Abstract
This clinical study aimed to compare a novel and conventional artificial pancreas (AP) used in surgical patients for perioperative glycemic control, with respect to usability, blood glucose measurements, and glycemic control characteristics. From July in 2010 to March in 2015, 177 patients underwent perioperative glycemic control using a novel AP. Among them, 166 patients were eligible for inclusion in this study. Intensive insulin therapy (IIT) targeting a blood glucose range of 80–110 mg/dL was implemented in 82 patients (49 %), and the remaining 84 patients (51 %) received a less-intensive regime of insulin therapy. Data were collected prospectively and were reviewed or analyzed retrospectively. A comparison study of 324 patients undergoing IIT for glycemic control using a novel (n = 82) or conventional AP (n = 242) was conducted retrospectively. All patients had no hypoglycemia. The comparison study revealed no significant differences in perioperative mean blood glucose level, achievement rates for target blood glucose range, and variability in blood glucose level achieved with IIT between the novel AP and conventional AP groups. The usability, performance with respect to blood glucose measurement, and glycemic control characteristics of IIT were comparable between novel and conventional AP systems. However, the novel AP was easier to manipulate than the conventional AP due to its smaller size, lower weight, and shorter time for preparation. In the near future, this novel AP system might be accepted worldwide as a safe and useful device for use in perioperative glycemic control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgical stress-induced hyperglycemia is a major cause of postoperative infection (POI) resulting in unfavorable surgical outcomes [1–4]. Although the optimal blood glucose range needed to avoid hyperglycemia and thus prevent POI remains unclear, such prevention is clearly desirable in critically ill surgical patients [5–8]. On the other hand, hypoglycemia with blood glucose levels less than 40 mg/dL or levels of 70 mg/dL associated with the conventional glycemic control methods, such as tight glycemic control (TGC) with an open-loop system and the sliding-scale method, can cause fatal complications in the presence of neurological disorders [9–12]. Furthermore, hypoglycemia was found to be more common in patients in surgical wards [9].
To solve the problem of hypoglycemia, we elucidated the perioperative glycemic control using a conventional artificial pancreas (AP) (STG-22, Nikkiso Co., Ltd, Tokyo) and closed-loop glycemic control system clinically [9–11]. Using this system, we achieved strict glycemic control approaching normoglycemia, such as targeting blood glucose ranging from 80 to 110 mg/dL corresponding intensive insulin therapy (IIT), without hypoglycemia and with less variability of blood glucose concentration [9–11].
To further improve the clinical usability of glycemic control, we recently explored a novel AP (STG-55) for use in our system [13, 14]. Our previous experimental and clinical evaluation of STG-55 in an animal model showed strong correlations in blood glucose concentrations with those achieved using the conventional STG-22 AP device [14]. Unfortunately, the previous clinical data were from low patient-number studies, with groups comprising no more than 5 patients [14]. This larger clinical study, therefore, compared STG-55 and STG-22 for usability, performance in blood glucose measurements, and glycemic control characteristics in surgical patients undergoing perioperative glycemic control.
Methods
Study population
From July in 2010 to March in 2015, 177 patients undergoing hepatic, pancreatic, or esophageal surgical resection underwent perioperative glycemic control using a novel AP (STG-55, Nikkiso Co., Ltd, Tokyo). Of these patients, 11 were excluded and 6 due to insufficient data resulting from blood sampling defects, while 5 cases underwent only blood glucose monitoring without perioperative glycemic control based on the targeted blood glucose range. The remaining 166 patients undergoing perioperative glycemic control using the novel AP were enrolled in this study, with data collected prospectively and reviewed or analyzed retrospectively. Among them, 82 (49.4 %) received glycemic control targeting a blood glucose range of 80–110 mg/dL, so-called IIT, and 84 patients (50.6 %) were treated using a less-intensive insulin therapy to target a higher blood glucose range (Fig. 1). For our AP comparative study, we used retrospective data from 242 patients administered IIT using the conventional AP system (STG-22, Nikkiso Co., Ltd, Tokyo) during similar surgical procedures from August in 2006 to July in 2012.
Study design
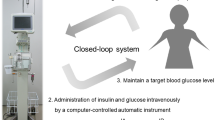
Perioperative glycemic control using any type of AP (Fig. 2) is typically performed 1–2 days before starting general anesthesia and continued until the end of post-surgical intensive care. The detailed mechanisms and characteristics of an AP were reported previously [9–11, 13, 14].
Briefly, an AP device comprises a measurement unit, control unit, and infusion unit, all of which are connected in a closed-loop system designed to maintain a specific blood glucose target level. The control unit receives electrical signals from the measurement unit and interprets these in accordance with the internal intravenous insulin and glucose infusion algorithms that are programmed for specific parameters. Every minute, the control unit instructs the insulin pump and the glucose pump to deliver insulin and glucose, respectively, with the amount varying according to the blood glucose level and to its change rate. There were no differences between the STG-22 and the STG-55 in the infusion parameters of insulin and glucose, because both systems have the same algorithm.
Thus, peripheral venous blood was sampled continuously at less than 2 mL/h for glucose monitoring, with the AP able to continuously measuring blood glucose using the glucose oxidase sensors and automatically infuse insulin and/or glucose to adjust blood glucose levels and maintain the target blood glucose value; this setup comprises the closed-loop system. The continuously measured intraoperative and postoperative blood glucose levels and achievement rate of the target blood glucose range were analyzed and calculated retrospectively. We previously established the perioperative accuracy and reliability of continuous blood glucose monitoring using an AP, intra-operatively [15] and postoperatively [16, 17].
In perioperative nutritional support care, calorie intake in all patients is calculated using the Harris–Benedict formula [18], and such unified standard calorie intake and standard care were provided to all our patients. On surgical days, patients were fed continuously with intravenous total parental nutrition. Combined intravenous total parental nutrition and enteral feeding were provided on the next day. After staying in the ICU for 1–2 days, patients were moved to the hospital ward, and total enteral feeding was attempted as early as possible.
Data collection
Patients were informed of the purpose and details of the study, and written informed consent was obtained from them prior to enrolment. The study was approved by the local ethics committee at the Kochi Medical School and carried out in accordance with the Helsinki Declaration. All the patients of this study were enrolled prospectively at the Kochi Medical School between August in 2006 and March in 2015.
Data were collected prospectively for retrospective review and/or analysis. The primary end point was hypoglycemia less than 70 mg/dL. Secondary end points included a blood glucose target range of 80–110 mg/dL and stable glycemic control with low variability in blood glucose concentrations.
Statistical analysis
Blood glucose data and infusion rates of insulin/glucose used in this study were stored electronically. Rate of achieving the target blood glucose range of IIT was calculated using the following formula: (total time of glycemic control) − (time out of range of 80–110 mg/dL)/(total time of glycemic control) × 100 (%), as described previously [9].
Data are presented as mean ± standard deviation (SD). The statistical analyses were performed using a statistical software package (JMP 8; SAS Institute Japan, Tokyo, Japan). Changes in the glucose level at all times points were analyzed by the repeated-measures analysis of variance (ANOVA) with post hoc testing. In the comparison study of IIT between the novel AP group and the conventional AP group, the statistical differences of intraoperative, postoperative, and perioperative mean blood glucose levels and achievement rates targeting blood glucose range or differences in disease site between the groups were analyzed by unpaired Student’s t test and Mann–Whitney U test, respectively. P values less than 0.05 were considered statistically significant.
Results
Patient characteristics between STG-55 and STG-22
Table 1 shows the characteristics of the patients who underwent IIT with either the STG-55 or STG-22. Of the 82 patients involving the STG-55, 48 patients (58.5 %) underwent hepatic resection for liver disease, 20 (24.4 %) underwent pancreatic resection for pancreatic disease, and 5 (6.1 %) with bile ductal disease underwent pancreaticoduodenectomy (PD). Nearly, all patients had malignant neoplasms, except those who underwent emergency operation. None of the 82 patients had hypoglycemia, which as defined by the American Diabetes Association, is blood glucose levels not only less than 40 mg/dL but also less than 70 mg/dL.
Of the 242 patients treated using the STG-22, 124 patients (51.2 %) underwent hepatic resection for liver disease, 50 (20.7 %) underwent pancreatic resection for pancreatic disease, and 25 (10.3 %) with esophagus disease underwent esophageal resection. Similar to the STG-55 group, no patient in the STG-22 group showed hypoglycemia. Although the incidence of esophageal disease seemed to be higher in the STG-22 group (10.3 %) than in the STG-55 group (2.4 %), there was no significant difference in disease sites, including the esophagus between two groups.
The comparison of the general concept between the STG-55 and STG-22 devices is shown in Table 2. The STG-55 was of markedly smaller size and lower weight than the STG-22, and required a shorter time for preparation. In addition, the STG-22 was not equipped with the battery and touch panel monitors found in the STG-55.
IIT outcomes between STG-55 and STG-22 surgeries
Figure 3 shows the perioperative continuous blood glucose levels in 82 patients administered IIT using the STG-55. Stable glycemic control was achieved within the target blood glucose range in every patient, with mean intraoperative, postoperative, and perioperative mean blood glucose levels of 94.9 ± 8.2, 109.9 ± 9.8, and 103.6 ± 14.6 mg/dL, respectively. Intraoperative, postoperative, and perioperative achievement rates of the target blood glucose range of 80–110 mg/dL were 93.0 ± 10.0, 83.4 ± 20.0, and 89.4 ± 14.3 %, respectively. The operation time of surgery and the operating time of the STG-55 were 315.9 ± 116.6 and 1473.2 ± 153.2 min, respectively. The amount doses for 24 h were 131.5 ± 113.1 U/day for insulin and 16.1 ± 29.3 g/day for glucose, respectively.
Figure 4 depicts the perioperative continuous blood glucose levels in the 242 patients administered IIT using the STG-22. Stable glycemic control was also achieved within the target blood glucose range in every patient, with mean intraoperative, postoperative, and perioperative mean blood glucose levels of 98.2 ± 12.7, 102.2 ± 16.2, and 100.5 ± 11.9 mg/dL, respectively. Intraoperative, postoperative, and perioperative achievement rates of the target blood glucose range of 80–110 mg/dL in all patients were 90.7 ± 12.4, 86.3 ± 18.9, and 88.1 ± 16.0 %, respectively. The operation time of surgery and the operating time of the STG-22 were 292.1 ± 120.5 and 1449.3 ± 151.9 min, respectively. The amount doses for 24 h were 158.2 ± 135.4 U/day for insulin and 8.0 ± 9.3 g/day for glucose, respectively.
Thus, there were no significant differences in perioperative mean blood glucose levels, achievement rates of target blood glucose range, variability in blood glucose levels, operation time of surgery, the operating time of the system, and the amount doses of insulin and glucose for 24 h between the STG-55 and STG-22 groups. Moreover, there were no significant differences in perioperative mean blood glucose levels at each hourly time point between the groups.
Discussion
In this study, perioperative IIT and less-IIT using a novel AP (STG-55) with closed-loop system had no hypoglycaemia, while all the previous reports of IIT and less-IIT with open-loop system had hypoglycemia ranging from 5 to 18.7 % and from 0.5 to 3.1 %, respectively. In addition, we found that perioperative IIT and less-IIT using STG-55 had not only higher achievement rates targeting blood glucose range of approximately 90 %, but also less variability of blood glucose concentration compared with former reports using the conventional glycemic control.
Tsukamoto et al. [14] performed an in vivo comparative study in dogs of a novel AP (STG-55) vs. conventional AP (STG-22) in terms of blood glucose measurement and glucose infusion rate (GIR) values during glucose-clamp in-animal experiments. The results showed strong correlation in blood glucose concentrations and GIR between STG-55 and STG-22 usage. This clinical comparison study demonstrated that the eligibility of perioperative glycemic control was comparable between the two devices. Coupling these findings with the physical and functional advantages of the STG-55 AP over the STG-22 in terms of size, weight, and preparation time, as well as comparable usability, accuracy, and feasibility between devices, we anticipate that the novel STG-55 will be acceptable as a progressive AP in the near future, at least instead of STG-22.
The conventional IIT with an open-loop glycemic control system has been associated with several serious issues, including hypoglycemia resulting in neurological critical events, low achievement rates in blood glucose range targeting, and high variability in blood glucose concentrations [19–24]. To date, we have tried to solve these issues using an AP with a closed-loop glycemic control system [9–11, 24]. In addition, in this study, we found that using the STG-55 produced higher achievement rates (~90 %) for blood glucose range targeting and less variability in blood glucose concentrations compared with former reports using the conventional glycemic control. Of note, no patients in this study had hypoglycemia, while the conventional IIT with an open-loop system has reportedly resulted in hypoglycemia if 5.0–18.7 % [25–29] of the patient population. These similar findings were shown in our previous reports using an STG-22 [9–11, 20]. Thus, glycemic control systems using an AP should achieve real-time continuous blood glucose monitoring, achieve stable glycemic control without hypoglycemia according to the targeted blood glucose range, and prevent surgical stress-induced hyperglycemia leading to morbidity and mortality [9–11, 13–17, 20]. Moreover, it could potentially reduce workload, anxiety, and the need for frequent blood glucose measurements by medical staff, especially ICU nurses [30, 31]. Our former report [30] suggested that using AP for glucose management in ICU patients increased the degree of attention given by nurses to glucose management and contributed to improved patient security, resulting in a reduced overall workload of ICU nurses compared to the sliding-scale method. We would like to expect that these characteristics of STG-22 will be replaced by STG-55 with an added sophistication of design and consequent clinical benefits for patients.
Achieving stable glycemic control with low variability in blood glucose concentration is very important for prognosis in critically ill patients, more so than hypoglycemia and/or hyperglycemia [9, 22, 23]. Of note, performing glycemic control using an AP is not warranted for all surgeries, because stable glycemic control is possible to achieve without this device, especially in non-diabetic patients undergoing minimally invasive surgery [9, 11]. Currently, we indicate this option mainly for patients undergoing hepatic, pancreatic, or esophageal resections who tend toward hyperglycemia and/or hypoglycemia, as well as unstable blood glucose levels. Consequently, pancreatogenic diabetes after pancreatic resection is the best indication of this method, because the conventional method cannot achieve stable glycemic control near normoglycemia, especially in patients undergoing total pancreatectomy (TP), for whom pancreatogenic diabetes is unavoidable in 100 % of cases [10, 32, 33]. Recently, we suggested that perioperative IIT using an AP enables stable glycemic control not only without hypoglycemia and hyperglycemia, but also with less variation in blood glucose concentration from the target blood glucose range, even in patients with the most serious form of diabetes, so-called “brittle diabetes,” and undergoing TP [10]. Therefore, AP was shown to be safe and efficient in achieving stable perioperative IIT even immediately after TP. The next most prominent indication is hepatectomy, because IIT by the conventional methods could not be administered until at least within 16 h after surgery [9, 33]. In addition, we suggest liver and pancreas transplantation, cardiac surgery, and esophageal resection as other indications, as well as Type 2 diabetic or elderly patients undergoing surgery with glucose intolerance and/or severe infection, such as pan-peritonitis [9]. Based on the rapidly increasing incidence of diabetic and elderly surgical patients and the advancement of STG-55, the indications for using an AP will swell in the near future.
In the present study, there was a significant difference in disease site incidence between the STG-55 and STG-22 groups, with a higher incidence of esophageal disease in the latter group. However, since disease site was liver and pancreas in >80 % of the STG-55 group and 70 % of the STG-22 group, such a bias might be acceptable. The main considerable trouble during the operation of both the conventional and novel artificial pancreas is the issues regarding insufficiency of blood sampling. In fact, 6 patients were excluded in this study due to insufficient data resulting from blood sampling defects. Further development of more sophisticated mechanical devices, including venous catheter and glucose sensor, is expected.
What should we do to advance the progression of STG-55 use? While the accuracy of blood glucose measurements, stability of blood glucose range achievement rates, and safety without hypoglycemia are acceptable, some urgent problems remain unsolved. First, the durable time remains short at 1–2 days immediately after surgery, and the clinical setting times of at least 1 week are necessary, especially in patients undergoing TP or major hepatectomy. Second, a fault in blood removal often occurred with the device during continuous blood glucose measurements, especially in elderly patients with unhealthy vessels, in which the blood glucose sensor line must be inserted. Solving this issue might require an alternative development, such as a peripheral venous line or novel equipment enabling use of the central venous line. Third, preparation time still remains long because we need to further reduce the labor burden of medical stuff, although the STG-55 time was shorter (1–1.5 h) than required for the STG-22 (3–12 h) [13, 14]. Ideally, preparation time needs to be as short as possible. Finally, the miniaturization of the AP is essential for its future use and development, especially in Type 1 or Type 2 diabetic patients.
Conclusion
We found comparable usability, performance in blood glucose measurement, and glycemic control characteristics of IIT between the novel and conventional AP. In addition, the novel AP was easier to use than the conventional AP due to it smaller size, weight, and time for preparation. In the near future, such a novel AP may gain acceptance worldwide as a safe and useful device for use in perioperative glycemic control.
References
McCowen KC, Malhorta A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17:107–24.
Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. JAMA. 2003;290:2041–7.
Rady MY, Johnson DJ, Patel BM, et al. Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus. Mayo Clin Proc. 2005;80:1558–67.
Gabbanelli V, Pantanetti S, Donati A, et al. Correlation between hyperglycemia and mortality in a medical and surgical intensive care unit. Minerva Anesthesiol. 2005;71:717–25.
Ramos M, Khalpey Z, Lipsitz S, et al. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg. 2008;248:585–91.
Ambiru S, Kato A, Kimura F, et al. Poor postoperative blood glucose control increases surgical site infections after surgery for hepato-biliary-pancreatic cancer: a prospective study in a high-volume institute in Japan. J Hosp Infect. 2008;68:230–3.
Eshuis WJ, Hermanides J, van Dalen JW, et al. Early postoperative hyperglycemia is associated with postoperative complications after pancreatodudenectomy. Ann Surg. 2011;253:739–44.
Ata A, Lee J, Bestle SL, et al. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg. 2010;145:858–64.
Hanazaki K, Kitagawa H, Yatabe T, et al. Perioperative intensive insulin therapy using an artificial endocrine pancreas with closed-loop glycemic control system: the effects of no hypoglycemia. Am J Surg. 2014;207:935–41.
Hanazaki K, Yatabe T, Kobayashi M, et al. Perioperative glycemic control using an artificial endocrine pancreas in patients undergoing total pancreatectomy: tight glycemic control may be justified in order to avoid brittle diabetes. Biomed Mater Eng. 2013;23:109–16.
Hanazaki K, Namikawa T. Development of perioperative glycemic control using an artificial endocrine pancreas. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:5719–22.
Murad MH, Coburn JA, Coto-Yglesias F, et al. Glycemic control in non-critically ill hospitalized patients: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97:49–58.
Tsukamoto Y, Okabayashi T, Hanazaki K. Progressive artificial endocrine pancreas: the era of novel perioperative blood glucose control for surgery. Surg Today. 2011;41:1344–51.
Tsukamoto Y, Kinoshita Y, Kitagawa H, et al. Evaluation of a novel artificial pancreas: closed loop glycemic control system with continuous blood glucose monitoring. Artif Organs. 2013;37:E67–73.
Yamashita K, Okabayashi T, Yokoyama T, et al. The accuracy of continuous blood glucose monitor during surgery. Anesth Analg. 2008;106:160–3.
Yamashita K, Okabayashi T, Yokoyama T, et al. Accuracy and reliability of continuous blood glucose monitor in post-surgical patients. Acta Anaesthesiol Scand. 2009;53:66–71.
Yatabe T, Yamazaki R, Kitagawa H, et al. The evaluation of the ability of closed-loop glycemic control device to maintain the blood glucose concentration in intensive unit patients. Crit Care Med. 2011;39:575–8.
Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci USA. 1918;4:370–3.
Workgroup on Hypoglycemia. American Diabetes Association. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28:1245–9.
Hanazaki K, Maeda H, Okabayashi T. Tight perioperative glycemic control using an artificial endocrine pancreas. Surg Today. 2010;40:1–7.
Duncan A. Hyperglycemia and perioperative glucose management. Curr Pharm Des. 2012;18:6195–203.
Egi M, Bellomo R, Stachowski E, et al. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. 2006;105:244–52.
Hermanides J, Vriesendorp TM, Bosman RJ, et al. Glucose variability is associated with intensive care unit mortality. Crit Care Med. 2010;38:838–42.
Munekage M, Yatabe T, Kitagawa H, et al. An artificial pancreas provided a novel model of blood glucose level variability in beagles. J Artif Organs. 2015;18:387–90.
Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–67.
Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in medical ICU. N Engl J Med. 2006;354:449–61.
Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300:933–44.
The NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–97.
Griesdale DE, de Souza RJ, van Dam RM, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180:821–7.
Mibu K, Yatabe T, Hanazaki K. Blood glucose control using an artificial pancreas reduces the workload of ICU nurses. J Artif Organs. 2012;15:71–6.
Maeda H, Hanazaki K. Pancreatogenic diabetes after pancreatic resection. Pancreatology. 2011;11(268–7):6.
Maeda H, Okabayashi T, Yatabe T, et al. Perioperative intensive insulin therapy using artificial endocrine pancreas in patients undergoing pancreatectomy. World J Gastroenterol. 2009;15:4111–5.
Okabayashi T, Nishimori I, Maeda H, et al. Effect of intensive insulin therapy using a closed loop glycemic control system in hepatic resection patients: a prospective randomized clinical trial. Diabetes Care. 2009;32:1425–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was supported by the Japan Ministry of Education, Culture, Sports, Science, and Technology, The Kochi University President’s Discretionary Grant, and grant from Nikkiso CO., Ltd.
Rights and permissions
About this article
Cite this article
Namikawa, T., Munekage, M., Kitagawa, H. et al. Comparison between a novel and conventional artificial pancreas for perioperative glycemic control using a closed-loop system. J Artif Organs 20, 84–90 (2017). https://doi.org/10.1007/s10047-016-0926-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-016-0926-5