Abstract
The overall pre-clinical process of determining the blood compatibility of any medical device involves several stages. Although the primary purpose is to protect the patients, laboratory testing has been over-utilized for many years with a huge number of unnecessary animal tests being done routinely. Recently, the elimination of needless testing has become important in controlling the cost of healthcare and in addressing many issues related to the ethics of animal research. With this in mind, we designed a new in situ porcine closed-circuit system to study the complex interplay between platelets, coagulation proteins, and other cellular elements in pigs. We proved that this system can be implemented in blood compatibility testing and minimize the number of animals used in the experiments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first synthetic blood vessel was reported in 1952 by Voorhees et al. [1]. Now, polyethylene terephthalate (PET, terylene, or dacron) and polytetrafluoroethylene (PTFE, teflon, or gore-tex) are mainly used as synthetic vascular grafts [2]. These materials work well for large-diameter and high-flow conduits, but for small-diameter applications (< 6 mm), their use has produced disappointing patency rates [3,4,5].
When a new material is developed, researchers initially test in vitro coagulation and inflammation markers on cells. These simplified tests mainly attempt to mimic the performance of blood vessels by examining the response of only a few blood components, and are often inaccurate [6]. Hence, animal testing has become an essential step in simulating human physiology [7]. However, there is no ideal animal model that is suitable for the assessment of all performance aspects of novel conduits. Cost, availability, and ease of handling during examinations are criteria often used to guide animal selection, although the primary importance is to simulate the relevant aspect of human physiology correctly [7,8,9]. Hemocompatibility is recognized as being extremely species-specific and thus, animal models fail to give predictive results [7, 10]. Moreover, hemocompatibility and thrombogenicity are not defined physical quantities [11]; therefore, new materials need to be compared with several references that have been identified as well-known thrombogenic and hemocompatible surfaces (negative and positive controls). Therefore, the main problems in in vivo testing are the lack of a standardized approach to the selection, the utilization of appropriate animal models [7, 10], and the large number of animals required in the screening process.
Several approaches have been developed to improve pre-clinical in vivo testing. For example, Noishiki [12] used sutures coated with test polymers and inserted these sutures into the lumen of peripheral dog veins, claiming that the method was a simple procedure with minimal surgical intervention. However, this method required a large number of dogs to test all the samples and controls. Subsequently, other researchers used a series of ex vivo shunts in dogs [13] and pigs [14]. In these experiments, animals were used to find out what happens in the entire living body, but the researchers did not try to minimize the frequent use of living animals to drastically decrease the resources necessary to obtain successful clinical products. Deirdre et al. [6] performed in vitro analyses of thrombosis and inflammation in parallel with in vivo responses in non-human primate models to identify in vitro predictive parameters for thrombogenicity. Determining the correlation between in vitro properties and positive in vivo outcomes is essential, but experiments on large animal models, in particular non-human primates, have several disadvantages and their use has long been the subject of heated debate.
Considering the ethical concerns arising in many areas of research regarding animal experiments and the bias in the results, in this work we designed a new in situ porcine closed-circuit system to study the complex interplay between platelets, coagulation proteins, and other cellular elements in pigs. This device will allow large numbers of samples (up to 16) to be studied using the fewest number of animals possible.
Materials and methods
Phage library, bacteria, and cells
The linear heptapeptide (Ph.D.-7) phage-display libraries kits (New England Biolabs, Beverly, MA, USA) were used for the in vitro biopanning experiments. Phage was propagated in Escherichia coli strain ER2738, which was provided with the kit. Human endothelial progenitor outgrowth cells (EPOCs, Catalog #: Z7030001) were obtained from BioChain Institute Inc. (Newark, CA, USA) and cultured in Cell Growth Media Kits EGM-2 SingleQuot Kit Suppl. and Growth Factors (Catalog #CC-4147, LONZA) at 37 °C with 5% CO2. Cells were detached using 0.05% trypsin–ethylenediaminetetraacetic acid (Gibco, Life Technologies, Carlsbad, CA, USA) and passaged. Biopanning was conducted following a modified protocol from New England Biolabs (Ipswich, MA, USA). The Ph.D.-7 library was used in four rounds of biopanning against adherent EPOCs. Phages at 2 × 1011 pfu were incubated with EPOCs plated on a collagen type I dish for 1 h at 37 °C, with occasional mixing. Cells were washed three times with TBSS [50 mM Tris–HCl (pH 7.5), 150 mM NaCl. 0.1% (v/v) Tween-20] and the bound phages were eluted with 0.2 M glycine–HCl (pH 2.2). The eluted phages were amplified in E. coli purified by polyethylene glycol (PEG) precipitation and used for subsequent enrichment rounds. After each round, individual clones were isolated, amplified, and purified by PEG precipitation. The following abbreviations for the peptides sequences were used: A, GQSEKHL; B, HGGVRLY; D, SLSKWSF; E, KIAVIST; H, VSRDTPQ (Fig. S1).
Peptide immobilization onto expanded PTFE (ePTFE) suture
GORE® PRECLUDE® suture (ePTFE, Catalog Number 4N04J) was purchased from Gore Creative Technologies Worldwide (W. L. Gore and Associates, Inc., Newark, DE, USA). N-ε-maleimidocaproic acid (ECMA) was purchased from Funakoshi (Tokyo, Japan). 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), 2-(N-morpholino) ethanesulfonic acid (MES), borane solution (BH3 in 1 M tetrahydrofuran, THF) and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used as received. Synthesized peptides were obtained from SCRUM Inc. (Tokyo, Japan). Each peptide contained the specific sequences of interest followed by the C-terminal sequence GGGC for maleimide–thiol coupling.
ePTFE disks (diameter of 12 mm) were cut from a 15 cm × 20 cm × 0.1 mm sheet (PSM-01200, W. L. Gore and Associates, Inc., Newark, DE, USA) and prepared as previously described [15]. The ePTFE sutures were cleaned by repeated washing in methanol in an ultrasonic bath. Dry samples were immersed in 1 M BH3 solution in THF for 1 h at room temperature. The samples were then washed in THF and water, and oxidation was performed in H2O2 (30%) containing 10% NaOH for 1 h in ice, followed by rinsing with water. The samples containing surface-bound OH groups were reacted with ECMA (1 mg/mL) and EDC (2 mg/mL) in 0.1 M MES (pH 6) for 1 h, washed with water, incubated with 1.5 mg/mL peptides in phosphate-buffered saline (PBS) at pH 7.4 for 1 h, and washed again with water. The samples were sterilized using ethylene oxide gas and stored under vacuum [15].
Measurement of blood recalcification profiles
Whole blood was collected from pigs using acid citrate dextrose (ACD) anticoagulant. The anticoagulated whole blood samples were recalcified with the addition of 0.1 M CaCl2. The modified ePTFE samples were rinsed with sterile water and placed in 24-well polystyrene (PS) plates. The disks were immobilized on the bottom of each well using a stainless-steel ring. The modified and non-modified ePTFE-containing wells as well as empty wells were incubated for 5, 10, and 15 min at 37 °C with 15 µL of recalcified blood. The whole blood incubated with PS completely clotted in 15 min and, therefore, it was used as the reference thrombogenic material (negative control). Then, 300 µL of distilled water was added to the wells and incubated at room temperature for 10 min to lyse red blood cells that were not trapped in the thrombus [16, 17]. All samples were appropriately diluted, and the concentration of released hemoglobin was measured by transferring the supernatant to a 96-well plate followed by spectrophotometric analysis. The absorbance was read at 380, 415, and 450 nm against a distilled water blank in a Varioskan microplate reader (ThermoFisher, Waltham, MA USA), according to the method devised by Harboe [18,19,20]. The percentage of clotting was defined as:
where Hb is the calculated hemoglobin content, N is the generic recalcified sample, PS is the control (polystyrene), and “Non-calcined whole blood” is the non-calcined sample.
Platelets adhesion assay
Platelet-rich plasma (PRP) was obtained from pig blood with ACD buffer as an anticoagulant. The blood was centrifuged for 4 min at 600×g. The clear PRP supernatant was removed and the number of platelets was counted with a Z2 coulter particle counter and size analyzer from BioChain Institute Inc. (Newark, CA, USA). Part of the PRP was centrifuged at 1300×g for 10 min to obtain platelet-poor plasma (PPP). The concentration of PRP was adjusted to 2 × 107 platelets with PPP. The samples were incubated for 1 h at 37 °C in 5% CO2. The samples were then fixed with formaldehyde (3.7% v/v in PBS) for 10 min, dehydrated with graded ethanol (50, 60, 70, 80, 90, and 100%), dried in vacuum, coated with gold by sputtering (EIKO IB3 Ion coater, EIKO Engineering Co., Ltd., Japan), and subjected to scanning electron microscopy (SEM, JCM 5700, JEOL, Japan). The reliable number of adhered platelets on the samples was calculated from five SEM images of each sample at a magnification of 2000×.
Surgical procedure and in situ experimental design
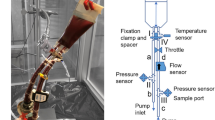
All animal experiments were conducted in accordance with the Guidelines for Animal Experiments established by the Ministry of Health, Labor, and Welfare of Japan and by the National Cerebral and Cardiovascular Center Research Institute (Osaka, Japan). The protocol was approved by the Committee on the Ethics of Animal Experiments of the National Cerebral and Cardiovascular Center Research Institute (Permit Number: 16,550). Goettingen minipigs, purchased from Ellegaard Goettingen Minipigs A/S (Dalmose, Denmark), were anesthetized with 100 mg/h intravenous injection of 1% propofol (Diprivan; AstraZeneca, Wilmington, DE, USA). A single injection of heparin (Novo-heparin; Novo Nordisk, Copenhagen, Denmark) was administered to achieve an activated clotting time (ACT) of greater than 250 s. The ACT (Medtronic Inc., Minneapolis, MN, USA) was monitored every 30 min. To evaluate the very early stage of thrombogenesis, the sutures were exposed to blood flow for 120 min in a modified heart–lung machine (Fig. 1a). After surgery, the sutures were fixed with formaldehyde (3.7% v/v in PBS) overnight. The sutures were stained with the primary antibody fluorescein isothiocyanate-tagged CD41 and examined using confocal microscopy (Olympus IX81; Olympus, Hamburg, Germany). The area covered by the adherent platelets was quantified using ImageJ software. Samples used for SEM were fixed with formaldehyde (3.7% v/v in PBS) for 10 min, dehydrated with graded ethanol (50, 60, 70, 80, 90, and 100%), dried in vacuum, coated with gold by sputtering (EIKO IB3 Ion coater, EIKO Engineering Co., Ltd., Tokyo, Japan), and subjected to SEM observation (JCM 5700 JEOL, Tokyo, Japan).
a Outline of the experimental set-up. The extracorporeal bypass circuit connects the superior vena cava and aorta in the pig. The black triangles represent the tubing forceps used to control blood flow. b, c, f, m show the schematic of system 1. All measurements are expressed in mm. d, e, g, h, l are in vivo photos
In situ porcine closed-circuit system design
Closed-circuit system 1
The system is a polycarbonate straight connector (Senko Medical instrument Mfg. Co., Ltd., Tokyo, Japan) with a diameter of 10 mm and a height of 80 mm (Fig. 1m). The sutures were inserted with a needle on two polycarbonate rings (Fig. 1l), and these rings were then inserted inside the straight connector at the opposite extremities. This module was connected to the extracorporeal blood circulation system (Fig. 1a, d, e, g, h).
Closed-circuit system 2
The system is a cylindrical polycarbonate block (Senko Medical instrument Mfg. Co., Ltd., Tokyo, Japan) with an outer diameter of 14 mm and a height of 70 mm (Fig. 2a, b). The sutures were inserted from top to bottom using a needle at an orientation angle of approximately 20° (α in Fig. 2b). Two connectors were inserted at the extremities of the tube and fixed by a cable tie. Two modules were inserted in parallel circuit and connected to the extracorporeal blood circulation system as shown in Figs. 1a and 2h.
Closed-circuit system 3
The system is a cylindrical polycarbonate block with an outer diameter of 14 mm and a height of 70 mm. The sutures were inserted using a needle at an orientation angle of approximately 20° (α in Fig. 2b, c). Two connectors were inserted at the extremities of the tube and fixed by a cable tie (Fig. 2d). This module was connected to the extracorporeal blood circulation system as reported in Figs. 1a, 2c, d.
Results and discussion
Several attempts have been made to suppress the thrombogenicity of ePTFE by immobilizing chemicals or anticoagulants to the graft lumen. Early studies have shown decreased platelet deposition on carbon-coated ePTFE grafts, but the overall patency rates have not improved [2]. Here, we report experiments to quantify the thrombogenicity of the sample surfaces: (1) the area of adherent platelets was calculated from SEM images (Fig. 3a, f), (2) the in vitro clotting kinetics were assessed against whole pig blood (Fig. 3b), and (3) the in situ thrombogenicity of the samples was assessed in minipigs (Fig. 4). In these experiments, the REDV sequence was used as a reference sample (low adhesion of platelets) [21,22,23] and naked ePTFE was used as the untreated sample [24,25,26]. In Fig. 3a, it is possible to see that ePTFE-B had the lowest number of platelets per unit area. However, although the other surfaces suppressed platelet adhesion, they could potentially stimulate platelet activation [17]. Since platelets undergo a change in shape upon activation, images of platelets were acquired by SEM, analyzed, and classified based on the categories of activation, as shown in Fig. 3f. Prior to activation, platelets are discoid in shape. Upon activation, they exhibit a progression of morphology from discoid to a fully spread form. These morphologies can be classified as round (inactivated), dendritic (minimally activated), spread dendritic (activated, one or more pseudopodia flattened), spread (activated), and fully spread (activated, hyaloplasm spreading) [27].
Material characterization and in vitro thrombogenic results. a Number of adherent platelets per mm2. b Assessment of clotting kinetics using whole blood recalcification assay. c Contact angle on various surfaces. d X-ray photoelectron spectroscopy of various surfaces, N1s spectra. e Adherent platelets per category expressed as percentages. R round, D dendritic, SD spread dendritic, S spread, FS fully spread. f Table with abbreviations used in this study. Additionally, a small platelet aggregate is labeled as A
The platelets that adhered to ePTFE-H and ePTFE presented pseudopodia and they were mostly in the activated state. On ePTFE-B, all platelets were in the inactivated state.
Figure 3b shows the kinetics of the clotting process expressed as percentages. PS and naked ePTFE acted as negative controls, and ePTFE-REDV was considered as the positive control (Fig. 3d). In fact, the REDV sequence provided an anti-thrombogenic surface and improved the in vivo patency [21,22,23]. The presence of peptides on the surface of ePTFE slowed down the clotting process. In particular, after 10 min, almost all the blood was clotted on PS, whereas on ePTFE, 65% of the blood formed a clot. Only 35% of the blood formed a clot on the surface covered by peptides (ePTFE-H). After 15 min, ePTFE-REDV and naked ePTFE behaved very similarly to the control PS sample. Instead, lower thrombogenicity was observed in ePTFE-A and ePTFE-B. This study was conducted under static conditions; therefore, the platelets could freely interact with the material surfaces. However, it is unclear exactly what would happen in vivo. Although these experiments suggested that peptide B might be a good choice for the surface modification of ePTFE, the in vivo hemocompatibility should ultimately be investigated.
No single model is optimal for studying the biocompatibility, thrombogenicity, and hemodynamics of ePTFE-modified surfaces. Therefore, selection must be made carefully and with specific criteria in mind. In this paper, we reported only the results of five samples and two controls. However, researchers often have dozens of samples to test. Studying these samples in small animal models, which often employ a short graft, does not ensure that human response is modeled because the grafts can spontaneously endothelialize [2]. Therefore, small animal models cannot be used as a screening method. To address this limitation, we outlined the experimental set-up for the screening of a large number of samples in a single pig. The extracorporeal bypass circuit connects the superior vena cava and aorta in the pig. Pigs were selected because of the similarities between their cardiovascular system and that of humans, as well as their ability to provide the large volume of blood needed to fill the systems. The final experimental set-up was realized as an evolution of three different designs. All the configurations were characterized by a parallel system, where the in situ porcine closed-circuit system was set in a branch, and a straight polycarbonate tube was on the other branch (Fig. 1a). This is necessary because it is essential to stabilize the flow condition after the surgery to insert the cardiopulmonary bypass and set-up the perfect condition for the heart–lung machine. During this first stage, there is no blood flow running thought the in situ porcine closed-circuit system, which was previously filled with saline solution. When the conditions become stable, blood is allowed to pass through the in situ porcine closed-circuit system and the other branch is closed. Few adjustments are necessary to stabilize the blood flow in the circuit. In this way, it is possible to control the time of exposure of the sutures to blood flow.
During the experiments, the in situ porcine closed-circuit system 1 (Fig. 1e, g, h, and Table S1) showed high bubbling in the tubes due to the blood flow. The tubing lines of the bypass circuit should be connected as smoothly as possible to prevent the formation of vortices. Flow separation tends to occur along curved walls, at branch points, and with changes in tube diameter. When flow separation occurs, vortices are commonly observed. Vortices are detrimental because of their ability to generate large fluctuations in force and pressure. Moreover, persistent turbulence can damage red blood cells and activate platelets. Therefore, the design of this tubing system was not suitable for the study of platelet adhesion on the sutures.
In the experimental set-up of closed-circuit system 2 (Fig. 2c, h, f, and Table S1), high bubbling in one of the two bioreactors was observed. Because the bubbling was not constant, we concluded that (1) the pressure inside the system was not high enough, and (2) the Y-junctions were too close to each other. The connection points along the circuit should produce a smooth path, and junctions that are spaced too closely can produce undesirable effects and cause increasing turbulence. In this bioreactor, to decrease the possible effect of diameter changes on the blood flow, the sutures were inserted directly in the tube with at an angle α, which guaranteed that the surface of the sutures was completely in contact with the blood flow (Fig. 2d and Table S1).
The closed-circuit system 3 (Fig. 2c–e) was the final design of the circuit system. The bioreactor was inserted in the circuit to keep the tubing system in a straight path. It was possible to insert only one device in the bloodstream of a minipig. However, in each bioreactor, it is possible to insert several samples (in Fig. 2d, it is possible to see 13 sutures inserted in the tube, but 16 sutures can easily be inserted). The blood flow was smooth and constant during the two hours of the experiments.
Figure 4a shows the SEM images of the suture surface tested for 2 h in system 3. Clots were present on ePTFE-A, ePTFE-E, ePTFE-H, and ePTFE, whereas only protein deposits were visible on ePTFE- B and ePTFE-REDV. To reveal the distribution of platelets, we stained the modified suture surfaces with platelet marker CD41 (Fig. 4b). In these images, platelet counting is an intricate procedure and background fluorescence subtraction was made only with manually optimized parameters. However, platelets are too small and after activation they appear to form complexes that are too difficult to be assessed. Therefore, here we only expressed the area of adherent platelets as percentages using ImageJ (Fig. 4c). Platelets covered greater areas on ePTFE-A and ePTFE-D. Moreover, from Fig. 4b, it is possible to see that platelet aggregations on the surfaces of the samples were different. Large and numerous aggregations were observed on ePTFE-A and ePTFE-H. However, on ePTFE-D, ePTFE-E, ePTFE-REDV, and naked ePTFE, many platelets seemed to attach to the surface but very few and small aggregates were detected. On ePTFE-B, a lower quantity of platelets was detected, confirming the SEM image in Fig. 4a, where almost no platelets were visible, although some protein started to adhere to the surfaces.
Conclusion
Ongoing innovation in biomaterial design requires pre-clinical assessment in vivo, and a large number of animal models have been used for this purpose. Although animals have been used since the second century, nowadays it is important to eliminate unnecessary testing on animals. This study presents the design of a simple in situ porcine closed-circuit system to explore the interaction between several samples and blood flow in minipigs. This device will support fast pre-clinical screening of several samples (i.e., 13 or 16) using the fewest number of animals possible. The reported results proved that HGGVRLY is most the appropriate choice for the modification of ePTFE surfaces to improve hemocompatibility.
References
Voorhees AB Jr, Jaretzki A 3rd, Blakemore AH. The use of tubes constructed from vinyon “n” cloth in bridging arterial defects. Ann Surg. 1952;135:332–6.
Chlupác J, Filová E, Bačáková L. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol Res. 2009;58:119–39.
Zilla P, Bezuidenhout D, Human P. Prosthetic vascular grafts: wrong models, wrong questions and no healing. Biomaterials. 2007;28:5009–27.
Kakisis JD, Liapis CD, Breuer C, Sumpio BE. Artificial blood vessel: the Holy Grail of peripheral vascular surgery. J Vasc Surg. 2005;41:349–54.
Pashneh-Tala S, MacNeil S, Claeyssens F. The tissue-engineered vascular graft—past, present, and future. Tissue Eng Part B Rev. 2016;22(1):68–100.
Anderson DEJ, Glynn JJ, Song HK, Hinds MT. Engineering an endothelialized vascular graft: a rational approach to study design in a non-human primate model. PLoS One. 2014;9:1–23.
Byrom MJ, Bannon PG, White GH, Ng MKC. Animal models for the assessment of novel vascular conduits. J Vasc Surg. 2010;52:176–95.
Ratner BD. Blood compatibility—a perspective. J Biomater Sci Polym Ed. 2000;11:1107–19.
Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (eds). PART III: practical aspects of biomaterials implants & devices—special concerns. In: Biomaterials science: an introduction to materials in medicine. 2nd ed. Elsevier Academic Press; 2004.
Greek R. A discussion of the role of complex evolved systems in the development of invasive cardiovascular interventions as illustrated by the Blalock-taussig shunt and intra-arterial stents. Biol Syst. 2014;3:1–27.
Jung F, Braune S, Lendlein A. Haemocompatibility testing of biomaterials using human platelets. Clin Hemorheol Microcirc. 2013;53:97–115.
Noishiki Y. in vivo evaluation method of a new antithrombogenicity using the peripheral vein. J Bioact Compat Polym. 1986;1:147–61.
Silver JH, Hergenrother RW, Lin J-C, Lim F, Lin H-B, Okada T, et al. Surface and blood-contacting properties of alkylsiloxane monolayers supported on silicone rubber. J Biomed Mater Res. 1995;29:535–48.
Badimon L, Vilahur G, Padro T. Atherosclerosis and thrombosis: insights from large animal models. J Biomed Biotechnol. 2011;2011:1–13.
Munisso MC, Yamaoka T. Novel peptides for small-caliber graft functionalization selected by a phage display of endothelial-positive/platelet-negative combined selection. J Mater Chem B. 2017;5:9354–64.
Leitão AF, Gupta S, Pedro J, Reviakine I, Gama M. Hemocompatibility study of a bacterial cellulose/polyvinyl alcohol nanocomposite. Colloids Surf B Biointerfaces. 2013;111:493–502.
Motlagh D, Yang J, Lui KY, Webb AR, Ameer GA. Hemocompatibility evaluation of poly (glycerol-sebacate) in vitro for vascular tissue engineering. Biomaterials. 2006;27:4315–24.
Noe DA, Weedn V, Bell WR. Direct spectrophotometry of serum hemoglobin: an Allen correction compared with a three-wavelength polychromatic analysis. Clin Chem. 1984;30:627–30.
Cookson P, Sutherland J, Cardigan R. A simple spectrophotometric method for the quantification of residual haemoglobin in platelet concentrates. Vox Sang. 2004;87:264–71.
Han V, Serrano K, Devine DV. A comparative study of common techniques used to measure haemolysis in stored red cell concentrates. Vox Sang. 2010;98:116–23.
Mahara A, Somekawa S, Kobayashi N, Hirano Y, Kimura Y, Fujisato T, et al. Tissue-engineered acellular small diameter long-bypass grafts with neointima-inducing activity. Biomaterials. 2015;58:54–62.
Hubbell J, Massia S, Desai N, Drumheller P. Endothelial cell-selective materials for tissue engineering in the vascular graft via a new receptor. Biotechnology. 1991;9:568–72.
Massia S, Hubbell J. Vascular endothelial-cell adhesion and spreading promoted by the peptide Redv of the iiics region of plasma fibronectin is mediated by integrin alpha-4-beta-1. J Biol Chem. 1992;267:14019–26.
Merhi Y, King M, Guidoin R. Acute thrombogenicity of intact and injured natural blood conduits versus synthetic conduits: neutrophil, platelet, and fibrin(ogen) adsorption under various shear-rate conditions. J Biomed Mater Res. 1997;34:477–85.
Tatterton M, Wilshaw S, Ingham E, Homer-Vanniasinkam S. The use of antithrombotic therapies in reducing synthetic small-diameter vascular graft thrombosis. Vasc Endovasc Surg. 2012;46:212–22.
Lee JH, Khang G, Lee JW, Lee HB. Platelet adhesion onto chargeable functional group gradient surfaces. J Biomed Mater Res. 1998;41:304–11.
Goodman SL. Sheep, pig, and human platelet–material interactions with model cardiovascular biomaterials. J Biomed Mater Res. 1999;45:240–50.
Acknowledgements
We gratefully acknowledge the financial support of the S-innovation Research Program for the “Development of the biofunctional materials for realization of innovative medicine”, Japan Agency for Medical Research and Development (AMED).
Author information
Authors and Affiliations
Contributions
MCM: conception and design, data collection, data analyses and interpretation, animal experiments, and manuscript writing. AM: animal experiments. TY: conception and design, data interpretation, and manuscript correction.
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Munisso, M.C., Mahara, A. & Yamaoka, T. Design of in situ porcine closed-circuit system for assessing blood-contacting biomaterials. J Artif Organs 21, 317–324 (2018). https://doi.org/10.1007/s10047-018-1042-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-018-1042-5