Abstract
Systemic sclerosis (SSc) is an autoimmune disease of unknown etiology characterized by progressive fibrosis. Activated fibroblasts are mainly responsible for fibrosis in SSc. Galectin-3, a β-galactoside-binding lectin, plays many important regulatory roles in both physiological and pathological processes including proliferation, apoptosis, inflammation, and fibrosis. The purpose of this study was to assess the serum galectin-3 levels in patients with SSc. Thirty-seven SSc patients, 23 systemic lupus erythematosus (SLE) patients (serving as patient control group), and 28 healthy volunteers were enrolled in this study. Disease activity and severity scores were detected with Valentini disease activity index and Medsger disease severity scale in the SSc group and SLE disease activity index and Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index in the SLE group. The serum levels of galectin-3, vascular endothelial growth factor, transforming growth factor-β, and interleukin-6 were determined. Compared to the control group, the galectin-3 levels were higher in the SSc and SLE groups. The galectin-3 levels were not correlated with the disease activity and severity indexes in both patient groups. But, the serum galectin-3 levels were higher in the active SSc and SLE subgroups than in the inactive SSc (4.6 ± 5.8 vs. 1.3 ± 1.1 ng/ml, p = 0.015) and SLE (17.4 ± 11.3 vs. 6.5 ± 8.9 ng/ml, p = 0.019) subgroups. These results suggest that galectin-3, which is associated with fibrosis and inflammation by previous studies, may be a prominent biomarker of disease activity in SSc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic sclerosis (SSc) is a multisystemic disorder characterized by vasculopathy, inflammation, and progressive fibrosis of the skin and internal organs [1, 2]. The pathogenesis of SSc is still not fully understood. Previous studies have shown that an initial vascular damage leads to an inflammatory response that finally promotes the development of an intense fibrosis [3]. In SSc patients, activated fibroblasts are responsible for the development of fibrosis and accumulation of extracellular matrix molecules [4]. There are many growth factors, chemokines, and cytokines associated with the pathogenesis of fibrosis in SSc [3].
Galectin (gal) is a β-galactoside-binding lectin, composed of two domains: the carboxyl-terminal and the amino-terminal domains [5]. To date, 15 members of the Gal family have been identified. This family can be divided into three main groups based on the structure of gal. Gal-3, the only chimeric one [6], has been first identified on the surface of murine thioglycollate-elicited macrophages as a specific macrophage surface marker, named Mac-2 in 1982 [7]. Nowadays, gal-3 is known to be expressed on a variety of tissues and cells including monocytes, macrophages, dendritic cells, mast cells, eosinophils, and B and T lymphocytes. It has a variety of effects on biological and pathological processes including inflammation, autoimmunity, fibrosis, cell adhesion, proliferation, differentiation, and tumor invasion [8, 9].
It has been previously documented that gal-3 plays an important role on the development of lung fibrosis by interacting with various cytokines and chemokines [10]. Moreover, it has also been reported that the blocking of gal-3 gene inhibits myofibroblast activation, decreases type I procollagen expression in in vitro and in vivo settings, and attenuates liver fibrosis [11]. Therefore, the purpose of our study was to assess the serum gal-3 level and its potential associations with disease activity and severity indexes in patients with SSc.
Materials and methods
Participants
Thirty-seven patients with SSc, 23 patients with systemic lupus erythematosus (SLE), and 28 healthy controls (HCs) were included in this study. SSc and SLE were diagnosed according to the established criteria [12, 13]. Moreover, SSc patients were classified as having diffuse cutaneous or limited cutaneous SSc [3]. The protocol of this study was approved by the institutional ethics committee, and all the participants gave informed consent before enrolling in the study.
Detailed histories of all participants were obtained, and systemic and rheumatologic examinations were performed. Glucocorticoid (GC) and disease-modifying antirheumatic drug (DMARD) usages were recorded. For each patient, the Valentini disease activity index, Medsger disease severity scale, and modified Rodnan skin score (mRSS) in the SSc group [14, 15] and the SLE disease activity index (SLEDAI) and the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index in the SLE group [16, 17] were determined. The diseases were considered as active if Valentini disease index was ≥3 in SSc [14] and SLEDAI was ≥6 in SLE [18].
Laboratory analysis
Blood samples were drawn from all the participants, who had fasted overnight. Serum samples were stored at –20 °C until further analysis. Routine laboratory evaluation of complete blood cell count, hepatic and renal function tests, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) were assessed in patients and controls using standard laboratory methods.
Antinuclear antibody and anti-centromere antibody were detected by indirect immunofluorescence using human epithelial cells (Hep-2); the level of anti-topoisomerase (Scl-70) antibody was determined by a commercially available enzyme-linked immunosorbent assay (ELISA) in patients with SSc. Pulmonary function tests, diffusion capacity of carbon monoxide, and high-resolution computed tomography were performed to detect pulmonary involvement. Echocardiogram was also performed. The result with >35 mmHg right ventricle systolic pressure was defined as pulmonary hypertension.
Serum gal-3, vascular endothelial growth factor, transforming growth factor-β (Bender MedSystems GmbH, Vienna, Austria) and interleukin (IL)-6 (BioSource International, Inc. Camarillo, CA, USA) levels were measured using appropriate commercial kits by ELISA method.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS 16.0, Chicago, IL, USA) was used for analysis. Normal distributions were tested with the Kolmogorov–Smirnov test with Lilliefors correction, and logarithmic transformations were applied to the skewed data to normalize before entering the analysis. Quantitative data with normal distribution were presented as mean ± standard deviation. Statistical differences among the groups were identified with one-way analysis of variance (ANOVA) followed by the Tukey's post hoc test. Chi-square test was used to compare the categorical variables. Correlation analysis was performed using the Pearson correlation coefficient. The p values less than 0.05 were considered as significant.
Results
The demographics
The demographical and laboratory data of the study groups are summarized in the Table 1. Age, sex ratio, and body mass indexes were similar in among the study groups. The mean disease durations were 4.2 ± 4.6 and 5.1 ± 4.4 years in the SSc and SLE groups, respectively (p = 0.079). The characteristics of the patients with SSc were summarized in the Table 2.
Serum galectin-3 levels
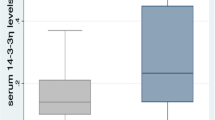
Compared to the HC group, in the SSc and SLE groups, the gal-3 levels were higher (p < 0.05, p < 0.001, respectively). The gal-3 levels were 4.2 ± 6.7 and 2.9 ± 2.8 ng/ml in diffuse cutaneous SSc (n = 15) and limited cutaneous subtype (n = 22), respectively (p = 0.490). And, its level was higher in both subgroups compared to the HC group (p = 0.041 and p = 0.015, respectively). In the study groups, we did not find any significant differences between men and women in terms of the gal-3 levels. In addition, gal-3 levels were not correlated with the inflammatory markers or the cytokines in the SSc group.
The association of galectin-3 with disease activity indices
The gal-3 levels were not directly correlated with the disease activity and severity indexes in both groups of patients. However, the gal-3 level in the active (n = 22) SSc subgroup was significantly higher than inactive (n = 15) subgroup (4.6 ± 5.8 vs. 1.3 ± 1.1 ng/ml, p = 0.015). Similarly, its level was higher in the active SLE (n = 15) subgroup than the inactive (n = 8) SLE subgroup (17.4 ± 11.3 vs. 6.5 ± 8.9 ng/ml, p = 0.019). Gal-3 level was not significantly different between patients with and without pulmonary involvement, intestinal involvement, and digital ulcer.
The treatment modalities
There were no differences in GC usage and mean dose of GC between SSc and SLE groups (Table 3). In both groups of patients, there was no significant difference in terms of gal-3 level both between receiving and not receiving GC and between receiving and not receiving at least one DMARD (data not shown).
Discussion
The present study demonstrated that the serum gal-3 levels were higher in the SSc and SLE groups compared to the healthy subjects. Moreover, active SSc and SLE patients had higher serum gal-3 levels than in inactive ones.
Tissue fibrosis, the hallmark of SSc, is responsible for the most of clinical manifestations. The extent and severity of tissue fibrosis correlates with prognosis and mortality in SSc [19]. The activation of fibroblasts which is triggered by growth factors/cytokines leads to the overproduction of extracellular matrix [20]. The behavior of fibroblasts is changed by many mediators including cytokines, chemokines, and growth factors [21, 22].
Gal-3 plays an important role in the development of inflammation by interacting with various cytokines and chemokines [23, 24]. In an experimental study [23], gal-3 has been shown to be predominantly expressed by mononuclear cells infiltrating into necrotic areas in liver injury induced by acetaminophen. On the other hand, acetaminophen-induced hepatotoxicity has been attenuated in gal-3-deficient mice [23]. Moreover, several studies [25–27] have shown that gal-3 null mice exhibit a reduced inflammatory response compared with wild-type mice. These results suggest the pro-inflammatory nature of this protein [23, 25–27].
In our study, the gal-3 levels were higher in the patients with SSc and SLE. Similarly, previous studies [28–30] documented the increased serum gal-3 levels in inflammatory disorders SLE, rheumatoid arthritis, and Behçet's disease. Gal-3 expression has been induced by tumor necrosis factor-alpha (TNF-α) and interferon-γ in a monocytic cell line U937 [9, 10]. On the other hand, gal-3 has induced the mRNA expression and protein production of TNF-α and IL-8 in a macrophage cell line THP-1 [9, 10]. Thus, it may be concluded that the cause of increased gal-3 level in SSc and SLE may be the inflammatory nature of the diseases.
Gal-3 has also stimulated NIH-3T3 fibroblast to induce migration and collagen synthesis in vitro, suggesting direct effects of gal-3 on fibrogenesis [10]. In vivo and in vitro profibrotic potential of gal-3 has been demonstrated in the experimental models of liver [23], renal [31], and vascular fibrosis [24]. The level of gal-3 increases in fibrotic diseases [11], and gal-3 activates fibroblastic cells [10, 11, 32]. Moreover, the silencing of the gal-3 gene inhibits fibroblastic activity [11], and gal-3 knockout mice are resistant to development of fibrosis [11, 25]. Moreover, Nishi et al. [10] have reported that the concentrations of gal-3 in bronchoalveolar lavage fluid are significantly higher in patients with idiopathic pulmonary fibrosis and interstitial pneumonia associated with collagen tissue diseases than in the control group. These results suggest that gal-3 level has to be increased in SSc a fibrotic disorder as in our study.
In contrast to present results, Taniguchi et al. [33] have reported that serum gal-3 levels are significantly lower in patients with diffuse cutaneous SSc than in healthy subjects and limited cutaneous SSc. However, its level is significantly and positively correlated with total skin score [33]. It is anticipated that serum gal-3 level is higher in SSc, as in our study, in case of that gal-3 level is positively correlated with skin score. Taniguchi et al. [33] have concluded that the contradictorily lower gal-3 level may be associated with disease duration. Conversely, gal-3 level was not related with disease duration in our cohort. The cause of discrepancy for gal-3 level may be the difference of genetic background and/or the difference in clinical involvement. Taniguchi et al. [33] have also reported that pulmonary hypertension and digital ulcer affect the gal-3 level. However, these clinical involvements were not associated with altered gal-3 levels in our study.
Measuring disease activity in SSc has been particularly difficult. This difficulty is influenced by several facts, as follows: SSc has an indolent course and the assessment of vasculopathy and fibrosis are more difficult. Moreover, when it becomes measurable, the disease is often progressed to permanent damage [34–36]. There are currently three tools that are used to measure disease activity in SSc including physician global assessment, the Valentini disease activity index, and the Medsger disease severity scale. Although the physician global assessment has been commonly used, it has not been formally evaluated. The application of others is difficult in clinical practice, since they take a lot of time. In addition, these index and score have failed to accurately measure activity in SSc due to the fact that these reflect damage and severity rather than activity [34, 37]. Disease activity is potentially reversible under drug treatment or even spontaneously; however, damage measures irreversible tissue injury [34]. mRSS is the outcome measure mostly used in clinical trials of SSc. Although mRSS is noninvasive and cost effective, it is not possible to differentiate fibrotic skin thickening from that resulting from tissue edema, inflammation, or skin tethering [19, 34].
Nonspecific markers of inflammation such as ESR and CRP could assess activity. However, they have not been shown to predict outcome [34, 38]. Up to now, in SSc, several biomarkers have been evaluated as potential markers [19]; those can be grouped as fibrotic, vascular, and prognostic biomarkers. Despite extensive studies to develop outcome measures for SSc, fully validated biomarkers that allow early diagnosis and assessment of disease activity or that carry a predictive prognostic value are not available [19]. Gal-3 which is also an important fibroblast product may be a potential biomarker to reflect the fibrotic and inflammatory process in SSc. Indeed, gal-3 level is reported to be correlated with the extent of skin involvement and with the severity of SSc [33]. In our study, serum gal-3 levels are higher in SSc patients when compared with HC. In addition, gal-3 levels are higher in active SSc patients than in inactive patients. This result suggests that gal-3 may be a potential biomarker for assessment of disease activity in SSc. However, extensive studies will be required to validate whether measurements of serum level of gal-3 may be useful biomarkers for the process of fibrosis in the disease.
There are no effective therapies for reversing or even controlling fibrosis of SSc although the pathogenesis of SSc is continually being better understood. Traditional medications are disappointing in clinical practice despite anecdotal evidence of benefit. TD139, a novel synthetic inhibitor of gal-3, has ameliorated the bleomycin-induced pulmonary fibrosis [32]. This result suggests that the blocking of gal-3 function may be an exciting novel therapeutic target to treat the fibrotic disorders.
We realize that the present preliminary study has some limitations. Firstly, the sample size could be small to reach definitive judgment. Secondly, the local production of gal-3 in affected and unaffected skin could be analyzed.
In conclusion, the serum gal-3 level increases in SSc. Moreover, active SSc patients have higher serum gal-3 levels. Therefore, it can be suggested that galectin-3 may be a prominent surrogate biomarker of activity in SSc.
References
Gabrielli A, Avvedimento EV, Krieg T (2009) Scleroderma. N Engl J Med 360(19):1989–2003
Rabquer BJ, Koch AE (2012) Angiogenesis and vasculopathy in systemic sclerosis: evolving concepts. Curr Rheumatol Rep 14(1):56–63
LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, Rowell N, Wollheim F (1988) Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 15(2):202–5
LeRoy EC (1974) Increased collagen synthesis by scleroderma skin fibroblasts in vitro: a possible defect in the regulation or activation of the scleroderma fibroblast. J Clin Invest 54(4):880–9
Henderson NC, Sethi T (2009) The regulation of inflammation by galectin-3. Immunol Rev 230(1):160–71
Barondes SH, Cooper DN, Gitt MA, Leffler H (1994) Galectins. Structure and function of a large family of animal lectins. J Biol Chem 269(33):20807–10
Ho MK, Springer TA (1982) Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J Immunol 128(3):1221–8
Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, Urashima T, Oka T, Futai M, Muller WE, Yagi F, Kasai K (2002) Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim Biophys Acta 1572(2–3):232–54
Dhirapong A, Lleo A, Leung P, Gershwin ME, Liu FT (2009) The immunological potential of galectin-1 and -3. Autoimmun Rev 8(5):360–3
Nishi Y, Sano H, Kawashima T, Okada T, Kuroda T, Kikkawa K, Kawashima S, Tanabe M, Goto T, Matsuzawa Y, Matsumura R, Tomioka H, Liu FT, Shirai K (2007) Role of galectin-3 in human pulmonary fibrosis. Allergol Int 56(1):57–65
Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T (2006) Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci 103(13):5060–5
Hochberg MC (1997) Update of the 1982 American College of Rheumatology Revised Criteria for Classification of Systemic Lupus Erythematosus. Arthritis Rheum 40:1725
Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee (1980) Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 23(5):581–90
Valentini G, Della Rossa A, Bombardieri S, Bencivelli W, Silman AJ, D'Angelo S, Cerinic MM, Belch JF, Black CM, Bruhlmann P, Czirják L, De Luca A, Drosos AA, Ferri C, Gabrielli A, Giacomelli R, Hayem G, Inanc M, McHugh NJ, Nielsen H, Rosada M, Scorza R, Stork J, Sysa A, van den Hoogen FH, Vlachoyiannopoulos PJ (2001) European multicentre study to define disease activity criteria for systemic sclerosis. II. Identification of disease activity variables and development of preliminary activity indexes. Ann Rheum Dis 60(6):592–8
Medsger TA Jr, Silman AJ, Steen VD, Black CM, Akesson A, Bacon PA, Harris CA, Jablonska S, Jayson MI, Jimenez SA, Krieg T, Leroy EC, Maddison PJ, Russell ML, Schachter RK, Wollheim FA, Zacharaie H (1999) A disease severity scale for systemic sclerosis: development and testing. J Rheumatol 26(10):2159–67
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH (1992) Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 35(6):630–40
Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, Bacon P, Bombardieri S, Hanly J, Hay E, Isenberg D, Jones J, Kalunian K, Maddison P, Nived O, Petri M, Richter M, Sanchez-Guerrero J, Snaith M, Sturfelt G, Symmons D, Zoma A (1996) The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 39(3):363–9
Abrahamowicz M, Fortin PR, du Berger R, Nayak V, Neville C, Liang MH (1998) The relationship between disease activity and expert physician's decision to start major treatment in active systemic lupus erythematosus: a decision aid for development of entry criteria for clinical trials. J Rheumatol 25(2):277–84
Castro SV, Jimenez SA (2010) Biomarkers in systemic sclerosis. Biomark Med 4(1):133–47
Moinzadeh P, Denton CP, Abraham D, Ong V, Hunzelmann N, Eckes B, Krieg T (2012) Biomarkers for skin involvement and fibrotic activity in scleroderma. J Eur Acad Dermatol Venereol 26(3):267–76
Abraham DJ, Krieg T, Distler J, Distler O (2009) Overview of pathogenesis of systemic sclerosis. Rheumatology 48:iii3–iii7
Shi-Wen X, Denton CP, Dashwood MR, Holmes AM, Bou-Gharios G, Pearson JD, Black CM, Abraham DJ (2001) Fibroblast matrix gene expression and connective tissue remodeling: role of endothelin-1. J Invest Dermatol 116(3):417–25
Dragomir AC, Sun R, Mishin V, Hall LB, Laskin JD, Laskin DL (2012) Role of galectin-3 in acetaminophen-induced hepatotoxicity and inflammatory mediator production. Toxicol Sci 127(2):609–19
Calvier L, Miana M, Reboul P, Cachofeiro V, Martinez-Martinez E, de Boer RA, Poirier F, Lacolley P, Zannad F, Rossignol P, López-Andrés N (2013) Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol 33(1):67–75
Colnot C, Ripoche MA, Milon G, Montagutelli X, Crocker PR, Poirier F (1998) Maintenance of granulocyte numbers during acute peritonitis is defective in galectin-3-null mutant mice. Immunology 94(3):290–6
Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung-Leung WP, Liu FT (2000) Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol 156(3):1073–83
Nieminen J, St-Pierre C, Bhaumik P, Poirier F, Sato S (2008) Role of galectin-3 in leukocyte recruitment in a murine model of lung infection by Streptococcus pneumoniae. J Immunol 180(4):2466–73
Ohshima S, Kuchen S, Seemayer CA, Kyburz D, Hirt A, Klinzing S, Michel BA, Gay RE, Liu FT, Gay S, Neidhart M (2003) Galectin 3 and its binding protein in rheumatoid arthritis. Arthritis Rheum 48(10):2788–95
Lee YJ, Kang SW, Song JK, Park JJ, Bae YD, Lee EY, Lee EB, Song YW (2007) Serum galectin-3 and galectin-3 binding protein levels in Behçet's disease and their association with disease activity. Clin Exp Rheumatol 25(4 Suppl 45):S41–5
Kang EH, Moon KC, Lee EY, Lee YJ, Lee EB, Ahn C, Song YW (2009) Renal expression of galectin-3 in systemic lupus erythematosus patients with nephritis. Lupus 18(1):22–8
Okamura DM, Pasichnyk K, Lopez-Guisa JM, Collins S, Hsu DK, Liu FT, Eddy AA (2011) Galectin-3 preserves renal tubules and modulates extracellular matrix remodeling in progressive fibrosis. Am J Physiol Renal Physiol 300(1):F245–53
Mackinnon AC, Gibbons MA, Farnworth SL, Leffler H, Nilsson UJ, Delaine T, Simpson AJ, Forbes SJ, Hirani N, Gauldie J, Sethi T (2012) Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am J Respir Crit Care Med 185(5):537–46
Taniguchi T, Asano Y, Akamata K, Noda S, Masui Y, Yamada D, Takahashi T, Ichimura Y, Toyama T, Tamaki Z, Tada Y, Sugaya M, Kadono T, Sato S (2012) Serum levels of galectin-3: possible association with fibrosis, aberrant angiogenesis, and immune activation in patients with systemic sclerosis. J Rheumatol 39(3):539–44
Hudson M, Steele R, Canadian Scleroderma Research Group (CSRG), Baron M (2007) Update on indices of disease activity in systemic sclerosis. Semin Arthritis Rheum 37(2):93–8
Medsger TA Jr (2003) Natural history of systemic sclerosis and the assessment of disease activity, severity, functional status, and psycho logic well-being. Rheum Dis Clin North Am 29(2):255–73
Valentini G, Silman AJ, Veale D (2003) Assessment of disease activity. Clin Exp Rheumatol 21:S39–41
Clements PJ (1995) Measuring disease activity and severity in scleroderma. Curr Opin Rheumatol 7(6):517–21
Hummers LK (2010) The current state of biomarkers in systemic sclerosis. Curr Rheumatol Rep 12(1):34–9
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koca, S.S., Akbas, F., Ozgen, M. et al. Serum galectin-3 level in systemic sclerosis. Clin Rheumatol 33, 215–220 (2014). https://doi.org/10.1007/s10067-013-2346-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-013-2346-8