Abstract
Over the last years, different case reports/studies have demonstrated that in patients with acute pulmonary embolism (PE) and refractory shock mechanical circulatory support (MCS) with Impella RP® (Abiomed, Inc, Danvers, Mass) increases the chances of survival, significantly unloading the right ventricle and improving both the cardiac output and the mean pulmonary artery pressure. We reviewed the medical literature about the use of Impella RP in patients with acute PE and refractory shock using PubMed (MEDLINE), Scopus, Cochrane library, and Google Scholar databases. The final research was conducted in July 2019. The results evidenced that available data are currently scant to definitively assess the real role Impella RP® in patient with acute PE and refractory shock. However, preliminary data seems to be very promising. Further larger studies are needed to confirm the safety and efficacy of MCS in these patients. A multidisciplinary assessment, using the PERT team, must be performed case by case to determine the need of MCS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute pulmonary embolism (PE) remains the third leading cause of cardiovascular mortality after acute myocardial infarction and stroke in western countries [1, 2]. According to the prognostic classification recommended by the European Society of Cardiology, patients are labelled as having a high-risk PE in the presence of hemodynamic instability, defined as systolic blood pressure < 90 mmHg, or a systolic pressure drop ≥ 40 mmHg for more than 15 min, not caused by new-onset arrhythmia, hypovolemia or sepsis [3, 4]. This clinical situation, which is encountered in about 5% of all PE cases, remains associated with a higher short-term mortality especially during the first hours after admission [5]. In these patients, expedited life-saving revascularization procedures, as systemic thrombolysis, catheter-direct therapy or surgical embolectomy are mandatory to improve the survival [6]. Indeed, from a pathophysiological point of view, the onset and progressive impairment of right ventricular function represent the main pathophysiological mechanisms of cardiogenic shock which is the main cause of the early cardiovascular mortality in these subjects. Intriguingly, over the latest years, a growing number of reports have highlighted the potential benefit of mechanical circulatory support (MCS) in high-risk PE patients who are refractory to medical and/or interventional treatments [7, 8]. In this regard, the use of Impella RP® (Abiomed, Inc, Danvers, Mass) has been successfully described but only in the form of isolated case reports [9,10,11]. In fact, no trials or larger prospective investigations have been performed on this issue, limiting the application of the MCS in daily clinical practice. Moreover, the absence of international recommendations further limits the potential use of the Impella RP® in selected PE patients. Aim of the present manuscript is to review the available medical literature on the use of Impella RP® in hemodynamically unstable PE patients also discussing its safety, effectiveness and drawbacks according to the available data.
Impella RP®: an overview
The Impella RP® (Abiomed, Inc, Danvers, Mass) currently represents the only percutaneous, minimally invasive micro-axial-pump approved by the Food and Drug Administration (FDA), able to provide temporary right ventricular support for up to 14 days in patients with acute right heart failure (Table 1).
From an interventional point of view, the Impella RP® is inserted percutaneously through a standard catheterization procedure via the femoral (generally the right) vein into the right atrium using a single 23 F vascular access. The pump inflow is placed in the inferior vena cava while the outflow in the pulmonary artery over a 0.018-inch wire. The device can propel blood from the inferior vena cava to the pulmonary artery bypassing the decompensated right ventricle, supplying up to 4.0 L/min. After an adequate weaning, the Impella RP is removed and the venous access site is closed with manual compression and a purse-string or using deep mattress suture [12].
Searching strategies
A review of the medical literature based on PubMed (MEDLINE), Scopus, Cochrane library and Google Scholar databases was performed to locate published investigations in English language reporting data on the use of Impella RP® in patients with acute PE.
Specifically, the relevant articles, case reports or case series were identified using a combined text word and MeSH (Medical Subject Headings) search strategy. The following combination of keywords were used: “Pulmonary Embolism and Impella RP”; “Impella and acute pulmonary embolism”; “mechanical circulatory support and acute pulmonary embolism”. Moreover, we searched the bibliographies of target studies for additional references. The final research was conducted in July 2019.
Study selection and data extraction
Titles and abstracts retrieved from the search were reviewed. Articles were included if a) they reported the use of Impella RP® in patients with acute PE, b) patients have an objectively confirmed diagnosis of acute PE, and c) data on the short-term outcome were reported. Final determination of article inclusion was based on consensus by authors. Extracted data included: demographic issues, comorbidities, hemodynamic profile at admission, transthoracic echocardiogram (TTE) findings, Pulmonary Embolism Severity Index (PESI) score, use of computed tomography angiography (CTA) for the diagnosis, interventional treatments, dose of thrombolytic, duration and improvement with the Impella RP® support, short-term mortality and cardiovascular drug used for vasopressor and/or inotropic support. This study is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [13].
Article selection and registries included in the analysis
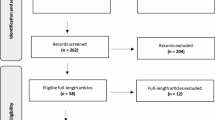
A total of 91 articles were identified. After excluding the duplicates obtained using the different searching MeSH (n = 36), 55 articles were screened. Specifically, 31 have been excluded for not meeting inclusion criteria and/or the article type (editorial, letter, comments general review on the use of the device) or article not in English language. As result, 24 articles were assessed for eligibility and carefully reviewed. Of them 40 were excluded because they not provided data on the use of Impella RP® in PE patients (Fig. 1). Finally, 4 investigations in the form of case reports or series, met the inclusion criteria and were included in the analysis.
Literature results
The current medical literature revealed 9 patients, reported in 4 investigations, treated with Impella RP® during acute PE [9,10,11, 14] (Table 2).
The mean age was 53.4 ± 13.6 years; 7 patients (77.7%) were aged < 65 years old. Unfortunately, since the largest case series reviewed did not provide the gender of patients enrolled, we cannot assess the overall gender distribution. About half of patients were hemodynamically stable at admission (55.5%) and deteriorated to high-risk over the following hours. The mean PESI was 133.6 ± 52.3; specifically, 6 (66.6%), 2 (22.2%) and one patient (11.1%) were classified as PESI class V, III and I, respectively [15]. Arterial hypertension was the most frequent cardiovascular comorbidity observed (33.3%). Moreover, three patients (33.3%) reported a previous history of venous thromboembolism while 5 subjects (55.5%) had a confirmed deep venous thrombosis during the hospitalization. Right ventricular dysfunction was detected in all patients at TTE while the final diagnosis of acute PE was made using computed tomography angiography in 88.8% of cases (n = 8). Only one patient, who experienced a cardiac arrest and remained hemodynamically unstable at admission, received the PE diagnosis due the presence of PE echocardiographic signs [14]. Eight on nine patients received a catheter-directed ultrasound-accelerated thrombolysis using the EKOS Ekosonic® Endovascular system. Among these, 5 subjects (62.5%) received a bilateral endovascular treatment with different rates of alteplase infusion. Due the presence of refractory shock, the Impella RP® was implanted for a mean period of 3.5 ± 1.8 days (range 1–6 days), generating a significant hemodynamic improvement. The vasopressor and/or inotropic agents administered, with the relative dosage, are presented in Table 3 [9,10,11, 14]. Regarding the short-term outcome, only one patient died after three months but not for reasons related to the acute PE [9].
Impella RP® in acute pulmonary embolism: when it should be considered?
Current international guidelines on the management of acute PE have not yet provided any recommendations regarding the use of Impella RP® [15]. Surely, in hemodynamically unstable PE patients who are refractory to the “traditional” volume expansion and inotropic support, or when the reperfusion treatments have failed, the Impella RP® could be considered. A potential algorithm to potentially evaluate the need of this device in PE patients has been provided in Fig. 2. In this regard, a patient-tailored evaluation balancing the potential benefits and risks appears. Probably, a special category of patients is represented by those admitted after having an out-of-hospital cardiac arrest since the diagnosis of acute PE might be delayed and their neurological status could influence their prognosis and suitability for mechanical support. However, a multidisciplinary evaluation, maybe conducted by a local PERT team, should be encouraged before supporting the patient with the Impella RP ® [16]. It is also true that the device is currently unavailable in most institutions and sometimes also underused. The creation of a dedicated Hub and spoke network, could be also considered to allow an adequate availability of the device, especially in emergent scenarios [13].
Potential algorithm to evaluate for the evaluation of the need on the Impella RP® in hemodynamically unstable PE patients with refractory shock. After having identify those patients with right ventricular failure/dysfunction, current international guidelines recommend a prompt revascularization using systemic thrombolysis (ST), catheter-direct treatment (CDT) or surgical embolectomy evaluating patient’s specific absolute and/or relative contraindications. Volume status must be carefully assessed and corrected, if needed, achieving a central venous pressure (CVP) between 12 and 15 mmHg. In hypervolemic patients the diuresis must be promote while in hypovolemic subjects volume resuscitation must be cautiously performed since after the first attempt the risk of increase the preload, which lead to a further deterioration in the ventricular hemodynamic due the interventricular interdependence, is high. Vasopressors and/or in inotropic agents can be administered tailoring the specific patient’s needs. An adequate ventilation must be guarantee because both atelectasis or higher positive-end-respiratory pressure (PEEP) values may increase the afterload. When the interventional and medical support have failed, and patients exhibit refractory shock the use of direct (e.g., Impella RP) RV mechanical circulatory support could be considered with potential further escalation to ECMO.TTE: Transthoracic echocardiogram; TEE: Transoesophageal Echocardiogram; RHC: Right heart catheterization
Potential advantages of Impella RP in patients with pulmonary embolism
The use of MCS in patients with refractory shock after acute PE represents one of the biggest advances in the treatment of hemodynamically unstable PE patients achieved during the last years, as well as the use of extracorporeal membrane oxygenation (ECMO). Initial experience with Impella RP® after acute PE seems to be promising [6, 17]. Indeed, the implantation and device preparation times are lower compared to other device used to provide circulatory support such as veno-arterial (V-A) ECMO. Moreover, the post-implant management appears to be simple, associated with lower costs and vascular access complications since requiring a single vascular access [18].
Potential pitfalls of Impella RP in patients with pulmonary embolism
Some pitfalls must be considered using the Impella RP. First, the need of fluoroscopy for the device implantation, which requires a non-neglectable period of time, is a significant limitation since it requires a cath-lab as well as to transfer and treat a hemodynamically unstable patient. Moreover, since the Impella RP ® is not able to deliver any respiratory support, the presence of concomitant hypoxaemia or more in general respiratory failure, must be managed using the traditional invasive approach.
Tricuspid valve regurgitation represents a contraindication to the use of the device according to the manufacturer. However, tricuspid regurgitation is generally observed in EP patients being a sign of right ventricular dysfunction [19, 20]. In this regard, some authors have suggested that a tricuspid valve regurgitation could be considered as a “warning” rather than an absolute contraindication to the use of Impella RP which requires a case by case evaluation [21]. Conversely, any tricuspid valve prosthesis represents a major contraindication for the use of the device.
A serial multiparametric evaluation of right ventricular function based on TTE and/or transoesophageal echocardiography (TEE) as well as central venous pressure, cardiac output and invasive pulmonary artery pressure measurements is required in patients supported with Impella RP®. For the latter, the tip of the Swan-Ganz catheter must be positioned into the right pulmonary artery, while the tip of Impella RP ® in the main pulmonary artery, directed toward the left pulmonary artery. Moreover, the presence of any clots into the main pulmonary artery must be carefully excluded before positioning the outflow cannula. Indeed, as suggested by the investigations reviewed, a catheter-directed treatment is generally perform before positioning the device since a clot in the main pulmonary artery can deteriorate the blood flow generated by the axial-pump as well as the afterload of the right ventricle. Similarly, any thrombus located into the inferior vena cava must be preliminary removed.
Other critical aspects that can be encountered during the circulatory support in intensive care unit and need immediate actions could range from displacement or thrombosis of the device, pump failure, arrhythmias, cardiac perforation and tamponade, haemolysis, thrombocytopenia, bleeding at cannula insertion site, pulmonary valve insufficiency, tricuspid valve injuries,, phlegmasia cerulens dolens as well as to infections at the cannula insertion [11, 14, 17].
The weaning procedure represents another key step in the circulatory management of these patients. Physiologically, the pulmonary circulation is a low-pressure system, so any reduction in the pump’s speed could leads to small reduction of the flow. As consequence a gradual and patient-tailored weaning must be performed.
Conclusions
The results evidenced that available data are currently scant to definitively assess the real role Impella RP® in patient with acute PE and refractory shock. However, preliminary data seem promising. Reviewed manuscripts demonstrated a significant hemodynamic improvement with a concomitant reduction of the right ventricle afterload in all patients which exhibit a low mortality rate in the short-term period. Intriguingly, the majority of PE patients included in the reviewed case reports were hemodynamically stable at admission and further deteriorate to refractory cardiogenic shock over the following hours. This aspect emphasises the dynamic evolution of acute PE. However, the absence of large studies as well as mid- and long-term data on the outcome of these patients represent the major limitations on the diffusion of this mechanical circulatory support. There is an urgent need of further studies on this issue to provide more specific recommendations on the selection of PE patients and confirm both the safety and efficacy of the device in this clinical scenario.
References
Zuin M, Rigatelli G, Picariello C, Carraro M, Zonzin P, Roncon L. Prognostic role of a new risk index for the prediction of 30-day cardiovascular mortality in patients with acute pulmonary embolism: the Age-Mean Arterial Pressure Index (AMAPI). Heart Vessels. 2017;32:1478–87.
Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379:1835–46.
Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–3069, 3069a–3069k.
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52.
Jiménez D, Bikdeli B, Barrios D, Quezada A, Del Toro J, Vidal G, Mahé I, Quere I, Loring M, Yusen RD, Monreal M; RIETE investigators. Epidemiology, patterns of care and mortality for patients with hemodynamically unstable acute symptomatic pulmonary embolism. Int J Cardiol. 2018. https://doi.org/10.1016/j.ijcard.2018.07.059(Epub ahead of print)
Elder M, Blank N, Shemesh A, Pahuja M, Kaki A, Mohamad T, Schreiber T, Giri J. Mechanical circulatory support for high-risk pulmonary embolism. Interv Cardiol Clin. 2018;7:119–28.
Watts JA, Marchick MR, Kline JA. Right ventricular heart failure from pulmonary embolism: key distinctions from chronic pulmonary hypertension. J Card Fail. 2010;16:250–9.
Desai H, Natt B, Bime C, Dill J, Dalen JE, Alpert JS. Pulmonary embolism with right ventricular dysfunction: who should receive thrombolytic agents? Am J Med. 2017;130:93.
Kumar Bhatia N, Dickert NW, Samady H, Babaliaros V. The use of hemodynamic support in massive pulmonary embolism. Catheter Cardiovasc Interv. 2017;90:516–20.
Elder M, Blank N, Kaki A, Alraies MC, Grines CL, Kajy M, Hasan R, Mohamad T, Schreiber T. Mechanical circulatory support for acute right ventricular failure in the setting of pulmonary embolism. J Interv Cardiol. 2018;31:518–24.
Shokr M, Rashed A, Mostafa A, Mohamad T, Schreiber T, Elder M, Kaki A. Impella RP support and catheter-directed thrombolysis to treat right ventricular failure caused by pulmonary embolism in 2 patients. Tex Heart Inst J. 2018;45:182–5.
Kapur NK, Esposito ML, Bader Y, Morine KJ, Kiernan MS, Pham DT, Burkhoff D. Mechanical circulatory support devices for acute right ventricular failure. Circulation. 2017;136:314–26.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100.
Youssef A, Selle A, Ende G, Ibrahim K. The successful use of the Impella RP after a long cardiopulmonary resuscitation and systemic thrombolytic therapy in a patient with a fulminant pulmonary embolism: the first case report. Eur Heart J Case Rep. 2018;2(1):ytx023. https://doi.org/10.1093/ehjcr/ytx023
Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Ní Áinle F, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2019. https://doi.org/10.1093/eurheartj/ehz405(Epub ahead of print)
Root CW, Dudzinski DM, Zakhary B, Friedman OA, Sista AK, Horowitz JM. Multidisciplinary approach to the management of pulmonary embolism patients: the pulmonary embolism response team (PERT). J Multidiscip Healthc. 2018;11:187–95.
Pieri M, Pappalardo F. Impella RP in the Treatment of Right Ventricular Failure: What We Know and Where We Go. J Cardiothorac Vasc Anesth. 2018. https://doi.org/10.1053/j.jvca.2018.06.007. (Epub ahead of print)
Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64:1407–15.
Torbicki A. Echocardiographic diagnosis of pulmonary embolism: a rise and fall of McConnell sign? Eur J Echocardiogr. 2005;6:2–3.
Kurnicka K, Lichodziejewska B, Goliszek S, Dzikowska-Diduch O, Zdończyk O, Kozłowska M, Kostrubiec M, Ciurzyński M, Palczewski P, Grudzka K, Krupa M, Koć M, Pruszczyk P. Echocardiographic pattern of acute pulmonary embolism: analysis of 511 consecutive patients. J Am Soc Echocardiogr. 2016;29:907–13.
Pieri M, Pappalardo F. Impella RP in the treatment of right ventricular failure: what we know and where we go. J Cardiothorac Vasc Anesth. 2018. https://doi.org/10.1053/j.jvca.2018.06.007(Epub ahead of print)
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zuin, M., Rigatelli, G., Daggubati, R. et al. Impella RP in hemodynamically unstable patients with acute pulmonary embolism. J Artif Organs 23, 105–112 (2020). https://doi.org/10.1007/s10047-019-01149-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-019-01149-9