Abstract
Background
Lumbar hernias that occur after surgery are called lumbar incisional hernias. Recently, laparoscopic repair of these hernias has been reported with excellent outcomes. This is a retrospective study of our series of patients with lumbar incisional hernias.
Patients and methods
We managed 11 patients with lumbar incisional hernias from 1996–2006. All the patients had undergone either nephrectomy or pyeloplasty in the past. Laparoscopic suturing of the defect and reinforcement with mesh were successfully performed for all the patients.
Results
There were more males than females, the age range was 42–65 years, and mean operating time was 120 min; discharge was at 1–2 postoperative days. There was no recurrence or mortality. Three cases had seroma, out of which two required aspiration after 60 days.
Discussion
Laparoscopic repair provides all the benefits of minimally invasive surgery, and the principles involved in repair of ventral hernias are applied in lumbar incisional hernias as well. Our technique involved suturing of the defect before placing a mesh over the defect. We theorize that approximating the ends of the muscles restores normal anatomy and results in functional improvement. For the larger hernias, we used two meshes to cover the defect—polypropylene and Parietex™, sizes being 15 × 15 cm.
Conclusion
Laparoscopic repair with prosthetic reinforcement is feasible and effective in the treatment of lumbar incisional hernias. Also, suturing of the defect may provide additional benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lumbar hernia is a rare hernia that accounts for less than 1.5% of the total hernia incidence, and so far only 200–300 cases have been reported in the literature [1]. Lumbar hernia per se does not include hernias that can occur following incisions in the lumbar area, for example, operations on the kidneys. Therefore, to differentiate one from the other, the term ‘lumbar incisional hernia’ is used. The other terms in use are secondary or acquired lumbar hernias. Lumbar hernias often pose a challenge for repair. Because of the surrounding anatomy, adequate surgical herniorraphy is often difficult. Minimally invasive surgery has become an option for these hernias. Some surgical repair procedures have been described, the most frequently used being either the open technique with primary closure or the use of prosthetic material [2]. Despite the wide use of the laparoscopic techniques for treating ventral abdominal hernias, there are only a few reports in the literature regarding the laparoscopic approach for correction of lumbar defects [3,4]. We present a retrospective series of patients from our institute treated with the laparoscopic repair of lumbar incisional hernias.
Materials and methods
We managed 11 patients with lumbar incisional hernias from 1996–2006. The retrospective study included patients with acquired traumatic lumbar hernias secondary to a lumbar incision for conventional renal (nephrectomy or pyeloplasty) surgery. All the procedures were performed by a single surgeon at our institute. Clinically, all patients presented with a swelling and cough impulse in the lumbar area, the range of duration of symptoms being 3–5 years. These were taken as indications for surgery. There were no cases of irreducibility or strangulation. Preoperative workup included routine blood and urine tests, EKG, chest radiograms, ultrasonography (USG) and computerized tomography (CT) scan. There was a distinct defect in nine patients and diffuse atrophy possibly due to denervation in two cases. Surgery was performed for these two patients as well even though they did not have a distinct defect as the area of muscle weakness was large and for cosmesis. Moreover, they requested surgery after having had explained to them that absence of a defect was a contraindication. After anesthetic fitness was obtained, all patients underwent the same operative procedure—laparoscopic suture and mesh repair. Patients were placed in the right lateral position at a 60° angle, almost similar to the laparoscopic splenectomy position. Pneumoperitoneum was established via a Veress needle in the umbilicus. The port positions were as follows (Fig. 1: for left-sided hernias):
-
10-mm port: to the left and above the umbilicus; for camera/laparoscope
-
5-mm port: below and left of the umbilicus in the left midclavicular line; for right working hand
-
5-mm port: midline, 2 cm from xiphoid; for left working hand
-
5-mm port: right of the umbilicus; for retraction of the large bowel
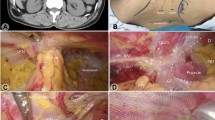
The first step was to perform adhesiolysis and reduction of hernial contents, if there were any, so that the entire defect/weakness was isolated (Fig. 2-arrows denote the superior border of the defect). At this point, the insufflation pressure was reduced to 9 mmHg to facilitate suturing. We then commenced intercorporeal suturing of the defect with one ‘loop’ ethilon (Fig. 3). The full extent of the defect could now be visualized (Fig. 3—white line). The needle was brought into the peritoneal cavity through a stab incision in the abdominal wall, and the end of the suture material was secured with a clamp outside the abdomen. Suturing was started from the lateral end. Once the medial end was reached, another line of sutures was made by going back to the lateral end (starting point). Now the needle was cut and the suture material was brought out of the abdomen using a port-closure needle. The final knot was made outside on the abdominal wall and buried within the subcutaneous fat. Normal anatomy of the muscle is restored once this is completed (Fig. 4). A polypropylene mesh was placed over the sutured defect, the size of the mesh being equal to the size of the defect before closure (Fig. 5a). Another mesh (ParietexTM) of size 15 × 15 cm was placed over the polypropylene mesh and secured with sutures to the intercostal space superiorly, iliac crest periosteum inferiorly, and rectus muscle anteriorly (Fig. 5b). Posteriorly, the mesh was secured to psoas major fascia with intercorporeal sutures to avoid nerve injury. This was achieved by mobilizing with harmonic shears the splenic flexure and left colon medially by dividing the white line of Toldt in the left paracolic gutter.
Results
There were seven males and four females. The age range was 42–65 years. As far as the previous surgery was concerned, four patients had undergone a left-sided nephrectomy, three patients had left pyeloplasty and four patients had right nephrectomy. The main findings of age, sex, BMI, size of the defect and duration of the hernia are listed in Table 1. It is obvious that most patients were either overweight or obese, which is a risk factor for hernia according to the literature. Mean size of the hernial orifice was 58.5 cm2 (40–77 cm2), and mean duration of the hernia was 45.5 (31–65) months. All cases were completed laparoscopically with no conversions. Operating times were in the range of 75–120 (mean−97.5) min. Normal diet was commenced on the 1st postoperative day (POD) for all the patients. Discharge from the hospital occurred between the first and the second POD. There were no intraoperative complications or perioperative mortality. The mean follow-up was 22.61 months. The patients were asked to review at 7 days, 30 days, 90 days and 1 year. Six patients were followed up for a total of 28 months, four patients for 45 months, and the rest lost to follow-up. There was no hernia recurrence in any case. Postoperatively, there was seroma in three cases, one resolved spontaneously after 60 days and two patients required aspiration. The return to usual activities occurred on an average of 3 weeks following surgery.
Discussion
The lumbar area is bounded by the 12th rib, the iliac crest, the erector spinae and the external oblique muscle. Flank incisions may be associated with flank hernias, which may be complicated by incarceration (25%) and strangulation (8%) [5]. Lumbar hernias can be classified into congenital (10–20%) or acquired (80–90%) hernias [6]. Acquired hernias are divided into two types—spontaneous and traumatic (incisional) [7]. According to published material, most postoperative incisional hernias occur in nephrectomy or aortic aneurysm repair incisions [8]. In our series, all the patients had undergone renal surgery previously. Lumbar incisional hernias are often diffuse with fascial defects that are usually hard to appreciate. This was evident in two of our patients, who did not have a distinct hernial orifice. CT scan is very useful, as it differentiates lumbar hernias from abdominal wall musculature denervation atrophy complicating flank incisions. We did CT scans for all our patients, as it is also useful in patient follow-ups to check the adequacy of the repair and recurrences. Whether surgery is needed for diffuse muscular atrophy alone is controversial, somewhat like the controversy over the management of divarication of the abdominal recti muscles. In both these conditions, surgery could be indicated for cosmesis, and as long as laparoscopy is being used, it is relevant. Since all these patients had a cough impulse and swelling at the site of incision, we considered this as an indication for surgery. The postoperative outcome was similar to that of patients with distinct hernial defects. Repairing lumbar incisional hernias is often thought to be difficult due to the surrounding structures. If permitted by the patient’s general condition, the lumbar hernia always has surgical indication with several techniques being described in the literature. Due to its rarity, there is no standardized technique. The difficulty in determining the margins of the fascial defect, the weakness of the involved structures, the participation of a bone element and the surgeon’s expertise are all elements taken into account during surgical planning. For all practical purposes, even though the etiology of primary lumbar and secondary lumbar hernias may be different, the techniques of repair remain the same. Repair with synthetic material is now accepted as the gold standard in the management of all hernias [9]. With the intention of reducing the morbidity observed with the conventional technique while maintaining the results from open surgery with mesh, the laparoscopic access has been recently described. The repair of lumbar hernia by laparoscopic approach was first published in 1996 by Burick and Parascandola [10]. The following year, Arca and Heniford published their experiences in patients with lumbar hernias treated by the laparoscopic approach [11]. Using the expertise in repair of ventral hernias that has been accumulated in many centers worldwide, these principles could be applied to the treatment of lumbar hernias as well. The laparoscopic intraperitoneal approach in the lateral decubitis position that we used in all our cases causes the intraperitoneal viscera to be displaced medially away from the hernia. The creation of a wide peritoneal flap around the hernial defect helps in mobilization of the colon, increased length of margin is available for coverage of mesh and more importantly for secure fixation of the mesh to the underlying fascia. In our center, we always suture the defect close with intercorporeal continuous sutures using #1 monofilament non-absorbable material. Chelala et al. reported that non-absorbable sutures are mandatory for closure of large defects to avoid having to ultimately extrude the mesh [12]. They also used sutures to fix the mesh in all their 120 patients. We have reported our findings in a retrospective study of 721 patients with midline incisional hernias, where we sutured the defect before placing the mesh [13].This is the technique that we follow in all patients who undergo laparoscopic repair of ventral hernias at our institute. We have incorporated this technique in the repair of lumbar incisional hernias as well. Theoretically, we believe that this restores the normal anatomy, thereby improving muscular function of the area, at the cost of the repair not being “tension-free.” The disadvantages are that the ‘rooftop’ suturing can be quite cumbersome, and the repair is not tension-free. In eight patients with a large area of weakness around the defect, two meshes were placed—one polypropylene and one Parietex™ composite mesh over it. The polypropylene mesh was used to cover the defect, while the larger composite mesh covered the surrounding weakness. This assured the complete covering of the defect and weak area. In the other three patients, only the Parietex™ mesh was placed over the defect. In all the patients, the 15 × 15-cm size Parietex™ composite meshes were enough to provide the necessary 5-cm overlap. This was possible because the size of the defect was reduced by suturing it close [13]. The totally extraperitoneal approach to repair lumbar hernias was first described by Meinke in 2002, and ever since a few reports have appeared in the literature [14, 15]. It is tempting to say that this approach could be superior to the transperitoneal technique, but the learning curve is longer. In this technique, the defect is covered with a polypropylene mesh and a second (ePTFE) mesh is used if the retroperitoneal space cannot be closed. Initial experiences have shown significant advantages of the laparoscopic approach over conventional surgery [16]. Moreover, we observed in our patients an excellent exposure of structures and achieved perfect anatomical visualization of the hernia ring. It is safe and simple, and accurately differentiates lumbar incisional hernia from muscle atrophy with no fascial defect. Larger comparative studies are needed to confirm the superiority of the laparoscopic approach with the open technique and to say that the laparoscopic procedure is the method of choice. The increasing use of laparoscopic and percutaneous surgery for treating surgical conditions of the kidneys and adrenal glands will certainly reduce the occurrence of such complications.
Conclusion
The laparoscopic repair of lumbar incisional hernia is a minimally invasive procedure with moderate complexity. As the defects in lumbar hernias are usually large, suturing of the defect before placing the mesh restores the normal muscular anatomy of the area and prevents mesh migration. The laparoscopic approach provides excellent exposure and definition of the defect and avoids the significant morbidity associated with open repair.
References
Bickel A, Haj M, Etian A (1997) Laparoscopic management of lumbar hernias. Surg Endosc 11:1129–1130
Salameh JR, Salloum EJ (2004) Lumbar incisional hernias: diagnostic and management dilemma. JSLS 8:391–394
Barry JM (2002) Transperitoneal preperitoneal laparoscopic lumbar incisional herniorrhaphy. J Urol 167:1800
Tobias-Machado M, Rincon FJ, Lasmar MT, Zambon JP, Juliano RV, Wroclawski ER (2005) Laparoscopic surgery for treatment of incisional lumbar hernia. Int Braz J Urol 31:309–314
Atul KM, Craig AT, Karen ES, Elizabeth PF, David ST (2006) Laparoscopic lumbar hernia repair. Am Surg 72(4):318–321
Shekarriz B, Graziottin TM, Gholami S, Lu HF, Yamada H, Duh QY, Stoller ML (2001) Transperitoneal preperitoneal laparoscopic lumbar incisional herniorrhaphy. J Urol 166:1267–1269
Sakarya A, Aydede H, Erhan MY, Kara E, Ilkgul O, Yavuz C (2003) Laparoscopic repair of acquired lumbar hernia. Surg Endosc 17:1494
Maeda K, Kanehira E, Shino H, Yamamura K (2003) Laparoscopic tension-free hernioplasty for lumbar hernia. Surg Endosc 17:1497
Burick AJ, Parascandola SA (1996) Laparoscopic repair of a traumatic lumbar hernia: a case report. J Laparoendosc Surg 6:259–262
Arca MJ, Heniford BT, Pokorny R, Wilson MA, Mayes J, Gagner M (1998) Laparoscopic repair of lumbar hernias. J Am Coll Surg 187:147–152
Heniford BT, Iannitti DA, Gagner M (1997) Laparoscopic inferior and superior lumbar hernia repair. Arch Surg 132:1141–1144
Chelala E, Gaede F, Douillez V, Dessily M, Alle JL (2003) The suturing concept for laparoscopic mesh fixation in ventral and incisional hernias: preliminary results. Hernia 7:191–196
Palanivelu C, Jani K, Senthilnathan P, Parthasarathi R, Madhankumar M, Malladi V (2007) Laparoscopic sutured closure with mesh reinforcement of incisional hernias. Hernia 13:17297570
Meinke AK (2003) Totally extraperitoneal laparoendoscopic repair of lumbar hernias. Surg Endosc 17:734–737
Habib E (2003) Retroperitoneoscopic tension-free repair of lumbar hernia. Hernia 7:150–152
Moreno-Egea A, Torralba-Martinez JA, Morales G, Fernandez T, Girela E, Aguayo-Albasini JL (2005) Open vs laparoscopic repair of secondary lumbar hernias: a prospective nonrandomized study. Surg Endosc 9:184–187
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palanivelu, C., Rangarajan, M., John, S.J. et al. Laparoscopic transperitoneal repair of lumbar incisional hernias: a combined suture and ‘double-mesh’ technique. Hernia 12, 27–31 (2008). https://doi.org/10.1007/s10029-007-0270-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-007-0270-z