Abstract
Decellularized tissues have attracted a great deal of attention as regenerating transplantation materials. A decellularizing method based on high hydrostatic pressure (HHP) has been developed, and the preparation of many types of decellularized tissues has been investigated, including aorta, cornea, and dermis. The preparation of a small-diameter vascular graft was studied using a carotid artery from the viewpoint of collagen denaturation and leakage. After HHP, the carotid artery was washed at two washing temperatures (37 and 4°C). Histological evaluation, collagen content measurement and circular dichroism (CD) measurement indicated that the washing temperatures clearly affected the collagen structure of the decellularized carotid artery. The amount of collagen decreased in the carotid artery decellularized by HHP washed at 37°C (HHP/37°C). On the other hand, the amount and structure of collagen were preserved in the carotid artery washed at 4°C after HHP (HHP/4°C). In rat carotid artery syngeneic transplantation, the HHP/37°C decellularized carotid artery occluded after 2 weeks, but the HHP/4°C decellularized one did not. These results indicate that collagen denaturation and leakage of the decellularized carotid artery affect the in vivo performance of the carotid artery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease is one of the leading causes of morbidity and mortality worldwide. Surgical therapies to replace lesion tissue with an artificial prosthesis or homograft and to create a bypass are the treatments most commonly used when the disease is severe. Each year, more than 500,000 coronary bypass surgeries are performed in the US [1], and 20,000 such procedures are carried out in Japan [2]. In small-diameter (inner diameter (ID) <6 mm) vascular prostheses, synthetic grafts such as Dacron® and expanded polytetrafluoroethylene (ePTFE) are not applicable, because it is well known that they soon occlude [3]. Autologous vessels have therefore been widely used as the standard grafts for coronary bypass surgery. However, autologous vessels require an invasive operation to harvest the vessels.
Tissue-engineered vascular prostheses have therefore attracted a great deal of attention as a possible solution to these problems, and have demonstrated the potential to regenerate vascular tissue using the host cells [4, 5]. The most successful study was reported by L’Heureux et al. [6, 7]. They fabricated a tissue-engineered vessel with an ID of 6 mm using a fibroblast cell sheet and autologous endothelial cells. This vessel showed 11-month patency during clinical use as a blood shunt for dialysis. They proved that the fabrication of a vessel using only host cells has enormous potential for small-diameter vascular prostheses; however, isolating cells from patients and culturing those cells require a great deal of time, and may increase the risk of infection and cell mutation [7].
Another strategy for regenerating vascular tissue has been investigated: the use of decellularized tissue. Decellularized human tissue is already in clinical use [8], and xenogeneic tissue is also expected to be an alternative source for decellularization [9]. CryoLife (CA, USA) developed the first commercially available decellularized human heart valve, and AutoTissue (Berlin, Germany) have been trying to apply the decellularized porcine heart valve to treat human heart disease. Our group has been investigating the decellularization method and the preparation of tissue for regeneration. Recently, we reported the preparation of decellularized aorta [10] and cornea [11] using a high hydrostatic pressure (HHP) method. This HHP method is a new technology for decellularizing various tissues. It is consists of two kinds of process. In a first step, the cells are destroyed by HHP at 980 MPa, and the residual cell debris is washed out in a rinsing process, which is the second step. HHP-decellularized tissues maintain their original physical properties, and an in vivo study revealed that these tissues could work as a good scaffold for tissue regeneration. In our studies, we adjusted the HHP process conditions to obtain optimal decellularized tissue; namely, we investigated the best pressurization conditions and washing procedure for the HHP process. In the present study, we attempt to prepare a decellularized small-diameter vascular graft using the HHP method. A carotid artery was selected as a candidate for a small-diameter vascular graft. The pressurization conditions for carotid artery decellularization were based on the previous aorta decellularization study, and the temperature used in the washing process was investigated (37 and 4°C were tested). Characterization of HHP-decellularized carotid arteries was performed in vitro and in vivo.
Methods
HHP decellularization
Porcine carotid arteries were purchased from a local slaughterhouse (Tokyo Shibaura Organ Co. Ltd., Japan). Rat carotid arteries were harvested from Wistar rats (male, 8–12 weeks old). The surrounding connective tissue and fat were trimmed, and the carotid arteries were immersed in Alsever’s solution (Sigma-Aldrich, Tokyo, Japan). The carotid arteries were put into polyethylene bags with Alsever’s solution, and the bags were sealed. Pressure was then applied at 980 MPa for 10 min at 30°C to destroy the cells in the tissue. The tissue was taken out of the plastic bag and subjected to the washing process. To wash out the cell debris in the tissue, we used a culture medium (EBM-2: Lonza, Basel, Switzerland) with 0.2 mg/ml DNase I (Roche, Switzerland), 0.4 μl/ml hydrocortisone, 1 μl/ml ascorbic acid, 1 μl/ml GA-1000, and 1 μl/ml heparin (Lonza, Basel, Switzerland). Two temperature conditions (37 and 4°C) were used in this study to gauge the best protocol for carotid artery decellularization. HHP/37°C: pressurized tissues were washed in EBM-2 at 37°C under shaking conditions for 14 days, and then washed in 80% ethanol in Alsever’s solution for 3 days at 37°C under shaking conditions. This procedure was carried out for the porcine aorta, and is abbreviated to HHP/37°C in this paper. HHP/4°C: the other washing procedure, abbreviated to HHP/4°C, was as follows: carotid arteries were washed in EBM-2 for 14 days and 80% ethanol in Alsever’s solution for 3 days at 4°C under static conditions. After both washing procedures had been completed, the tissues were stored in Alsever’s solution at 4°C.
TritonX-100/sodium deoxycholate decellularization
Detergent and nuclease treatments were also used for porcine carotid artery decellularization. The carotid arteries were trimmed of fat, immersed in isodine (Meiji Seika, Tokyo, Japan) for 10 min, and washed using 100 μg/ml gentamicin (Wako Chemical, Tokyo, Japan) in phosphate-buffered saline (PBS) overnight to sterilize the carotid arteries. Then the vessels were treated with 0.25% tert-octylphenylpolyoxyethylene (TritonX-100, Sigma-Aldrich, St. Louis, MO, USA) and 0.25% sodium deoxycholate (SDC, Wako Chemical) in PBS for 24 h at 37°C under shaking conditions. After treatment with the detergents, the carotids were washed in Hanks’ Balanced Salt Solution (HBSS, Invitrogen, Carlsbad, CA, USA) at 4°C for 72 h, and then put into 100 μg/ml RNase, 150 U/ml DNase Ι, and 50 mmol/l MgCl2 in PBS for 24 h at 37°C under shaking conditions. After these treatments, the tissues were stored in HBSS at 4°C [12].
Histological analysis
The samples were fixed with 10% neutral buffered formalin, dehydrated and embedded in paraffin, and then cut into 4–8 μm thick sections. These sections were stained with hematoxylin eosin (HE) and elastica van Gieson (EVG) staining. The stained sections were imaged using bright-field microscopy (Coolscope, Nikon Co., Ltd., Tokyo, Japan).
DNA quantification
Native and decellularized samples were lyophilized, and dry weights were measured. These samples were dissolved in 0.5 ml of the lysis buffer containing 50 μg/ml protease K, 50 mM Tris–HCl, 1%w/v SDS, 100 mM NaCl, and 20 mM disodium EDTA overnight at 55°C. The DNA in the solution was extracted by phenol/chloroform and purified by ethanol precipitation. The DNA content was measured by 260 nm absorbance using a spectrophotometer (FP-560, JASCO, Tokyo, Japan).
Collagen quantification
The collagen content of each sample was measured using a collagen staining kit (Cosmo Bio, Tokyo, Japan) [13]. To normalize the collagen content in tissue, a serial section was stained with HE, and the area of the section was measured using Image J software (NIH, USA). The collagen contents of the sections are given as collagen content (ng) per unit area (mm2) in this study.
Scanning electron microscopy (SEM)
The structure of the extracellular matrix (ECM) in the carotid artery was observed by SEM. Samples were fixed in 2.5% glutaraldehyde (Tokyo Kasei, Tokyo, Japan) in PBS. Tissues were freeze-fractured and dried under reduced pressure overnight. The dried samples were sputter-coated with gold, and microscopic evaluation was done by SEM (Hitachi).
Burst test
The burst pressure of the carotid artery was measured using a water pressure gauge. One end of the artery was clamped, and the other was connected to a silicone tube. The tube was filled with water and connected to a water pressure gauge and syringe. The carotid artery was pressurized with the water from the syringe, and the burst pressure was measured with the water pressure gauge. The burst pressure is defined as the pressure at which the artery ruptured and a water leak was observed.
Circular dichroism of collagen and elastin
The effect of HHP and the washing procedures on collagen and elastin were evaluated by circular dichroism (CD). Atelocell® (native collagen from bovine dermis: Koken, Tokyo, Japan) was diluted in water to 200 μg/ml. Water-soluble elastin (EPC, Owensville, MO, USA) solution was prepared at 10 μg/ml in water. These solutions were subjected to HHP (subjected to 980 MPa of pressure for 10 min at 30°C), and then kept at 37°C under shaking conditions (HHP/37°C) or 4°C under static conditions (HHP/4°C) for 14 days. Control collagen and elastin solutions were kept at 37°C under shaking conditions (37°C) or at 4°C under static conditions (4°C) for 14 days.
In the collagen CD spectra, the Rpn value (the ratio of the positive peak value to the negative peak value) is an indicator of collagen triple helix formation. This value represents the ratio of the molar extinction of the positive peak at 222 nm and the negative peak at 198 nm [14].
Endothelial cell seeding of HHP-decellularized carotids
Human umbilical vein endothelial cells (HUVEC, Lonza) were seeded onto the luminal surfaces of the HHP-decellularized porcine carotids to evaluate cell adhesion [15]. Decellularized porcine carotids (HHP/37°C and HHP/4°C) were longitudinally incised (20 × 20 mm) and placed in a 12-well culture plate (Falcon) with the luminal surfaces facing upward. A sterilized acrylic mold (diameter 20 mm, thickness 1.5 mm, 10 × 10 array) was set on the tissue to prevent floating, and 1 ml of HUVEC suspension in EBM-2 (5.0 × 105 cells/ml) was dropped onto the tissue surface. The samples were cultured for 10 days, and cell adhesion was then evaluated by HE and EVG staining and SEM.
In vivo evaluation using rat carotids
All animal experiments were approved by the ethical committees for animal welfare of Tokyo Medical and Dental University (Tokyo, Japan).
To evaluate the durability and biocompatibility of HHP-decellularized carotid arteries, a rat syngeneic transplantation model was performed. Carotid arteries subjected to HHP using two washing procedures were used in this transplant experiment in order to compare the in vivo behaviors of HHP/37°C and HHP/4°C arteries. Decellularized rat carotid arteries obtained using HHP/37°C and HHP/4°C treatments were implanted into rat carotids using the cuff method. The recipient Wistar rats (male, 10–12 weeks old, n = 3 for each condition) were anesthetized with medetomidine (0.4 ml/kg, Zenyaku Kogyo, Tokyo, Japan) and sodium pentobarbital (0.3 ml/kg, Kyoritsu Seiyaku, Tokyo, Japan). For each rat, a decellularized graft was implanted into the carotid using a cuff (ID 0.6 mm, outer diameter 0.9 mm) and 6-0 silk suture ligation. After certain periods (1 or 2 weeks), the recipient rats were sacrificed, and the grafts were extracted and evaluated by HE staining.
Results
Morphology and histology
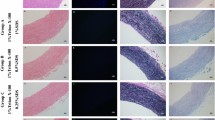
No nuclei were detected in sections of the decellularized porcine arteries (Fig. 1e–h), which means that the decellularization process was effective for all of the methods. However, there were some differences in macroscopic strength among the decellularization methods. Native artery and the HHP/4°C-decellularized artery maintained luminal structure (Fig. 1a, b), while the detergent-decellularized artery and the HHP/37°C decellularized artery did not maintain inner space (Fig. 1c, d). In EVG-stained sections, the native and HHP/4°C-decellularized arteries did not differ. Based on this observation, it became clear that the HHP/4°C-decellularized artery seemed to maintain collagen and elastin structures (Fig. 1i, l). On the other hand, it also became clear that the detergent decellularization damaged the aligned elastin fibers seen in the native tissue (Fig. 1j), and the HHP/37°C decellularization caused collagen leakage, even though the elastin fibers remained (Fig. 1k).
To evaluate whether the procedures established in this study were appropriate for the decellularization of the artery of another animal, rat carotid arteries were decellularized using HHP/37°C and HHP/4°C and tested. Figure 2 shows the results from HE and EVG staining of rat carotid arteries. In the HE sections, no nuclei were detected in either of the HHP-decellularized arteries (Fig. 2b, c). The decellularized rat carotid arteries showed the same features as those of the decellularized porcine carotid arteries, which were that HHP/37°C treatment induced collagen leakage and HHP/4°C treatment preserved the collagen and elastin structures (Fig. 2e, f).
From these results, the HHP/4°C procedure seemed to be most effective at preserving collagen structure. In vitro evaluations were performed using porcine carotid arteries to aid macroscopic handling, and the in vivo evaluation was performed using rat carotid artery to aid vascular diameter matching.
DNA quantification
The DNA contents of the decellularized porcine carotid arteries are shown in Fig. 3. The residual DNA contents in all arteries were drastically decreased to less than one-tenth that of the residual DNA in the native carotid arteries. The amounts of residual DNA in the detergent-decellularized artery and in the HHP/4°C-decellularized artery were not significantly different. The HHP/37°C method was the most effective at DNA removal.
Collagen quantification
The amount of residual collagen in decellularized porcine arteries was quantified (Fig. 4). The detergent and the HHP/37°C decellularization induced a significant reduction in collagen, whereas the HHP/4°C decellularization only slightly reduced the collagen content.
SEM observations
The surface structures and cross-sections of the decellularized porcine carotid arteries were observed by SEM. The luminal surface of the native porcine carotid artery was covered with endothelial cells (Fig. 5a), whereas endothelial cells were not observed in the decellularized arteries (Fig. 5b–d). The luminal surface of the HHP/4°C-decellularized artery was smooth (Fig. 5d) compared to the detergent- and the HHP/37°C-decellularized arteries, which had some cracks (Fig. 5b, c, arrows). From the cross-sectional views, it is clear that the native artery had a wave-shaped internal elastic lamina (Fig. 5e), and the same structure was also observed in the HHP/4°C-decellularized artery (Fig. 5h). On the other hand, disordered internal elastic laminas were observed for the detergent- and the HHP/37°C-decellularized arteries (Fig. 5f, g).
SEM images of porcine carotid arteries treated under various conditions. a–d Luminal surfaces and e–h cross-sections. a, e Native carotid artery; b, f detergent-decellularized carotid artery, c, g HHP/37°C-decellularized carotid artery; d, h HHP/4°C-decellularized carotid artery. Black scale bar 50 μm; white scale bar: 100 μm
Burst pressure test
The native porcine artery and the HHP-decellularized arteries (HHP/37°C and HHP/4°C) did not burst at 1750 mmHg (the limit of measurements in our lab). In contrast, the detergent-decellularized artery burst at 925 ± 47 mmHg.
CD spectra
Figure 6 shows the CD spectra of collagen and elastin under various treatments. In this experiment, the 4°C-treated collagen solution was regarded as the control of natural collagen. The Rpn values and crossover points calculated using the data of Fig. 6a are given in Table 1. The HHP/37°C- and 37°C-treated collagen solutions had completely different peak values from those of the HHP/4°C- and 4°C-treated collagen solutions (Fig. 6a). The Rpn value of the HHP/4°C-treated collagen solution was almost the same as that of the 4°C-treated collagen solution. This means that the HHP/4°C treatment did not affect the triple-helix structure of collagen. On the other hand, it was clear that the HHP/37°C and 37°C treatments caused drastic changes in the triple-helix structure of collagen (Table 1). In Fig. 6b, the elastin spectra of the four samples did not show any differences, which indicated that the HHP treatments did not affect the structure of elastin. These results from the CD measurements confirmed that the results from the histological evaluations of HHP-decellularized arteries (Figs. 1, 2) could be explained by structural changes in collagen caused by the decellularization process.
HUVEC seeding
To evaluate cell adhesion and proliferation in the decellularized tissues, HUVEC was cultured on their surfaces. The luminal surfaces of the decellularized arteries were covered with a monolayer of HUVEC after a 10-day culture period (Fig. 7a, d). From Fig. 7b, it is clear that the intima of the HHP/37°C-decellularized artery was decomposed, while the intima of the HHP/4°C-decellularized artery was well maintained (Fig. 7e). However, SEM observations revealed that the luminal surfaces of both decellularized arteries were covered with HUVEC, which indicated that the cells adhered and proliferated on each decellularized artery (Fig. 7c, f).
Syngeneic transplantation of decellularized rat carotid artery
The decellularization conditions for porcine carotid artery were estimated in the above in vitro studies. In order to study in vivo performance, a rat implantation model was performed as a preliminary study. First, we tried to use the decellularized porcine carotid artery as a graft to replace rat carotid artery, but the difference in the IDs of the carotid arteries was very large, so as an alternative study, the HHP-decellularized rat carotid arteries were used in vivo instead. These studies were performed in order to evaluate the difference in performance due to the decellularization of the artery in vivo.
All of the rats survived without suffering any infection, graft dilation, or rupture over the experimental period. Table 2 shows the results of the decellularized rat carotid artery syngeneic transplantations. After 1 week, an artery occluded in the HHP/37°C-decellularized group (Fig. 8a). At the 2-week evaluations, all of the HHP/37°C-decellularized arteries had occluded (Fig. 8d–f). On the other hand, the HHP/4°C-decellularized arteries were all patent and had not formed any clots after 2 weeks of implantation (Fig. 8g–i).
HE-stained sections of decellularized rat carotid arteries after syngeneic transplantations. a–c The HHP/37°C-decellularized carotid arteries after 1 week of implantation: a occlusion and clot formation; b, c patent. d–f the HHP/37°C-decellularized carotid arteries after 2 weeks of implantation—all arteries were occluded; g–i the HHP/4°C-decellularized carotid arteries after 2 weeks of implantation—all arteries were patent and there was no thrombosis. Scale bar 250 μm. * Luminal area
Discussion
In this study, we focused on the preparation of a small-diameter artery that achieves high blood compatibility, comparable mechanical properties and collagen and elastin structure preservation. In a former study, we demonstrated the good performance of the HHP-decellularized aorta in 6-month allo-transplantation [10]. Based on that study, we performed HHP decellularization of a small-diameter artery and evaluated its feasibility for clinical use. We selected carotid arteries as candidates for a small-diameter vascular graft. Porcine carotid arteries are sufficiently long and have fewer branches, making them suitable for use as a graft in a coronary artery bypass. To realize this concept, we optimized the HHP decellularization conditions for carotid arteries. The best decellularized conditions were judged based on the preservation of collagen and elastin structures and how comparable the mechanical properties of the decellularized arteries were to those of native tissue.
Ideal tissue-engineered cardiovascular grafts need to have many properties: mechanical strength to endure arterial blood pressure, compliance and elasticity resembling those of native arteries, no thrombogenicity or immunogenicity, and the potential to be remodeled by host cells. The crucial factor when selecting what to use for small-diameter vessels is the prevention of early thrombosis.
Factors affecting the mechanical properties of HHP-decellularized tissue are temperature, washing conditions, and pressurization. We investigated the effect of temperature by using 4°C for carotid artery decellularization. HHP/4°C decellularization showed a high level of cell removal, comparable to that obtained with the detergent and HHP/37°C decellularization (Figs. 1, 2, 3). DNase was added to the washing medium to remove the residual nucleic acids, whose optimal temperature was 37°C. Under the 4°C condition, however, the DNase activity was lowered. On the other hand, HHP/4°C decellularization gave high cell and DNA removal efficiencies and preserved mechanical properties. Based on these results, HHP/4°C was judged to be adequate for decellularizing carotid artery.
CD spectral measurements for collagen and elastin revealed that HHP/37°C was prone to inducing triple-helix structural changes in collagen. Meanwhile, HHP/4°C did not affect the collagen triple-helix structure. The carotid artery is a collagen-rich artery, whereas aorta is an elastin-rich artery [16]. From CD spectral measurements, collagen was found to be more sensitive to temperature than elastin, so HHP/4°C was considered to be effective at maintaining the extracellular matrix structure in the carotid artery (Figs. 1, 2, 5). Many decellularization studies have been performed at 37°C to ensure high enzyme activity and detergent efficiency. Based on the collagen CD data and histological evaluations, applying 4°C washing to other decellularized collagen-rich tissue could allow the preservation of collagen structure and mechanical properties. In a burst test, the HHP-decellularized artery exhibited a comparable value to that of human saphenous vein (1680 mmHg), which is currently used for coronary artery bypass grafts. On the other hand, the detergent-decellularized artery had a lower burst pressure than that of saphenous vein. The lower burst pressure of the detergent-decellularized artery seemed to be due to elastin fiber ablation and collagen leakage (Figs. 1, 4). Elastin is an important vascular extracellular matrix for vessel elasticity and ensuring that the vessel can endure the blood pressure. As the HHP treatments did not affect the elastin structure (Fig. 6b), it is believed that the burst pressures of HHP-decellularized vessels are maintained.
It is well known that endothelial cells adhering to the luminal surfaces of vessels play an important role in anticoagulation [17]. We investigated HUVEC adhesion and proliferation on HHP-decellularized arteries. An in vitro experiment showed that endothelial cells had attached to the luminal surfaces of the HHP/37°C- and HHP/4°C-decellularized arteries (Fig. 7). This suggested that the luminal structures were adhered to by HUVEC. The luminal structure of the artery is denser than that of tunica, so the HHP/37°C-decellularized artery maintains the luminal structure as a scaffold that was adhered to by HUVEC, although collagen did leak from the tunica.
These in vitro results indicate that the HHP/4°C-decellularization condition is the most effective method of carotid artery decellularization among the three conditions, as it maintains the collagen and elastin structure, mechanical properties, and cell adhesivity.
To evaluate the in vivo performances of the HHP-decellularized carotid arteries, rat syngeneic transplantations were performed. We first evaluated the graft patency for all samples at 2 weeks. A difference in patency was observed between the HHP/37°C- and HHP/4°C-decellularized arteries. Namely, all of the HHP/37°C-decellularized arteries were occluded. Therefore, in order to investigate the occlusion rate of the HHP/37°C-decellularized arteries, a 1-week experiment was performed. The results after 1 and 2 weeks of implantation are shown in Table 2. The HHP/37°C-decellularized arteries occluded after 2 weeks of implantation (Fig. 8). The reason for the thrombosis formation is not explained in detail in this investigation. The HHP/37°C decellularization causes collagen structural and mechanical property changes in the carotid arteries compared to those of native arteries. These may be the reasons for the low patency after transplantation of the HHP/37°C-decellularized arteries. On the other hand, the in vitro results suggest that HHP/4°C decellularization can maintain the collagen structure and mechanical properties, and the corresponding grafts were still patent after 2 weeks in vivo. The detailed reasons of this enhanced patency are unclear, although the preservation of vascular collagen and elastin structures such as basement membrane and inner elastic lamellae seem to be involved. We are continuing to investigate these mechanisms and to study small-diameter vascular grafts.
In conclusion, we suggest that it is important to optimize the conditions used during the decellularization of the carotid artery in order to preserve its mechanical properties and collagen and elastin structure. In contrast to the HHP-decellularized porcine aorta, the HHP/4°C condition was required for small-diameter arteries in order to preserve the collagen structure of the native artery. The results of this decellularization condition study should aid in the preparation of new vascular grafts.
References
MacNeill BD, Pomerantseva I, Lowe HC, Oesterle SN, Vacanti JP. Toward a new blood vessel. Vasc Med. 2002;7:241–6.
Yada I, Wada H, Fujita H. Thoracic and cardiovascular surgery in Japan during 2002: annual report by the Japanese Association for Thoracic Surgery. Jpn J Thorac Cardiovasc Surg. 2004;52:491–508.
Banz T, Reiben R. Endothelial cell protection in xenotransplantation: looking after a key player in rejection. Xenotransplantation. 2006;13(1):19–30.
Simionescu DT, Lu Q, Song Y, Lee JS, Rosenbalm TN, Kelley C, Vyavahare NR. Biocompatibitity and remodeling potential of pure arterial elastin and collagen scaffolds. Biomaterials. 2006;27:702–13.
Bergmeister H, Plasenzotti R, Walter I, Plass C, Bastian F, Rieder E, Sipos W, Kaider A, Losert U, Weigel G. Decellularized, xenogeneic small-diameter arteries: transition from a muscular to an elastic phenotype in vivo. J Biomed Mater Res Part B. 2008;87B:95–104.
Narita Y, Kagami H, Matsunuma H, Murase Y, Ueda M, Ueda Y. Decellularized ureter for tissue-engineered small-diameter vascular graft. J Artif Organs. 2008;11:91–9.
L’Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB. 1998;12:45–56.
Konertz W, Dohmen PM, Liu J, Beholz S, Dushe S, Posner S, Lembcke A, Erdbrügger W. Hemodynamic characteristics of the Matrix P decellularized xenograft for pulmonary valve replacement during the Ross operation. J Heart Valve Dis. 2005;14(1):78–81.
Sasaki S, Funamoto S, Hashimoto Y, Kimura T, Honda T, Hattori S, Kobayashi H, Kishida A, Mochizuki M. In vivo evaluation of a novel scaffold for artificial corneas prepared by using ultrahigh hydrostatic pressure to decellularize porcine corneas. Mol Vis. 2009;15:2022–8.
Funamoto S, Nam K, Kimura T, Murakoshi A, Hashimoto Y, Niwaya K, Kitamura S, Fujisato T, Kishida A. The use of high-hydrostatic pressure treatment to decellularize blood vessels. Biomaterials. 2010;31:3590–5.
Hashimoto Y, Funamoto S, Sasaki S, Honda T, Hattori S, Nam K, Kimura T, Mochizuki M, Fujisato T, Kobayashi H, Kishida A. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials. 2010;31:3941–8.
Rieder E, Kasimir MT, Silberhumer G, Seebacher G, Wolner E, Simon P, Weigel G. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiov Sur. 2004;127:399–405.
Leon ALD, Rojkind M. A simple micromethod for collagen and total protein determination in formalin-fixed paraffin embedded sections. J Histochem Cytochem. 1985;33:743–67.
Feng Y, Melacini G, Taulane JP, Goodman M. Acetyl-terminated and template-assembled collagen-based polypeptides composed of Gly-Pro-Hyp sequences. 2. Synthesis and conformational analysis by circular dichroism, ultraviolet absorbance, and optical rotation. J Am Chem Soc. 1996;118:10351–64.
Brown B, Lindberg K, Reing J, Stolz DB, Badylak SF. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12:519–26.
Fischer GM, Llaurado JG. Collagen and elastin content in canine arteries selected from functionally different vascular beds. Circ Res. 1966;19:394–9.
Rodenberg EJ, Pavalko FM. Peptides derived from fibronectin type III connecting segments promote endothelial cell adhesion but not platelet adhesion: implications in tissue-engineered vascular grafts. Tissue Eng. 2007;13:2653–66.
Acknowledgments
This study was supported in part by Research on the Human Genome, Tissue Engineering from the Ministry of Health, Labor and Welfare, and by the Core Research of Evolutional Science and Technology from the Japan Science and Technology Agency (JST-CRST).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Negishi, J., Funamoto, S., Kimura, T. et al. Effect of treatment temperature on collagen structures of the decellularized carotid artery using high hydrostatic pressure. J Artif Organs 14, 223–231 (2011). https://doi.org/10.1007/s10047-011-0570-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-011-0570-z