechanisms by which vitamin D deficiency might contribute to CNMP—disruption of calcium homeostasis and a loss of anti-inflammatory, anti-apoptotic or anti-fibrotic effects [13, 14]—imply that the primary cause of CNMP lies within the tissues of the body. Although such mechanisms seem intuitive, they are not necessarily consistent with modern models of CNMP and other chronic pain conditions. Although vitamin D deficiency, through disruption of immune regulation [13, 14], could disrupt the neuroimmunological processes that subserve pain [34], the assumption that this would increase pain rather than decrease it remains to be properly tested. The prevailing theories with regard to chronic pain place greater emphasis on enhanced sensitivity within the nociceptive system [35], increased contribution of non-nociceptive sensory inputs and associative learning [36], and cognitive mechanisms that emphasise perceived threat to body tissue and behavioural processes linking fear of pain, activity avoidance and catastrophising [37] and de-emphasise ongoing tissue pathology or damage (with some exceptions, for example, seronegative arthropathies) [38]. Indeed, CNMP is widely considered to be influenced by a wide range of biological, physical, psychological and social factors [7] and management approaches reflect this biopsychosocial framework [39, 40]. Perhaps, vitamin D supplementation might play a more important role in painful conditions that more obviously relate to tissue inflammation, for example, rheumatoid arthritis, although this remains to be determined.
The biological complexity of vitamin D effects leaves open the possibility that supplementation could offer benefit for people with CNMP and that the current research base is not sufficient to detect it. That is, protocols of the RCTs may have led to inadequate rise in serum vitamin D levels postsupplementation [41] due to noncompliance, although one might argue that such interventions are really “advice to take a particular action” rather than the action itself [42]. Different effects may also relate to heterogeneity of body mass index (BMI) between participants. Alternatively, standard doses of vitamin D supplementation may not always produce predictable increases in the vitamin D levels [41] or predictable rates at which vitamin D level changes [43], potentially masking positive effects. That other study designs, for example, clinical studies [1], observational [18], cross-sectional and case report studies [17], have demonstrated moderate benefit following supplementation may reflect an advantage of tailored supplementation regimes (although considering the findings of such studies, one should remember that these study designs are highly vulnerable and may overestimate true effects) [44].
The relative paucity of RCTs comparing vitamin D supplementation to placebo is surprising, considering the widespread clinical endorsement of the idea. The available data are also not very generalisable to all ages because most studies have investigated primarily postmenopausal women and compared nonstandard doses for which there is little justification. Estradiol is recognised as a physiological predictor of vitamin D binding protein [45], and postmenopausal women show a higher natural decline in vitamin D levels than premenopausal women [46], suggesting that it would be important to investigate the variance in vitamin D levels in premenopausal women with depleting estradiol levels as well as in younger women with normal estradiol levels. Furthermore, CNMP is highly prevalent in children and adolescents, but this group has not been investigated with regard to vitamin D.
There are several considerations, strengths and limitations of the current study. We included the Warner et al. study [31] even though they diagnosed participants with primary fibromyalgia, not CNMP. On closer appraisal, the participants in their study did not satisfy the ACR criteria for fibromyalgia but did satisfy criteria for CNMP. The strengths of this study are its focus on CNMP and inclusion of meta-analysis, as was recommended in a recent Cochrane review [19]; the absence of language or publication restrictions, giving confidence that we did not miss important studies; and the confinement of included studies to those that used a RCT design, because they provide the most rigorous method of verifying if a cause-effect relationship exists between the intervention and outcome [47]. We used SMD score to evaluate the clinical relevance and CI for inference because it focuses on the probability and significance of the intervention and helps to establish the clinical and statistical significance of the findings [48]. There are also limitations: the forest plot shows variability between the studies, and broad 95 % CIs show the imprecision of the results, a common problem with small sample sizes [48]. The most significant limitation is indeed the lack of source literature, which is particularly pertinent to the field because it contrasts with popular clinical belief.
This study shows that there is no proven effect of vitamin D supplementation on pain in people with CNMP, when compared to a placebo. We conclude that there is GRADE C (low) to level B (moderate) evidence that vitamin D supplementation is not helpful for people with CNMP. Clearly, more robust and nuanced RCTs might have an important impact on our confidence in the estimate of effect [33].
References
Kumar A, Gopal H, Khamkar K et al (2012) Vitamin D deficiency as the primary cause of musculoskeletal complaints in patients referred to rheumatology clinic: a clinical study. Indian J Rheumatol 7:199–203
O’Sullivan P, Beales D, Jensen L, Murray K, Myers T (2011) Characteristics of chronic non-specific musculoskeletal pain in children and adolescents attending a rheumatology outpatients clinic: a cross-sectional study. Pediatr Rheumatol Online J 9:3
de Vries HJ, Reneman MF, Groothoff JW, Geertzen JHB, Brouwer S (2012) Factors promoting staying at work in people with chronic nonspecific musculoskeletal pain: a systematic review. Disabil Rehabil 34:443–58
Konijnenberg AY, Uiterwaal CS, Kimpen JL, van der Hoeven J, Buitelaar JK, Graeff-Meeder ER (2005) Children with unexplained chronic pain: substantial impairment in everyday life. Arch Dis Child 90:680–6
Guite J, Logan D, Sherry D, Rose J (2007) Adolescent self-perception: associations with chronic musculoskeletal pain and functional disability. J Pain 8:379–86
de Vries HJ, Reneman MF, Groothoff JW, Geertzen JH, Brouwer S (2013) Self-reported work ability and work performance in workers with chronic nonspecific musculoskeletal pain. J Occup Rehabil 23:1–10
Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC (2007) The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 133:581–624
Perquin C, Hunfeld J, Hazeborek-Kampschreur A et al (2003) The natural course of chronic benign pain in childhood and adolescence: a two-year population-based follow-up study. Eur J Pain 7:551–9
Perquin C, Hunfeld J, Hazeborek-kampschreur A et al (2001) Insights in the use of health care services in chronic benign pain in childhood and adolescence. Pain 94:205–13
El-Metwally A, Salminen JJ, Auvinen A, Kautiainen H, Mikkelsson M (2004) Prognosis of non-specific musculoskeletal pain in preadolescents: a prospective 4-year follow-up study till adolescence. Pain 110:550–9
Plotnikoff GA, Quigley JM (2003) Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc 73:1463–70
Tague SE, Clarke GL, Winter MK, McCarson KE, Wright DE, Smith PG (2011) Vitamin D deficiency promotes skeletal muscle hypersensitivity and sensory hyperinnervation. J Neurosci 31:13728–38
Lai Y-H, Fang T-C (2013) The pleiotropic effect of vitamin D. ISRN Nephrol Article ID 898125
Zittermann A (2003) Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr 89:552–72
Lyman D (2005) Undiagnosed vitamin D deficiency in the hospitalized patient. Am Fam Physician 71:299–304
Body JJ, Bergmann P, Boonen S et al (2012) Extraskeletal benefits and risks of calcium, vitamin D and anti-osteoporosis medications. Osteoporos Int 23(Suppl 1):S1–23
Chaudhary WA, Arshad SH, Waqas S et al (2013) Vitamin D supplement as an adjuvant in the management of chronic musculoskeletal pain. Anaesth Pain Intensive Care 17:296–300
Knutsen KV, Brekke M, Gjelstad S, Lagerløv P (2010) Vitamin D status in patients with musculoskeletal pain, fatigue and headache: a cross-sectional descriptive study in a multi-ethnic general practice in Norway. Scand J Prim Health Care 28:166–71
Straube S, Derry S, Straube C, Moore RA (2015) Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev
Guyatt G, Gutterman D, Baumann MH et al (2006) Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians Task Force. Chest 129:174–81
Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PloS 6
Jadad AR, Moore A, Carroll D et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Cohen J (2013) Statistical power analysis for the behavioral sciences. Academic Press
Glass GV (1976) Primary, secondary, and meta-analysis of research. Educ Res 5:3–8
Hedges LV, Olkin I (2014) Statistical method for meta-analysis. Academic press
Higgins PT, Green S (2008) Cochrane handbook for systematic reviews of interventions. Wiley-Blackwell, Chichester, England
StataCorp (2011) Stata Statistical Software: Release 12. StataCorp LP, TX
Bailey KR (1987) Inter-study differences: how should they influence the interpretation and analysis of results. Stat Med 6:351–60
Sakalli H, Arslan D, Yucel AE (2012) The effect of oral and parenteral vitamin D supplementation in the elderly: a prospective, double-blinded, randomized, placebo-controlled study. Rheumatol Int 32:2279–83
Schreuder F, Bernsen RM, van der Wouden JC (2012) Vitamin D supplementation for nonspecific musculoskeletal pain in non-western immigrants: a randomized controlled trial. Ann Fam Med 10:547–55
Warner AE, Arnspiger SA (2008) Diffuse musculoskeletal pain is not associated with low vitamin D levels or improved by treatment with vitamin D. J Clin Rheumatol 14
Gendelman O, Itzhaki D, Makarov S, Bennun M, Amital H (2015) A randomized double-blind placebo-controlled study adding high dose vitamin D to analgesic regimens in patients with musculoskeletal pain. Lupus 24:483–9
Guyatt G, Oxman AD, Vist GE et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Br Med J 336:924
Grace PM, Hutchinson MR, Maier SF, Watkins LR (2014) Pathological pain and the neuroimmune interface. Nat Rev Immunol 14:217–31
Woolf CJ (2014) What to call the amplification of nociceptive signals in the central nervous system that contribute to widespread pain? Pain 155:1911–2
Moseley GL, Vlaeyen JWS (2015) Beyond nociception: the imprecision hypothesis of chronic pain. Pain 156:35–8
Vlaeyen JWS, Linton SJ (2012) Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain 153:1144–7
Moseley GL, Butler DS (2015) Fifteen years of explaining pain: the past, present, and future. J Pain 16:807–13
Butler DS, Moseley GL (2013) Explain pain, 2nd edn. Noigroup Publications, Adelaide
Meulders A, Harvie DS, Bowering JK, Caragianis S, Vlaeyen JW, Moseley GL (2014) Contingency learning deficits and generalization in chronic unilateral hand pain patients. J Pain 15:1046–56
Ryan PJ (2007) Vitamin D therapy in clinical practice. One dose does not fit all. Int J Clin Pract 61:1894–9
Moseley GL (2006) Do training diaries affect and reflect adherence to home programs? Arthritis Rheum 55:662–4
Abbasi M, Hashemipour S, Hajmanuchehri F, Kazemifar AM (2013) Is vitamin D deficiency associated with non specific musculoskeletal pain? Glob J Health Sci 5
O’Connell NE, Moseley GL, McAuley JH, Wand BM, Herbert RD (2015) Interpreting effectiveness evidence in pain: short tour of contemporary issues. Phys Ther 95:1087–94
Pop LC, Shapses SA, Chang B, Sun W, Wang X (2015) Vitamin D-binding protein in healthy pre- and postmenopausal women: relationship with estradiol concentrations. Endocr Pract 21:936–42
Kanwar G, Sharma N, Shekhawat M, Sharma P, Hada R, Chandel CS (2015) Comparison of vitamin D levels in pre and post menopausal type 2 diabetic females. IOSR-JDMS 14:70–3
Kendall JM (2003) Designing a research project: randomised controlled trials and their principles. Emerg Med J 20:164–8
Sim J, Reid N (1999) Statistical inference by confidence intervals: issues of interpretation and utilization. Phys Ther 79:186–95
Acknowledgments
MG is funded by Australian Postgraduate Award for her PhD. MNM is funded by John Lynch’s NHMRC Australian Fellow funding (ID 478115). GLM is supported by a Principal Research Fellowship from the NHMRC ID 1061279.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MG, SV, MM and NS declare that they have no conflict of interest. GLM—Noigroup Publications royalties, speaker’s fees and Pfizer consultancy. This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Rights and permissions
About this article
Cite this article
Gaikwad, M., Vanlint, S., Mittinity, M. et al. Does vitamin D supplementation alleviate chronic nonspecific musculoskeletal pain? A systematic review and meta-analysis. Clin Rheumatol 36, 1201–1208 (2017). https://doi.org/10.1007/s10067-016-3205-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-016-3205-1