Abstract
The objective of this study was to identify the most effective treatment by evaluating the different therapies used to treat mild, moderate, and severe Henoch–Schönlein purpura (HSP) patients. We performed a retrospective study of children discharged with a diagnosis of HSP. The study group consisted of 425 children divided into mild, moderate, and severe condition groups. Different therapeutic protocols of hydrocortisone sodium succinate (HCSS) therapy, methylprednisolone (MP) pulse therapy, and MP combination with tripterygium glycoside (TG) therapy were used to treat the different groups. The evaluation of curative effect was performed. After 4 weeks, all patients with no obvious recovery were treated by strengthening the different treatment intervention. The remission time of skin, joint, and gastrointestinal manifestations was evaluated, and the results of the follow-up were analyzed (remission time of proteinuria, relapse, and side effects of therapy). After 4 weeks, in the mild group, the difference of the curative effect between HCSS and MP therapy was not statistically significant. Moderate HSP patients were more likely to respond to MP therapy than HCSS therapy (P < 0.05). Severe HSP patients were more likely to respond to MP combination with TG than single MP therapy (P < 0.05). At last follow-up, they all had normal urinalysis. In the moderate HSP group, the mean duration of proteinuria was shorter in the MP pulse therapy group than in the HCSS therapy group (P < 0.05). In the mild group, the mean duration of purpura was shorter in HCSS therapy group than in the MP pulse therapy group (P < 0.05). At last follow-up, 99 patients had recurrences of purpura and/or proteinuria and 41 patients had liver functional impairment and/or hypertension. The relapse and side effects were all satisfactorily controlled, and the rates of relapse and side effects did not differ between groups with different therapies (P > 0.05). Our study has demonstrated a superior effect for HCSS therapy in patients with mild HSP disease, for MP therapy in patients with moderate disease, and for MP combined with TG therapy in patients with severe disease. MP therapy administered initially reduces the duration of urinary protein abnormality. The therapeutic protocols did not increase the risk of relapse and were safe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Henoch–Schönlein purpura (HSP) is a systemic vasculitic syndrome characterized by non-thrombocytopenic purpura, arthritis/arthralgia, abdominal pain, and glomerulonephritis caused by the inflammation of polymorphonuclear cells accumulated in the walls of small vessels [1]. This syndrome was first described by Heberden in 1801 [2]. Later, in 1837, Schönlein defined the association of purpuric cutaneous lesions and arthralgia [3]. In 1874, Henoch described purpura, abdominal pain, and melena as a syndrome and in 1895 glomerulonephritis was described as a complication of this syndrome [4, 5].
HSP is primarily a disease that occurs in children. Although most children with HSP recover completely, patients with severe gastrointestinal (GI) and renal manifestations should be carefully treated and followed up. During the acute period, massive GI bleeding and intussusception, which can be life threatening, may be observed [6]. Long-term prognosis is usually associated with the severity of renal involvement [7-9]. Although various regimens of treatment in HSP have been described, including anticoagulants, angiotensin-converting enzyme inhibitors (ACEIs), corticosteroids or intravenous administration of methylprednisolone (MP) followed by oral treatment with corticosteroids, and immunosuppressants, there has been no consensus on the protocols.
The objective of our retrospective study was to define the different treatments of HSP patients at our institution and identify, if possible, the most effective. To do that, we divided HSP patients into mild, moderate and severe groups according to the severity of their symptoms. Each group received different treatments. We evaluated the treatment protocols, clinical follow-ups, and prognosis of those with joint, GI, and renal manifestations.
Materials and methods
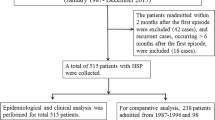
Hospital files of patients who were followed up with the diagnosis of HSP in the Pediatric Nephrology Clinics of the first affiliated hospital of Anhui Medical University between January 2003 and December 2007 were retrospectively evaluated.
Inclusion criteria
The criteria used were the EULAR/PReS endorsed consensus criteria for Henoch–Schönlein purpura proposed by Ozen et al. [10] as following: Palpable purpura (mandatory criterion) and the presence of at least one of the following four features: (1) diffuse abdominal pain, (2) any biopsy showing predominant IgA deposition, (3) arthritis or arthralgia, and (4) renal involvement (any haematuria and/or proteinuria).
Case definition
A retrospective analysis of all data of the selected patients was performed by the review of the medical charts. The clinical signs and symptoms of the acute phase of the disease were collected. According to their conditions, patients were divided into mild, moderate, and severe groups as following:
-
Mild: palpable purpura or petechia with one or more of the following signs and symptoms: abdominal pain and/or vomiting, arthritis/arthralgias (i.e., swelling and/or functional limitation of the joint), and nephritis (proteinuria<1.0 g/L and/or hematuria).
-
Moderate: besides mild patient manifestations, they also have one or more of the following signs and symptoms: gastrointestinal bleeding (a positive test for occult blood in the stool, or melena, or hematochezia), proteinuria 1–2 g/L or acute nephritic syndrome (microscopic or macroscopic hematuria with at least two of the following three findings: oliguria, hypertension, and raised serum urea or creatinine).
-
Severe: besides the above manifestations, they also have one or more of the following signs and symptoms: proteinuria ≥2.0 g/L, rapidly progressive glomerulonephritis, nephrotic syndrome, or a renal biopsy specimens showing cellular crescents.
Treatment
Mild and moderate groups were divided into hydrocortisone sodium succinate (HCSS) and MP pulse therapy groups. The severe group was divided into MP pulse and MP combination with tripterygium glycoside (TG) therapy groups.
Patients in the HCSS therapy group were initially treated intravenously with HCSS 4–8 mg/kg daily for 1–2 weeks, then administered oral steroid at 0.5–1 mg/kg daily; patients in MP pulse therapy group were initially treated intravenously with MP 10–15 mg/kg for 3 or 6 alternate days, then were given oral steroid at 0.5–1 mg/kg daily; patients in the MP combination with TG therapy group were initially treated intravenously with MP 10–15 mg/kg for 6 alternate days, then were given oral steroid at 0.5–1 mg/kg daily, while at the same time given oral TG at 1 mg/kg daily for 3–6 months. All patients received anticoagulant, antioxidant, and antianaphylactic therapy and an ACEI was given to patients with renal involvement.
Outcome
We evaluated patients after 4 weeks. Patients with remission of skin, joint, and gastrointestinal manifestations, and decreasing proteinuria were defined as obvious recovery; patients with any persistent symptoms described above or without decreasing proteinuria were defined as no obvious recovery. All patients with no obvious recovery were given intensified treatment: patients who failed to respond to HCSS were given MP, while those who failed MP were administered TG therapy. The remission time of skin, joint, and gastrointestinal manifestations was evaluated and the results of the follow-up were evaluated (remission time of proteinuria, relapse, and side effects of therapy).
Statistical analysis
Continuous data were described as means and standard deviations (mean ± SD); categorical variables were expressed as cases. Statistical analysis of data between group comparisons of categorical data parameters were performed by using the chi-square test, and comparisons of continuous data were done with the Mann–Whitney test. Statistical significance was taken as P < 0.05. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS 11.5; Chicago, IL).
Results
Over the 5-year period, we identified 425 patients that were enrolled, 270 were boys, and 155 were girls. The mean age at onset of HSP was 9.00 ± 2.53 years (ranging from 2 to 14 years). The mean follow-up was 45.22 ± 17.04 months (ranging from 18 to 78 months).
Short-term outcome
Treatment effects at 4 weeks are shown in Table 1. Three hundred fifty-seven patients (84%) had obvious recovery, and 68 (16%) had no obvious recovery. Among the 68 patients showing no obvious recovery, three in the mild group with HCSS therapy had continuous purpura, and the other 65 had persistently high proteinuria which did not decrease with treatment. In the mild group, the difference between HCSS and MP therapy was not statistically significant (P > 0.05). Patients with moderate disease were more likely to respond to MP therapy than to HCSS (P < 0.05). Patients with severe disease were more likely to respond to MP combined with TG than to single MP therapy (P < 0.05).
Three patients with persistent purpura were treated with MP pulse therapy. Two of them recovered, and the one resistant to MP therapy was successfully treated with TG. The remission time of skin, joint, and gastrointestinal manifestations is shown in Table 2. In almost all groups, the difference in remission time between two therapies was not statistically significant (P > 0.05), except in mild group, where the mean duration of purpura was shorter in HCSS therapy group than in the MP pulse therapy group (P < 0.05).
Long-term outcome
All patients had complete remission at their last follow-up (mean, 45.22 ± 17.04 months). In the mild group, five patients with HCSS resistance achieved complete remission after receiving MP pulse therapy, and two patients with MP resistance achieved complete remission after receiving oral TG. In the moderate group, four of 20 patients who responded to HCSS at the end of 4 weeks did not achieve complete remission during oral steroid. After MP pulse therapy was added, proteinuria diminished. All 48 patients resistant to HCSS were given MP pulse therapy; 38 of them achieved complete remission, the other ten achieved complete remission after receiving oral TG. Two patients in the MP pulse therapy group resistant to MP were given oral TG and achieved complete remission. In the severe group, four of six patients responding to MP at the end of 4 weeks did not achieve complete remission during oral steroid. After oral TG was added, proteinuria diminished. Eight patients resistant to MP achieved complete remission after receiving oral TG.
The time to remission of the proteinuria is shown in Table 2. In patients with mild and severe disease, using two different therapies and strengthened intervention, the duration of proteinuria was not statistically significant (P > 0.05). In patients with moderate disease, the mean duration of proteinuria was shorter in the MP pulse therapy group than in the HCSS therapy group (P < 0.05).
Relapse
A total of 99 patients had relapse of their disease (Table 3). Purpura was the most common recurrence, and proteinuria was the next. Most of the children had only one relapse, but ten had two or more. The number of recurrences ranged from one to five. The relapses were described as milder and shorter than the first episode in all patients, and they all achieved complete remission. There was no significant difference in relapse between different therapies (P > 0.05).
Side effects
The side effects of therapy are shown in Table 3. All 41 had liver functional impairment (increased serum ALT level) and/or hypertension; nine patients had increased serum ALT level, 35 patients had hypertension, and three patients had both hypertension and increased serum ALT level. The blood pressure and serum ALT level were all satisfactorily controlled by administration of antihypertensive or liver-protective medications. There was no significant difference in side effects between different therapies (P > 0.05).
Discussion
To the best of our knowledge, this study provides one of the most extensive therapeutic effectiveness reports on HSP in children. The objective of this study was to evaluate the results of treatment in 425 cases of HSP with follow up information. The clinical symptoms of patients with HSP have great diversity, varying from mild cutaneous lesions to severe gastrointestinal bleeding and renal failure, suggesting the need for more than one treatment protocol. The long-term prognosis of HSP is usually related to the severity of renal involvement. Patients with renal involvement may have an unsatisfactory long-term outcome and are at risk of developing chronic renal failure. Treatment strategies for HSP nephritis (HSPN) may be guided by the results of renal biopsy, but the majority of patients have a favorable prognosis [11, 12]. So for many patients with mild disease, renal biopsy usually is not necessary and some patients and their families refuse the examination. Ronkainen et al. [7] reported that the long-term outcome of HSP associated with symptoms at onset and the severity of the first kidney biopsy findings did not correlate with the long-term risk of a poor outcome. We therefore divided patients into three groups according to the severity of their clinical symptoms.
The goals of treating HSP are typically to (1) ameliorate acute symptoms, (2) mitigate short-term morbidity (such as abdominal complications that require surgery), and (3) prevent chronic renal insufficiency. Because HSP is characterized by leukocyte infiltration of the blood vessel walls along with immunoglobulin A deposition (which results in vascular injury and necrosis), and because corticosteroids inhibit inflammatory processes, early treatment with corticosteroids has been postulated to be effective for all 3 therapeutic goals. Corticosteroid treatment however, remains controversial. A meta-analysis of four randomized controlled trials [13] demonstrated no significant benefit of short-course prednisone, administered at presentation of HSP, for preventing persistent renal disease in children. However another meta-analysis, based on a comprehensive review of the literature, showed corticosteroids, given early in the course of illness, seem to produce consistent benefits for several major clinically relevant HSP outcomes [14]. Recently a prospective, placebo-controlled study reported that prednisone was effective in reducing the intensity of abdominal pain, joint pain, and treating renal symptoms, but did not prevent the development of renal symptoms [15].
In the present study, all patients were given corticosteroids. In the mild group, the majority of patients had complete remission using either HCSS therapy or MP pulse therapy. In addition, HCSS therapy reduced the time to remission of the purpura, suggesting this should be given first to patients with mild disease.
In patients with moderate disease, the majority given HCSS therapy had no obvious recovery after 4 weeks. Those patients were then given MP pulses strengthened therapy to avoid delaying the best treatment time. Majority of patients (94/122 ) given MP pulse therapy initially or after 4 weeks had complete remission. These results suggest that MP pulse therapy should be given first to patients with moderate disease.
Patients with severe HSP often have severe renal damage and there is little agreement about the best therapy. In our study all 38 patients with severe disease had heavy proteinuria (≥2.0 g/L), ten had nephrotic syndrome, and four patients undergoing renal biopsy had Class IIIb histopathology. Because the morbidity rate is high among patients with severe HSP and nephrotic range proteinuria, many investigators emphasize the importance of early and intensified treatment [8, 16-18], including steroids combined with immunosuppressive therapy. Various regimens have been described, such as intravenous administration of MP followed by oral treatment with corticosteroids, or corticosteroids in combination with azathioprine or cyclophosphamide, with or without anticoagulants, and with or without plasmapheresis, cyclosporine A or mycophenolate mofetil [17-20].
TG has been used to treat glomerulonephritis for more than 30 years in China with dramatic antiproteinuric effects. Triptolide is one of the major active components of TG. In puromycin aminonucleoside nephrotic rats, triptolide ameliorated the foot process effacement and podocyte injury [21]. In the present study, when severe HSP patients were treated with MP in combination with TG, they all had complete remission, while single MP pulse therapy was found to be effective in only six of 14 patients after 4 weeks. These results suggest a potential benefit in patients with severe disease who are treated initially with MP in combination with TG. As there is only one in vitro study proving the effectiveness of TG, further investigation including a prospective, randomized, controlled clinical trial is needed.
The long-term outcome of HSP includes renal symptoms. Extrarenal symptoms are rare. In the present study, extrarenal symptoms of almost all patients disappeared in the short term. HSPN patients may progress to end-stage renal disease (ESRD), especially in patients whose initial disease is severe. Lavjay et al. [22] reported 52 patients with HSPN at 20 years follow-up, in whom 21% had progressed to ESRD. Penina et al. [23] reported that of 56 patients with HSPN followed for up to 14 years, 39.3% of patients had persistent kidney abnormalities, while 12.5% of patients had progressed to ESRD and/or death. While we did not specifically study the long-term outcomes, in our study, the duration of proteinuria was associated with the extent of initial renal involvement. The more severe the renal involvement, the longer duration of proteinuria. In the moderate disease group, the mean duration of proteinuria was shorter in the MP pulse therapy group than in the HCSS therapy group, while in mild disease group, two therapies showed no significant difference, presumably due to the mild renal damage and rapid recovery. In the present study, all patients had normal urinalysis during follow-up. No cases progressed to ESRD, this may be due to our graded therapeutic protocols, timely (4 week) intensified treatment intervention, and our relatively short follow-up time.
The frequency of relapses varies from series to series. In the present study, 23.3% (99/425) patients had relapse. This percentage is lower than that reported by other studies [12, 24]; however, in the study by Calvino et al., cutaneous relapses occurred less frequently [25]. In our study, all relapses were milder and shorter than the first episode and all achieved complete remission. However relapse should be monitored in the very long term as suggested by Ronkainen et al., [7]; the authors discovered that all women even with mild renal symptoms at onset may have a poor outcome during pregnancy. Therefore, we recommend prolonged follow-up of all patients, including those who seem to have recovered.
The most common side effect in our study, likely related to corticosteroid therapy, is hypertension, followed by liver functional impairment. Liver functional impairment is also a common side effect of TG. In our study, the rate of side effects was 9.6% (41/425), which is lower than reported by Hari et al. [26]. All side effects were relatively mild and satisfactorily controlled. We therefore considered our therapeutic protocols as relatively safe.
In conclusion, our data suggest a graded treatment approach for children with HSP. Our study demonstrates a superior effect for HCSS therapy in patients with mild HSP disease, for MP therapy in patients with moderate disease, and for MP combined with TG therapy in patients with severe disease. Our study also shows that the majority of patients receiving this graded treatment had a good outcome at follow-up. It also shows that MP therapy administered initially could reduce the persistent time of urinary protein abnormity. The therapeutic protocols did not increase the risk of relapse and were safe.
References
Saulsbury FT (2001) Henoch–Schönlein purpura. Curr Opin Rheumatol 13:35–40
Heberden W (1801) Commertarii de Malbaun. Historia et curatione, Payne, London
Schönlein JL (1837) Allgemenie und specielle Pathologie und Therapie, vol 2, 3rd edn. Herisau, Wurzburg
Henoch EH (1874) Uber eine eigentumliche Form von Purpura. Berliner Klinische Wochenschrift 2:641–643
Henoch EH (1895) Vorlesungen uber Kinderkrankheiten. Hirschwald, Berlin
Szer IS (2002) Henoch Schönlein purpura. Curr Opin Rheumatol 6:25–31
Ronkainen J, Nuutinen M, Koskimies O (2002) The adult kidney 24 years after childhood Henoch–Schönlein purpura: a retrospective cohort study. Lancet 360:666–670
Ronkainen J, Ala-Houhala M, Huttunen NP et al (2003) Outcome of Henoch–Schönlein nephritis with nephrotic-range proteinuria. Clin Nephrol 60:80–84
Goldstein AR, White RH, Akuse R et al (1992) Long-term follow-up of childhood Henoch–Schönlein nephritis. Lancet 339:280–282
Ozen S, Ruperto N, Dillon MJ et al (2006) EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis 65:936–941
Peru H, Soylemezoglu O, Bakkaloglu SA et al (2008) Henoch Schönlein purpura in childhood: clinical analysis of 254 cases over a 3-year period. Clin Rheumatol 27:1087–1092
Trapani S, Micheli A, Grisolia F et al (2005) Henoch Schönlein purpura in childhood: epidemiological and clinical analysis of 150 cases over a 5-year period and review of literature. Semin Arthritis Rheum 35:143–153
Chartapisak W, Opastiraku SL, Willis NS et al (2009) Prevention and treatment of renal disease in Henoch–Schönlein purpura: a systematic review. Arch Dis Child 94:132–137
Weiss PF, Feinstein JA, Luan X et al (2007) Effects of corticosteroid on Henoch–Schönlein purpura: a systematic review. Pediatrics 120:1079–1087
Ronkainen J, Koskimies O, Ala-Houhala M et al (2006) Early prednisone therapy in Henoch–Schönlein purpura: a randomized, double-blind, placebo-controlled trial. J Pediatr 149:241–247
Foster BJ, Bernard C, Drummond KN et al (2000) Effective therapy for severe Henoch–Schönlein purpura nephritis with prednisone and azathioprine: a clinical and histopathologic study. J Pediatr 136:370–375
Niaudet P, Habib R (1998) Methylprednisolone pulse therapy in the treatment of severe forms of Schönlein–Henoch purpura nephritis. Pediatr Nephrol 12:238–243
Shin JI, Park JM, Shin YH et al (2005) Can azathioprine and steroids alter the progression of severe Henoch–Schönlein nephritis in children? Pediatr Nephrol 20:1087–1092
Shenoy M, Bradbury MG, Lewis MA et al (2007) Outcome of Henoch–Schönlein purpura nephritis treated with long-term immunosuppression. Pediatr Nephrol 22:1717–1722
Tanaka H, Suzuki K, Nakahata T et al (2003) Early treatment with oral immunosuppressants in severe proteinuric purpura nephritis. Pediatr Nephrol 18:347–350
Zheng CX, Chen ZH, Zeng CH et al (2008) Triptolide protects podocytes from puromycin aminonucleoside induced injury in vivo and in vitro. Kidney Int 74:596–612
Butani L, Morgenstern BZ (2007) Long-term outcome in children after Henoch–Schönlein purpura nephritis. Clin Pediatr (Phila) 46:505–511
Tarshish P, Bernstein J, Edelmann CM Jr (2004) Henoch–Schönlein purpura nephritis: course of disease and efficacy of cyclophosphamide. Pediatr Nephrol 19:51–56
Saulsbury FT (1999) Henoch–Schönlein purpura in children. Report of 100 patients and review of the literature. Medicine (Baltimore) 78:395–409
Calviño MC, Llorca J, García-Porrúa C et al (2001) Henoch–Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore) 80:279–290
Hari P, Bagga A, Jindal N et al (2001) Treatment of focal glomerulosclerosis with pulse steroids and oral cyclophosphamide. Pediatr Nephrol 16:901–905
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, F., Lu, L., Zhang, Q. et al. Henoch–Schönlein purpura in childhood: treatment and prognosis. Analysis of 425 cases over a 5-year period. Clin Rheumatol 29, 369–374 (2010). https://doi.org/10.1007/s10067-009-1329-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-009-1329-2