Abstract
Purpose
The use prosthetic materials for the surgical repair of abdominal wall defects has become almost standard practice. This study was designed to assess the expression of different growth factors (VEGF/TGF-β1) and macrophages during the early host tissue incorporation of several polypropylene lightweight (PP-LW)—including one partially absorbable—and heavyweight (PP-HW) prosthetic meshes.
Methods
Ventral defects were created in the anterior abdominal wall of New Zealand rabbits and repaired by fixing PP-LW meshes of different pore size and a low porosity PP-HW mesh to the edges of the defect. Following killing 14 days after implant, specimens were taken to examine TGF-β1/VEGF gene and protein expression by qRT-PCR and immunohistochemistry. The macrophage response was also assessed.
Results
All the materials showed good host tissue incorporation, with a more severe inflammatory reaction and greater numbers of macrophages recorded in the partially absorbable LW implants. Relative amounts of VEGF mRNA were significantly lower for the LW partially absorbable implants compared with the remaining LW meshes. Protein expression of VEGF showed undetectable or minimum staining in the different groups. TGF-β1 mRNA levels were also lower in the partially absorbable group compared with one of PP-LW type of mesh. Gene expression patterns were consistent with the TGF-β1 protein levels detected.
Conclusions
The results suggest that VEGF and TGF-β1 expression were independent of mesh pore size. The expression of both growth factors and the macrophage response were correlated with the presence of biodegradable material in the mesh. The presence of absorbable material in the LW mesh gave rise to a more intense inflammatory reaction and the reduced synthesis of growth factors known to contribute to neotissue maturation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of a prosthetic material for the surgical repair of an abdominal wall hernia has become almost standard practice [1, 2]. One of the most commonly used materials, polypropylene (PP), induces a desirable, rapid, acute inflammatory response followed by a chronic foreign body reaction that persists over months and years following surgery [3–6].This inflammatory reaction along with shrinkage of the biomaterial determines the formation of strong scar tissue but also increases the rigidity of the abdominal wall, producing a feeling of stiffness in the patient [7, 8].
Various strategies have been employed to avoid longer-term complications due to an exaggerated foreign body response. One solution is to limit the amount of PP in the mesh or to use absorbable biomaterials that will provide initial strength and then soon become reabsorbed, reducing local inflammation and the foreign body reaction.
With this goal in mind, the changes made to PP meshes over the years have included modifications in pore size or in the spatial arrangement or diameter of their filaments. These meshes have been accordingly classified as heavyweight (HW) or lightweight (LW) depending on their weight and density [7–9]. Theoretically, low-density meshes should optimize the foreign body reaction induced by reducing the mass of the material used thus diminishing its contact surface with the host tissue [10]. Several studies have shown that the LW mesh elicits a reduced acute inflammatory response compared with its HW counterpart [11, 12]. In effect, the development of reduced-material implants adapted to the physiological requirements of the anterior abdominal wall has improved biocompatibility as well as patient comfort [13, 14].
Other studies [15] have tried to assess the effects of varying the amount of material implanted by comparing PP-LW meshes composed solely of non-absorbable materials and PP-LW that have both absorbable and non-absorbable components. One of the aims of using a partially absorbable mesh has been to try to reduce the amount of material that persists in the host following their placement. The rationale for this is that by reducing the amount of biomaterial in the host, the foreign body reaction induced will be diminished and the repair process will probably generate less fibrosis in the recipient [16, 17]. In one of our experimental studies with a 90-day follow-up period [18], we observed the similar post-implant tensile strength of PP-HW and partially absorbable PP-LW meshes. In another study, at short term, we were also able to demonstrate that LW meshes have several advantages over HW implants in terms of their early collagenization and improved tensile strength [19].
When a prosthetic material is implanted in the abdominal wall, inflammatory cells, and fibroblasts migrate to the site of injury, and macrophages start to release several cytokines and growth factors. Growth factors are peptide mediators involved in cell proliferation, cell cycling and apoptosis. Among the various pro-angiogenic mediators involved in wound healing, VEGF appears to be the most important. In zones of tissue damage, VEGF is a powerful inducer of angiogenesis and of the blood vessel patency that is essential in the early proinflammatory response. Transforming growth factor beta (TGF-β1) is a pleiotropic factor that has the capacity to control cell processes that span from proliferation, differentiation, and apoptosis in most cell types, to complex physiologic processes such as the immune response, inflammation, wound scarring, and tissue repair. TGF-β1 is a potent antiinflammatory agent; it promotes fibroblast chemotaxis and increases the expression of collagen, fibronectin, and proteoglycans playing a role in the development of fibrosis in several chronic inflammation processes. Here, we examine the expression of different growth factors to improve our understanding of the tissue regeneration process. Few studies have dealt with changes in inflammatory marker and growth factor production after polypropylene mesh implant and existing reports have mostly focused on serum or wound drain fluids [20–22].
Our study was designed to assess the expression of these growth factors and the recruitment macrophages during the early host tissue incorporation of several polypropylene lightweight (PP-LW) and heavyweight (PP-HW) meshes and to correlated this expression with pore size and the presence of absorbable material in the implants.
Our interest in 14 days post-implant is focused on that the initial acute phase reaction after implantation of a mesh is really important because the good or bad integration of the biomaterial depend on it. In our opinion, the 14 days are critical from the standpoint of acute response in the healing phase. If the integration fails in the first 2 weeks, the process will be not successful.
Materials and methods
Experimental animals
The experimental animals were 24 male New Zealand White rabbits weighing approximately 2,500 g and caged under conditions of constant light and temperature according to European Union animal care guidelines (EEC 2871-22 A9).
Prosthetic materials
The biomaterials used were:
-
Surgipro® (Covidien, USA): HW polypropylene (85 g/m2) with a pore surface area of 0.26 ± 0.03 mm2.
-
Parietene® (Covidien, USA): LW polypropylene (38 g/m2) with a pore surface area of 1.15 ± 0.05 mm2.
-
Ultrapro® (Ethicon, Johnson & Johnson, USA): LW polypropylene and polyglecaprone 25 (28 g/m2) with a pore surface area of 3.45 ± 0.19 mm2. This prosthesis is partially absorbable.
-
Optilene Elastic® (B/Braun, Germany): LW polypropylene (48 g/m2) with a pore surface area of 7.64 ± 0.32 mm2.
Four study groups were established in which six animals were implanted with each of the meshes.
Surgical technique
The animals were anesthetized with a mixture of ketamine hydrochloride (70 mg/kg), diazepam (1.5 mg/kg), and chlorpromazine (1.5 mg/kg) administered intramuscularly. In some cases, an additional dose of anesthetic was injected directly in the abdominal cavity during the course of surgery.
Using a sterile surgical technique, a ventral defect (7 × 5 cm) was created in the anterior abdominal wall, comprising the aponeurotic, muscular, and peritoneal planes. These defects were then repaired by securing the different meshes to the edges of the defect with running 4/0 polypropylene suture. The animals were killing at 14 days post-implant.
Light microscopy
After the animals were killed, specimens were taken from the mesh areas and from the mesh/recipient tissue interface to assess neoformed tissue within the mesh by examination under a light microscope (Zeiss Axiophot, Carl Zeiss, Oberkochen, Germany). Specimens were fixed in Bouin’s fluid, embedded in paraffin and cut into 5-μm sections and stained with hematoxylin-eosin and Masson’s trichrome (Goldner-Gabe) stains.
Real time RT-PCR
Tissue fragments, 1 cm2 in size, were obtained from the mesh area, and stored at -80 °C until use. RNA was extracted using guanidine–phenol–chloroform isothiocyanate procedures with trizol (Invitrogen, Carlsbad, CA, USA). The RNA was recovered from the aqueous phase and precipitated by adding isopropanol and incubating overnight at -20 °C. Complementary DNA was synthesized using 200 ng of the total RNA by reverse transcription with oligo dT primers (Amersham, Fairfield, USA) and the M-MLV reverse transcriptase enzyme (Invitrogen). cDNA was amplified using the following primers: TGF-β1 (sense 5′-CGG CAG CTG TAC ATT GAC TT-3′ and antisense 5′-AGC GCA CGA TCA TGT TGG AC-3′); VEGF (sense 5′-GGA GTA CCC TGA TGA GAT CGA-3′ and antisense 5′-CTT TGG TCT GCA TTC ACA TTT GT-3′); GAPDH (sense 5′-TCA CCA TCT TCC AGG AGC GA-3′ and antisense 5′-CAC AAT GCC GAA GTG GTC GT-3′).
The RT-PCR mixture contained 5 μl of the inverse transcription product (cDNA) diluted 1:20, 10 μl of iQ SYBR Green Supermix (Bio-rad, Laboratories, Hercules, CA, USA), and 1 μl (6 μM) of each primer in a final reaction volume of 20 μl. RT-PCR was performed in a StepOnePlus Real-Time PCR System (Applied Biosystem, Foster City, California, USA). Samples were subjected to an initial stage of 10 min at 95 °C. The conditions for cDNA amplification were as follows: 40 cycles of 95 °C for 15 s, 60 °C (TGF-β1 and VEGF) or 55 °C (GAPDH) for 30 s and 72 °C for 1 min. Negative control with ultraPure™ distilled water DNase, RNase-free (Invitrogen) was added in each reaction. Products were submitted to 2 % agarose gel electrophoresis, stained with SYBR Green II RNA gel stain (Invitrogen) and visualized with UV light.
Gene expression was normalized against the expression recorded for the constitutive gene glyceraldehyde 3-phosphate-dehydrogenase.
Immunohistochemistry
For immunohistochemical analysis, after the animals were killed, specimens were taken from the central mesh areas and from the peripheral regions. Specimens were fixed in F-13 fluid, embedded in paraffin and cut into 5 μm-thick sections. The sections were deparaffinated, hydrated, equilibrated in PBS buffer and incubated with the different antibodies.
We used the following antibodies to detect the cells normally present in a chronic inflammatory reaction: goat monoclonal anti-LAP-TGF-β1 antibody (dilution 1:10; R&D Systems, Minneapolis, MN, USA), mouse monoclonal anti-TGF-β1 antibody (dilution 1:1000; Chemicon, Temecula, CA, USA), mouse monoclonal anti-VEGF (dilution 1:50; Abcam, Cambridge, UK) and monoclonal anti-rabbit macrophage RAM-11 (dilution 1:50; Dako, Carpinteria, CA, USA).
The alkaline phosphatase-fast red technique was used to detect the antigen–antibody reaction. Cell nuclei were counterstained with hematoxylin.
For each antibody, staining intensity was assessed by examining 10 digitalized histological images per animal captured using a digital camera fitted to the microscope (Axiocam HR, Zeiss, Germany) and analyzed using image analysis software Axiovision AC 4.1. Each section was divided into 4 zones and one microscope field (×200) was randomly selected from each zone to estimate staining intensity. A simple scale was used to perform a semiquantification of the staining. Results were expressed as follows: −, undetectable staining (<10 %); ±, minimum staining (10–25 %); +, moderate staining (25–50 %); ++, strong staining (50–75 %); +++, maximum staining (75–90 %) and ++++, almost complete staining (>90 %). Staining intensity was scored by two independent observers in a blinded fashion.
Statistical analysis
All data are expressed as mean ± SEM. The Mann–Whitney test was used to compare data among the different study groups. The level of significance was set at P < 0.05. All statistical analyses were performed using the Graph Pad Prism 4 package.
Results
Light microscopy
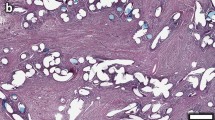
All the groups of meshes, regardless of pore size or the presence of biodegradable material, induced complete tissue colonization of the zones limited by the filaments comprising the implants. On light microscopy, all the prosthetic materials showed good host tissue ingrowth (Fig. 1). In these areas, we could observe neoformed tissue such that the implant surface overlying the visceral peritoneum appeared completely mesothelialized.
Light microscopy images of the different biomaterials 14 days after implant. a Surgipro®, b Parietene®, c Ultrapro®, and d Optilene Elastic®. All the groups of meshes induced complete tissue colonization of the zones limited by the filaments of the biomaterials (Original magnification ×100). (F filaments of the meshes, PG partially absorbable filaments of polyglecaprone, Ss subcutaneous side, Ps peritoneal side)
All the meshes induced inflammatory tissue reaction involving mainly macrophages and foreign body giant cells in perifilament zones. This reaction was greater in the Ultrapro group, which showed numerous inflammatory cells around the absorbable material that was gradually being degraded.
Real time RT-PCR
Both growth factors, VEGF and TGF-β1, showed gene expression in the different study groups. No significant expression differences in any of the growth factors attributable to mesh pore size were detected.
When we examined the expression of the gene coding for VEGF, we observed lower relative amounts of VEGF mRNA for the partially absorbable LW Ultrapro implants compared with the rest of the meshes, although the differences between Surgipro and Ultrapro showed no statistical significance, due to variability in the data from this last group. The rest of the groups with the exception of Ultrapro showed no significant differences in VEGF gene expression among themselves (Fig. 2a).
mRNA expression of VEGF (a) and TGF-β1 (b). Relative amount (Left pannel) and RT-PCR products of TGF-β1, VEGF and GAPDH (Right pannel) are shown. GAPDH gene was used as the endogene control. Values are expressed as the mean ± SEM. (N negative control; 1, 2 Surgipro®; 3, 4 Parietene®; 5, 6 Ultrapro®; 7, 8 Optilene Elastic®; MW molecular weight markers) (*P < 0.05)
Different behavior was observed when we examined the expression of the gene encoding TGF-β1. As for VEGF, the Ultrapro group of meshes showed lower TGF-β1 expression levels than the remaining groups. However, in this case, significant differences compared with Ultrapro only emerged in the Parietene group (Fig. 2b), and the rest of the groups showed no significant differences among each other, although Optilene showed a tendency to increase respect to the Ultrapro group.
Immunohistochemistry
Fourteen days after placement, the different types of meshes showed expression for TGF-β1 and VEGF. In all the groups, greater staining was detected for the active form of TGF-β1 than latent form (LAP-TGF-β1) for which staining was very weak. Protein expression of the active form of TGF-β1 (Fig. 3a–d) was observed in localized areas of the neoformed tissue among the prosthetic filaments, mainly in areas of neoangiogenesis close to these filaments. The Ultrapro group showed the lowest levels of active TGF-β1 protein (Fig. 3c; Table 1). Thus, we were able to correlate observed TGF-β1 gene expression and the levels of protein immunodetected. The latent form of the protein, LAP-TGF-β1, was weakly expressed in general across the implant groups, with staining limited to small areas close to the filaments of the prosthesis (Fig. 3e–h; Table 1). VEGF expression was observed mainly around the blood vessels and in some groups immunolabeling spread to nearby connective tissue (Fig. 4). This protein was also minimally expressed in the partially absorbable meshes, together with Parietene, with respect to the rest of the groups (Fig. 4c; Table 1), and was correlated with underexpression of the gene.
Immunohistochemical expression of the active form of TGF-β1 14 days after implant. a Surgipro®, b Parietene®, c Ultrapro® and d Optilene Elastic®. (×100) Immunohistochemical expression of the latent form of TGF-β1 (LAP-TGF-β1) 14 days after implant. e Surgipro®, f Parietene®, g Ultrapro® and h Optilene Elastic®. (Original magnification ×100). In all the groups, greater staining was detected for the active form of TGF-β1 (a–d) than latent form (LAP-TGF-β1) (e–h). The Ultrapro group showed the lowest levels of active TGF-β1 protein. (F filaments of the meshes, PG absorbable filaments)
Immunohistochemical expression of VEGF in the different study groups. a Surgipro®, b Parietene®, c Ultrapro® and d Optilene Elastic®. (Original magnification ×200). Expression was observed mainly around the blood vessels and was minimally expressed in the partially absorbable meshes. (F filaments of the meshes, PG absorbable filaments)
Macrophages
The presence of macrophages was observed in the neoformed tissue around the prosthetic filaments in all the study groups (Fig. 5). Greatest numbers of inflammatory cells appeared in areas close to the filaments where, besides macrophages, multinucleated foreign body giant cells, typical of the wound repair process triggered by the presence of a foreign body, could be seen (Fig. 5f). This type of cell was observed in greatest numbers around the filaments of absorbable material comprising the Ultrapro implants (Fig. 5e, f).
Macrophage response. Immunohistochemical staining with monoclonal anti-rabbit macrophage RAM-11 in the different study groups. a, b) Surgipro®, c, d) Parietene®, e, f) Ultrapro® and g, h) Optilene Elastic®. (Original magnification ×200; ×400). Graph showing mean percentages of RAM-11-positive-cells as mean ± SD (*P < 0.05, **P < 0.01)
Similar macrophage counts were obtained for the Parietene, Surgipro and Optilene Elastic groups (Fig. 5). For the Ultrapro group, significantly higher macrophage percentages were recorded compared with the other study groups (Fig. 5).
Discussion
Polypropylene (PP) is currently the material most commonly used for the repair of the abdominal wall damaged by an inguinal hernia. After mesh implantation, an inflammatory foreign body reaction regulated mainly by monocytes and macrophages occurs at the implant-tissue interface. These cells are responsible for releasing proinflammatory cytokines and growth factors, which, in turn, regulate wound healing and scar formation. Our interest in 14 days post-implant is focused on that the initial reaction after implantation of a mesh is really important and critical from the standpoint of acute response in the healing phase. If the integration fails in the first 2 weeks, the prosthetic implant will fail also.
Several authors have reported that the presence of a mesh in hernia repair compared with the more conventional suture repair method is generally associated with the enhanced production of inflammatory mediators [20]. Also, in patients undergoing either primary or incisional midline abdominal hernia repair, elevated VEGF levels in serum and wound fluids have been detected in the early proliferative stage of wound healing [23].
In an effort to improve the tissue incorporation of a prosthetic mesh and leave behind a minimal quantity of foreign material in the recipient, several structural modifications led to the introduction of the lightweight prosthetic implant [7]. To further improve the characteristics of these lightweight meshes, manufacturers have incorporated absorbable filaments into the weave of the mesh that add stiffness during the early post-implant period without compromising mechanical resistance.
Several studies [24–26] have shown that different factors are able to influence the inflammatory response to an implanted prosthesis such as density, pore size, type of material, fiber structure, texture and mesh construction. However, in a review [12] of the English and German medical literature on the outcome of groin and incisional hernia mesh repair, the authors claimed that mesh construction and composition, as characterized by pore size and filament structure, seemed to be the most important determinants of the foreign body reaction produced after implant.
In animal experiments, it was observed that material-reduced meshes achieved better host tissue incorporation and elicited a reduced foreign body reaction [12]. Other studies also showed that the apoptotic effects and inflammatory response after the implant of lightweight meshes were significantly reduced in comparison with traditional meshes [8, 15]. It should nevertheless be considered that in both these studies, the LW meshes employed (Vypro/Ultrapro) were partially absorbable and in the case of Ultrapro, the reduced inflammatory and fibrotic response observed was not statistically significant [8]. For the other partially absorbable implant (Vypro) results indicated that restriction of abdominal wall mobility was significantly reduced compared with that seen with HW meshes and the inflammatory reaction and connective tissue formation were markedly diminished at the study endpoint of 90 days [15]. Moreover, at a follow-up time similar to ours (21 days), percentages of lymphocytes, foreign body giant cells and macrophages were greater in their partially absorbable PP-LW group, in agreement with our results.
In our study, no significant differences in terms of the inflammatory response elicited were detected between the LW and HW meshes that could be attributed to their pore size or density. In effect, the percentages of macrophages, which perhaps play the most important role in a chronic inflammatory reaction, for the PP-LW and PP-HW meshes were similar and neither were significant differences detected in the gene or protein expression levels of VEGF and TGF-β1. In contrast, the behavior of Ultrapro (a partially absorbable LW mesh) was completely different and significantly higher percent macrophage values were observed compared with the other meshes, as well as a significant reduction in VEGF and TGF-β1 expression. This augmented inflammatory reaction is likely to delay neotissue maturation in the short term, although at later time points this effect would be compensated for as indicated in our prior work at 90 days [18], having the benefit that less foreign material remains in the recipient, without compromising mechanical resistance. Therefore, the findings of our study have not necessarily a negative impact on the biocompatibility of Ultrapro in the long term. Other studies [27] have also showed good behavior with Ultrapro in the long term showing for this mesh a very low shrinkage rate with a good biocompatibility 91 days post-implant.
Since Ultrapro contains rapidly absorbable material, it is not surprising that it elicits a more intense phagocytic response of the macrophages along with a lengthening of the inflammatory stage to the detriment of fibroplasia and neotissue maturation. This delayed tissue maturation in the short term would coincide with the reduced synthesis of VEGF and TGF-β1 observed here.
Data from experimental studies comparing the behavior of HW and LW implants with absorbable components have been controversial. Some authors feel that the incorporation of an absorbable material is not a real advantage for hernia surgery because of this more intense inflammation and lower biocompatibility [28, 29]. In fact, researchers testing HW, LW, LW partially absorbable and titanium-coated LW implants in pigs, noted that the partially absorbable mesh produced an intense inflammatory-fibrotic reaction, and that the titanium-coated LW mesh showed best behavior in terms of inducing a chronic inflammatory reaction [28].
In an experimental study conducted on rats 112 days after PP or PP-polyglactin mesh implant, Rosch et al. [30] observed short-term polyglactin-induced enhanced inflammation and fibrosis around the implanted meshes and concluded that in the long-term the presence of multifilament polyglactin (PG) does not impair mesh biocompatibility.
Another recent study [31] conducted in rats revealed that the implant of a mesh with a non-absorbable (PP) and an absorbable (PG) component induced an intense inflammatory reaction that was rich in granulomas and foreign body cells. These authors observed greater inflammation on the mesh surface and reduced tissue maturation, with less collagen deposition. Thus, the early absorbed material might cause a more intense phagocytosis reaction, with prolongation of the inflammatory stage of healing thus delaying fibroplasia and neotissue maturation. In the Pereira study, it was concluded that the inflammatory reaction was more intense in the animals that received PP + PG and was less intense in those receiving the high-density PP mesh. This was correlated with greater collagen deposition in the PP mesh group and lower deposition in the PP + PG group. These findings are consistent with our observation of a significantly higher percentage of macrophages in our Ultrapro group and lower amounts of the two growth factors examined compared with the remaining LW and HW implants. This, as mentioned earlier, would translate to a delay in neotissue maturation.
The above observations are also related to the findings of recent studies in which we examined mesh collagenization in the early post-implant stages. The results of this prior work indicated higher relative amounts of mRNA coding for collagen types 1 and 3 in PP-LW or PP+PG-LW implants compared with PP-HW meshes. However, these higher amounts did not correlate with protein levels of mature or type I, collagen in the implants with an absorbable component [19].
Further recent studies [22] in which the serum of patients implanted with PP or PP-PG meshes was analyzed to explore changes produced in leukocytes, cytokines, growth factors and acute phase proteins, revealed that leukocytes and acute phase proteins increased postoperatively in both patient sets, with slightly higher values recorded in the PP group. Cytokine levels, especially proinflammatory, were also significantly elevated in both groups, with a slightly higher increment in the PP-PG group, and growth factors were also significantly diminished in both groups. Such a reduction in the different growth factors is also in accordance with our results.
In summary, our findings indicate that
VEGF and TGF-β1 expression is independent of mesh pore size.
The expression of both growth factors and macrophages could be related to the presence of biodegradable material in the mesh.
The presence of re-absorbable material in the LW mesh gave rise to a more intense inflammatory reaction and diminished synthesis of growth factors known to contribute to neotissue maturation.
References
EU Hernia Triallists Collaboration (2000) Mesh compared with non-mesh methods of open groin hernia repair: systematic Review of randomized controlled trials. Br J Surg 87:854–859
Kingsnorth A, LeBlanc K (2003) Hernias: inguinal and incisional. Lancet 362:1561–1571
Di Vita G, Milano S, Frazzetta M et al (2000) Tension-free hernia repair is associated with an increase in inflammatory response markers against the mesh. Am J Surg 180:203–207
Di Vita G, Milano S, Patti R et al (2001) Cytokine modifications after tension-free hernioplasty or open conventional inguinal hernia repair. Am J Surg 181:487–491
Schachtrupp A, Klinge U, Junge K et al (2003) Individual inflammatory response of human blood monocytes to mesh biomaterials. Br J Surg 90:114–120
Arnaud JP, Eloy R, Adloff M et al (1978) Prosthetic materials and wound healing: critical evaluation of six different materials. Int Surg 63:7–9
Cobb WS, Kercher KW, Heniford BT (2005) The argument for lightweight polypropylene mesh in hernia repair. Surg Innov 12:63–69
Junge K, Rosch R, Krones CJ et al (2005) Influence of polyglecaprone 25 (Monocryl) supplementation on the biocompatibility of a polypropylene mesh for hernia repair. Hernia 9:212–217
Schumpelick V, Klinge U, Junge K et al (2004) Incisional abdominal hernia: the open mesh repair. Langenbecks Arch Surg 389:1–5
Klosterhalfen B, Junge K, Klinge U (2005) The lightweight and large porous mesh concept for hernia repair. Expert Rev Med Devices 2:103–117
O’Dwyer PJ, Kingsnorth AN, Molloy RG et al (2005) Randomized clinical trial assessing impact of a lightweight or heavyweight mesh on chronic pain after inguinal hernia repair. Br J Surg 92:166–170
Weyhe D, Belyaev O, Müller C et al (2007) Improving outcomes in hernia repair by the use of light meshes: a comparison of different implant constructions based on a critical appraisal of the literature. World J Surg 31:234–244
Klinge U (2007) Experimental comparison of monofile light and heavy polypropylene meshes: less weight does not mean less biological response. World J Surg 31:867–868
Schumpelick V, Klosterhalfen B, Müller M et al (1999) Minimized polypropylene mesh for preperitoneal net plasty (PNP) of incisional hernias. Chirurg 70:422–430
Junge K, Klinge U, Rosch R et al (2002) Functional and morphologic properties of a modified mesh for inguinal hernia repair. World J Surg 26:1472–1480
Klinge U, Junge K, Stumpf M et al (2002) Functional and morphological evaluation of a low-weight monofilament polypropylene mesh for hernia repair. J Biomed Mater Res 63:129–136
Cobb WS, Burns JM, Peindl RD et al (2006) Textile analysis of heavy weight, mid-weight, and light weight polypropylene mesh in a porcine ventral hernia model. J Surg Res 136:1–7
Bellón JM, Rodriguez M, Garcia-Honduvilla N et al (2007) Partially absorbable meshes for hernia repair offer advantages over nonabsorbable meshes. Am J Surg 194:68–74
Pascual G, Rodríguez M, Gomez-Gil V et al (2008) Early tissue incorporation and collagen deposition in lightweight polypropylene meshes: bioassay in an experimental model of ventral hernia. Surgery 144:427–435
Di Vita G, D’Agostino P, Patti R et al (2005) Acute inflammatory response after inguinal and incisional hernia repair with implantation of polypropylene mesh of different size. Langenbecks Arch Surg 390:306–311
Di Vita G, Patti R, D’Agostino P et al (2006) Modifications in the production of cytokines and growth factors in drainage fluids following mesh implantation after incisional hernia repair. Am J Surg 191:785–790
Di Vita G, Patti R, Barrera T et al (2010) Impact of heavy polypropylene mesh and composite light polypropylene and polyglactin 910 on the inflammatory response. Surg Innov 17:229–235
Karayiannakis AJ, Zbar A, Polychronidis A, Simopoulos C (2003) Serum and drainage fluid vascular endothelial growth factor levels in early surgical wounds. Eur Surg Res 35:492–496
Post S, Weiss B, Willer M et al (2004) Randomized clinical trial of lightweight composite mesh for Lichtenstein inguinal hernia repair. Br J Surg 91:44–48
Kayaoglu HA, Ozkan N, Hazinedaroglu SM et al (2005) Comparison of adhesive properties of five different prosthetic materials used in hernioplasty. J Invest Surg 18:89–95
Di Vita G, Patti R, Sparacello M (2008) Impact of different texture of polypropylene mesh on the inflammatory response. Int J Immunopathol Pharmacol 21:207–214
Schug-Pass C, Tamme C, Sommerer F et al (2008) A lightweight, partially absorbable mesh (Ultrapro) for endoscopic hernia repair: experimental biocompatibility results obtained with a porcine model. Surg Endosc 22:1100–1106
Scheidbach H, Tamme C, Tannapfel A et al (2004) In vivo studies comparing the biocompatibility of various polypropylene meshes and their handling properties during endoscopic total extraperitoneal (TEP) patchplasty: an experimental study in pigs. Surg Endosc 18:211–220
Klinge U, Klosterhalfen B, Birkenhauer V et al (2002) Impact of polymer pore size on the interface scar formation in a rat model. J Surg Res 103:208–214
Rosch R, Junge K, Quester R et al (2003) Vypro II mesh in hernia repair: impact of polyglactin on long-term incorporation in rats. Eur Surg Res 35:445–450
Pereira-Lucena CG, Artigiani-Neto R, Lopes-Filho GJ et al (2010) Experimental study comparing meshes made of polypropylene, polypropylene + polyglactin and polypropylene + titanium: inflammatory cytokines, histological changes and morphometric analysis of collagen. Hernia 14:299–304
Acknowledgments
This research was supported by the Spanish Ministry of Science and Technology through Project DPI2011-27939-C02-02.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pascual, G., Rodríguez, M., Sotomayor, S. et al. Inflammatory reaction and neotissue maturation in the early host tissue incorporation of polypropylene prostheses. Hernia 16, 697–707 (2012). https://doi.org/10.1007/s10029-012-0945-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-012-0945-y