Abstract
The objective of the study is to examine whether interleukin-10 (IL-10) promoter polymorphism is a marker of susceptibility of systemic lupus erythematosus (SLE) in Chinese patients in Taiwan. The study included 119 Chinese patients with SLE. One hundred unrelated healthy individuals living in central Taiwan served as control subjects. Each polymorphism was detected as a result of a polymerase chain reaction (PCR)-based restriction analysis. The PCR product length was determined to be 412 bp (CC) whereas two fragments of 236 and 176 bp were determined to be excisable lengths (AA). The relationship between the IL-10 gene polymorphism and clinical manifestations of SLE was evaluated. For the genotype and allelic frequency, there were statistically significant differences between the SLE patients and the normal control subjects (p=0.007 and 0.003, respectively). But we did not detect any association of carriage rate of the IL-10 polymorphism and the normal control subjects (p=0.077). Furthermore, we did not detect any association of IL-10 genotype with antinuclear antibody, malar rash, photosensitivity, discoid lupus, mucosal ulcer, arthritis, serositis, hematology, immunology, involvement of central nervous system, and renal disease involvement in the SLE patients. The significant relation of −627 IL-10 genotype and allelic frequency with SLE implies that the IL-10 gene polymorphism can serve as a candidate gene marker for further study in patients with SLE in Taiwan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is a prototype for systemic autoimmune disease with a wide spectrum of laboratory and clinical manifestations. It is known to involve multisystems and the production of autoantibodies to intracellular antigens. The etiology is largely unknown although valuable knowledge was accomplished through intensive research in this field in recent years. Interleukin-10 (IL-10) is produced at a high level by the B lymphocytes and monocytes of patients with SLE. The high IL-10 production contributes to the abnormal production of immunoglobulins (Ig) and of autoantibodies in SLE [1]. It may be a risk factor for susceptibility and severity of rheumatoid arthritis (RA) and SLE [2–4]. The spontaneous in vitro production of IgM, IgG, and IgA by peripheral blood mononuclear cells from SLE patients was weakly increased by recombinant IL (rIL)-6, but strongly by rIL-10. IL-10 is an important pleiotropic cytokine with anti-inflammatory and stimulatory activities [5]. It exerts its anti-inflammatory effect by inhibiting the synthesis of proinflammatory cytokines such as IL-α, IL-1β, IL-6, IL-8, IL-12, and TNF-α in activated macrophages [6]. IL-10 also enhances B cell proliferation, differentiation, and survival [7–9]. Levels of IL-10 production are critical in immunity regulation. Thus, the expression of IL-10 was implicated in a number of autoimmune disorders, including RA, Sjogren’s syndrome, and SLE [4]. This study has examined the polymorphism of the gene IL-10 to determine whether it was a marker of susceptibility to SLE by focusing on single nucleotide polymorphisms (SNPs). We compared allelic and genotypic frequencies between 119 Chinese patients with SLE and 100 healthy individuals in Taiwan.
Materials and methods
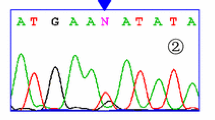
One hundred nineteen patients with definite SLE according to the 1982 revised American College of Rheumatology criteria for SLE [10] and 100 unrelated healthy individuals living in central Taiwan, who served as control subjects, were enrolled in this study. Informed consent was obtained from all patients involved. Clinical and serological data were available on these patients, including malar rash, photosensitivity, antinuclear antibody (ANA), involvement of central nervous system, and renal disease (defined as proteinuria >1 g/day). The involvement of the central nervous system was evaluated by a neurologist. The genomic DNA was prepared from peripheral blood by the use of a genomic DNA isolation reagent kit (Genomaker, Taiwan, Republic of China). The polymerase chain reaction (PCR) for IL-10 gene polymorphism was carried out on a total volume of 25 μl containing genomic DNA (2–6 pmol of each primer), 1X Taq polymerase buffer (1.5 mM MgCl2), and 0.25 U of AmpliTaq DNA polymerase (Perkin-Elmer, Foster City, CA, USA). The primer for the IL-10 promoter gene at position −627 was 5′-CCTAGGTCACAGTGACGTGG-3′ and 5′-GGTGAGCACTACCTGACTAGC-3. The PCR amplification was performed in a programmable thermal cycler GeneAmp PCR System 2400 (Perkin-Elmer). The cycling conditions for IL-10 polymorphism were set as follows: 1 cycle at 96°C for 5 min, 35 cycles at 96°C for 30 s, 35 cycles at 60°C for 30 s, 35 cycles at 72°C for 45 s, and 1 final cycle of extension at 72°C for 7 min. The PCR product of 412 bp was mixed with 2 U of Rsa I (New England Biolabs, Beverly, USA) and two fragments of 236 and 176 bp were present when the product could already be digested (AA homozygote). The reaction was incubated for 3 h at 37°C. Then 10 μl of the product was loaded onto a 3% agarose gel containing ethidium bromide for electrophoresis. The polymorphism was divided into digestible (AA homozygote), indigestible (CC homozygote), and A/C heterozygote.
The carriage rate of an allele is the number of individuals carrying at least one copy of the allele relative to the total number of individuals. Allelic frequency was expressed as a percentage of the total number of alleles. Results from the control subjects and the SLE patients were compared using the χ 2 test (3×2 and 2×2 contingency tables) for statistical significance. When the assumption of the χ 2 test was violated and one cell had an expected count of <1, or >20% of the cells had an expected count of <5, Fisher’s exact test was used. The distributions of the IL-10 gene polymorphisms in each group were evaluated. A p value of less than 0.05 was considered statistically significant. The odds ratios (OR) were calculated from allelic frequency with a 95% confidence interval (95% CI) for the polymorphism of the IL-10 gene.
Results
The frequencies of the genotype in the SLE and control groups are shown in Table 1. It shows that among the 119 SLE patients, 66 patients (55.5%) had IL-10 genotype AA, 48 patients (40.3%) had A/C, and 5 patients (4.2%) had CC. Among the 100 control volunteers, the IL-10 genotype AA was found in 40 (40.0%), A/C in 45 (45.0%), and CC in 15 (15.0%). There were significant differences in the distribution of the IL-10 gene polymorphism between the healthy control subjects and the SLE patients (p=0.007). In addition, there was a significant difference in the allelic frequency of the IL-10 between the SLE patients and healthy controls (p=0.003), giving an OR of 1.210 for A allele (95% CI 1.063–1.377). There was no significant association in the carriage rates of the IL-10 (p=0.077), giving an OR of 1.164 for A allele (95% CI 0.981–1.382).
Clinical manifestations and laboratory findings of the SLE patients are shown in Table 2. The associations of IL-10 with particular clinical features of SLE were examined in the 119 Chinese patients. We did not detect any association of IL-10 genotype with ANA, malar rash, photosensitivity, discoid lupus, mucosal ulcer, arthritis, serositis, hematology, immunology, involvement of central nervous system, and renal disease involvement in the SLE patients (all p>0.05).
Discussion
SLE is a prototype of autoimmune diseases that affects practically every organ in the body. There are strong epidemiological evidences that genes contribute to the risk of developing many common diseases. However, the genetic background of patients with SLE is still mostly unknown. To date, genetic researches of multifactorial diseases were studied with difficulty due to the vast uncertainty surrounding the presence of a polygenic trait. In the present study, we chose the IL-10 gene polymorphism to examine whether IL-10 gene polymorphism was a marker of susceptibility to SLE in Chinese patients in Taiwan.
IL-10 is a 36-kDa homodimeric cytokine, which is mainly produced by macrophage, monocytes, and lymphocytes. The IL-10 gene maps to the junction of 1q31–q32 [11]. IL-10 production appears to be controlled at the transcriptional level [12]. The IL-10 5′ flanking region, which controls transcription, is polymorphic, with two microsatellites between −4,000 and −1,100 and three single base pair substitutions that were described in the IL-10 promoter at positions −1,082 (G/A), −819 (C/T), and −592 (C/A) [13]. Reports from Lazarus et al. [14] found that IL-10-1082G, IL-10-819C, and IL-10-592C haplotypes were associated with Ro autoantibodies and renal involvement in white patients with SLE. In Chinese patients, a different IL-10-1087*A/-824*T/-597*A haplotype was also associated with renal involvement but not Ro autoantibodies [15]. These studies found no association with disease susceptibility. In contrast, Gibson et al. [16] found novel SNPs in the distal region of the IL-10 promoter significantly associated with SLE susceptibility in African-Americans. The study of Lim et al. [17] showed that −627*A allele is associated with severe asthma, which is detected with low levels of IL-10 [18]. Grove et al. [19] also showed that the −627*A IL-10 promoter gene is related to advanced alcoholic liver disease. They proposed that the −627*A allele is associated with low IL-10 expression, which will favor the inflammatory, immune-mediated, and profibrotic mechanisms of an alcohol-related liver injury. Guseva et al. [20] found that polymorphism of genes FcgRIIIA and IL-10 is associated with predisposition to development of SLE in the Kazakh population. The analysis of combined genotypes of the studied genes suggests a synergic action of genes FcgRIIIA and IL-10-627 with the risk to develop SLE [20].
To our knowledge, an association between SLE and the gene polymorphism of −627 IL-10 including genotype, allelic frequencies, and carriage rate has not been demonstrated before. In the present study, significant differences were observed in the allelic frequencies and genotype of IL-10 gene polymorphism between the patients with SLE and the healthy control subjects. We were unable to find significant differences in the carriage rate of IL-10 gene polymorphism between the normal controls and SLE patients. However, the possibility of a type II error must be kept in mind in view of the relatively small sample sizes. Recently, SNPs were used as tools for mapping the complex disease genes responsible for SLE [21–29]. SNPs provide a method for defining patients at risk from disease. In addition, they assist in determining the exact prognosis of patients, which in turn leads to the selection of a specific therapy based on specific genetic variations [30]. This study provides a useful method for the further study of genes in patients with SLE.
References
Llorente L, Zou W, Levy Y, Richaud-Patin Y, Wijdenes J, Alcocer-Varela J, Morel-Fourrier B, Brouet JC, Alarcon-Segovia D, Galanaud P et al (1995) Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med 181:839
Mongan AE, Ramdahin S, Warrington RJ (1999) Interleukin-10 response abnormalities in systemic lupus erythematosus. Scand J Immunol 46:406
Lacki JK, Samborski W, Mackiewicz SH (1997) Interleukin-10 and interleukin-6 in lupus erythematosus and rheumatoid arthritis, correlations with acute phase proteins. Clin Rheumatol 16:275
Llorente L, Richaud-Patin Y, Fior R, Alcocer-Varela J, Wijdenes J, Fourrier BM, Galanaud P, Emilie D (1994) In vivo production of interleukin-10 by non-T cells in rheumatoid arthritis, Sjogren’s syndrome, and systemic lupus erythematosus: a potential mechanism of B lymphocyte hyperactivity and autoimmunity. Arthritis Rheum 37:1647
Howard M, O’Garra A, Ishida H, de Waal Malefyt R, de Vries J (1992) Biological properties of interleukin 10. J Clin Immunol 12:239–247
Chernoff AE, Granowitz EV, Shapiro L, Vannier E, Lonnemann G, Angel JB, Kennedy JS, Rabson AR, Wolff SM, Dinarello CA (1995) A randomized, controlled trial of IL-10 in humans. Inhibition of inflammatory cytokine production and immune responses. J Immunol 154:5492–5499
Te Velde AA, de Waal Malefijt R, Huijbens RJ, de Vries JE, Figdor CG (1992) IL-10 stimulates monocyte FcγR surface expression and cytotoxic activity: distinct regulation of antibody-dependent cellular cytotoxicity by IFN-γ, IL-4, and IL-10. J Immunol 149:4048
Lalani I, Bhol K, Ahmed AR (1997) Interleukin-10: biology, role in inflammation and autoimmunity. Ann Allergy Asthma Immunol 79:469
Llorente L, Zou W, Levy Y, Richaud-Patin Y, Wijdenes J, Alcocer-Varela J, Morel-Fourrier B, Brouet JC, Alarcon-Segovia D, Galanaud P et al (1995) Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med 181:839
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25:1271
Eskdale J, Kube D, Tesch H, Gallagher G (1997) Mapping of the human IL10 gene and further characterization of the 5′ flanking sequence. Immunogenetics 46(2):120–128
Bienvenu J, Doche C, Gutowski MC, Lenoble M, Lepape A, Perdrix JP (1995) Production of pro-inflammatory cytokines and cytokines involved in the TH1/TH2 balance is modulated by pentoxifylline. J Cardiovasc Pharmacol 25(Suppl 2):80–84
Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV (1997) An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet 24(1):1–8 (Feb)
Lazarus M, Hajeer AH, Turner D, Sinnott P, Worthington J, Ollier WE, Hutchinson IV (1997) Genetic variation in the interleukin 10 gene promoter and systemic lupus erythematosus. J Rheumatol 24:2314–2317
Mok CC, Lanchbury JS, Chan DW, Lau CS (1998) Interleukin-10 promoter polymorphisms in Southern Chinese patients with systemic lupus erythematosus. Arthritis Rheum 41:1090–1095
Gibson AW, Edberg JC, Wu J, Westendom RG, Huizinga TW, Kimberly RP (2001) Novel single nucleotide polymorphism in the distal IL-10 promoter affect IL-10 production and enhance the risk of systemic lupus erythematosus. J Immunol 166:3915–3922
Lim S, Crawley E, Woo P, Barnes PJ (1998) Haplotype associated with low interleukin-10 production in patients with severe asthma. Lancet 352(9122):113 (Jul 11)
Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S (1996) Interleukin-10 regulation in normal subjects and patients with asthma. J Allergy Clin Immunol 97(6):1288–1296 (Jun)
Grove J, Daly AK, Bassendine MF, Gilvarry E, Day CP (2000) Interleukin 10 promoter region polymorphisms and susceptibility to advanced alcoholic liver disease. Gut 46(4):540–545 (Apr)
Guseva IA, Omarbekova Zh, Myakotkin VA (2003) Polymorphism of FcgRIIIA-158F/V and promotion region of IL-10 genes in systemic lupus erythematosus in Kazakhs. Therapeutic Archives 5:36
Eskdale J, Gallagher G, Verweij CL, Keijsers V, Westendorp RG, Huizinga TW (1998) Interleukin 10 secretion in relation to human IL-10 locus haplotypes. Proc Natl Acad Sci USA 5:9465
Mehrian R Jr, Quismorio FP, Strassmann G, Stimmler MM, Horwitz DA, Kitridou RC, Gauderman WJ, Morrison J, Brautbar C, Jacob CO (1998) Synergistic effect between IL-10 and bcl-2 genotypes in determining susceptibility to systemic lupus erythematosus. Arthritis Rheum 41:596
Eskdale J, McNicholl J, Wordsworth P, Jonas B, Huizinga T, Field M, Gallagher G (1998) Interleukin-10 microsatellite polymorphisms and IL-10 locus alleles in rheumatoid arthritis susceptibility. Lancet 352:1282
Keijsers V, Verweij CL, Westendorp RGJ, Breedveld FC, Huizinga TWJ (1997) IL-10 polymorphisms in relation to production and rheumatoid arthritis. Arthritis Rheum 40(Suppl 9):S179
Lazarus M, Hajeer AH, Turner D, Sinnott P, Worthington J, Ollier WE, Hutchinson IV (1997) Genetic variation in the interleukin 10 gene promoter and systemic lupus erythematosus. J Rheumatol 24:2314
Mok CC, Lanchbury JS, Chan DW, Lau CS (1998) Interleukin-10 promoter polymorphisms in Southern Chinese patients with systemic lupus erythematosus. Arthritis Rheum 41:1090
Eskdale J, Wordsworth P, Bowman S, Field M, Gallagher G (1997) Association between polymorphisms at the human IL-10 locus and systemic lupus erythematosus. Tissue Antigens 49:635
Crawley E, Kay R, Sillibourne J, Patel P, Hutchinson I, Woo P (1999) Polymorphic haplotypes of the interleukin-10 5′ flanking region determine variable interleukin-10 transcription and are associated with particular phenotypes of juvenile rheumatoid arthritis. Arthritis Rheum 42:1101
D’Alfonso S, Rampi M, Bocchio D, Colombo G, Scorza-Smeraldi R, Momigliano-Richardi P (2000) Systemic lupus erythematosus candidate genes in the Italian population: evidence for a significant association with interleukin-10. Arthritis Rheum 43:120
Pratt RE, Dzau VJ (1999) Genomics and hypertension concepts, potentials, and opportunities. Hypertension 33:238–247
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, PW., Huang, CM., Huang, CC. et al. The association of −627 interleukin-10 promoter polymorphism in Chinese patients with systemic lupus erythematosus. Clin Rheumatol 26, 298–301 (2007). https://doi.org/10.1007/s10067-006-0329-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-006-0329-8