Abstract
Biological soil crusts (BSCs) are a key biotic component of dryland ecosystems worldwide. However, most studies carried out to date on carbon (C) fluxes in these ecosystems, such as soil respiration, have neglected them. We conducted a 3.5-year field experiment to evaluate the spatio-temporal heterogeneity of soil respiration in a semiarid Stipa tenacissima steppe and to assess the contribution of BSC-dominated areas to the annual soil respiration of the whole ecosystem. We selected the six most frequent microsites in the study area: Stipa tussocks (ST), Retama sphaerocarpa shrubs (RS), and open areas with very low (<5% BSC cover, BS), low, medium and high cover of well-developed BSCs. Soil respiration rates did not differ among BSC-dominated microsites but were significantly higher and lower than those found in BS and ST microsites, respectively. A model using soil temperature and soil moisture accounted for over 85% of the temporal variation in soil respiration throughout the studied period. Using this model, we estimated a range of 240.4–322.6 g C m−2 y−1 released by soil respiration at our study area. Vegetated (ST and RS) and BSC-dominated microsites accounted for 37 and 42% of this amount, respectively. Our results indicate that accounting for the spatial heterogeneity in soil respiration induced by BSCs is crucial to provide accurate estimations of this flux at the ecosystem level. They also highlight that BSC-dominated areas are the main contributor to the total C released by soil respiration and, therefore, must be considered when estimating C budgets in drylands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil CO2 efflux, commonly called soil respiration, is a major component of the biosphere’s carbon (C) cycle and represents about three-quarters of total ecosystem respiration (Law and others 2001). Despite its significance, we only have a limited understanding of the magnitude and controlling factors of soil respiration, particularly in arid, semiarid, and dry-subhumid ecosystems (drylands hereafter). These areas cover 41% of Earth’s land surface and host more than 38% of the world’s population (Reynolds and others 2007), yet soil respiration has been much less studied in dryland than in temperate and tropical ecosystems. As an example, drylands constitute less than 8% of case studies of a global soil respiration database accounting for more than 818 studies (Bond-Lamberty and Thomson 2010).
Soil respiration accounts for major C losses from dryland soils (Conant and others 2000), with global estimates averaging over 80.4 Pg CO2-C emitted annually, which is approximately 10-fold greater than CO2 emissions from fossil fuel combustion and deforestation sources combined (Raich and Schlesinger 1992; Raich and others 2002). Given the relatively limited pool of organic C stored in drylands (West and others 1994; Lange 2003), even small changes in current soil respiration rates could have a large impact on their soil C stocks. Therefore, these ecosystems are highly sensitive to changes in temperature and rainfall patterns predicted by future climate change scenarios (West and others 1994; Körner 2000). Carbon stocks in drylands are also being affected by phenomena such as shrub encroachment, which has potential implications for C storage and loss through C accrual in soil and woody biomass and changes in soil respiration (Pacala and others 2001; Jackson and others 2002; Maestre and others 2009). Important research efforts have been devoted in the last decade to understand the sensitivity of soil respiration to changes in precipitation and temperature in drylands (for example, Conant and others 2000; Han and others 2007; Almagro and others 2009; Zhang and others 2010). Other studies have attempted to elucidate the role of biotic community attributes such as species composition and diversity on soil respiration (Maestre and others 2005, 2009). However, most previous research has neglected the role of biological soil crusts (hereafter BSCs) on soil respiration dynamics (but see Maestre and Cortina 2003; Thomas and others 2008; Thomas and Hoon 2010). BSCs are specialized communities dominated by mosses, lichens, liverworts, cyanobacteria, and other organisms that may constitute as much as 70% of the living cover in dryland ecosystems (Belnap and Lange 2003). They also affect multiple ecosystem functions, including soil stability and erosion (Bowker and others 2008; Chaudhary and others 2009), infiltration and runoff (Belnap 2006; Eldridge and others 2010), nitrogen fixation and cycling (Belnap 2002; Delgado-Baquerizo and others 2010; Maestre and others 2010), and C cycling (Zaady and others 2000; Thomas and others 2008; Grote and others 2010). Indeed, BSCs have recently been suggested as one of the factors responsible for large annual CO2 net uptake rates recorded in desert ecosystems from the USA and China (Wohlfahrt and others 2008; Xie and others 2009).
Previous research on the role of BSCs on the C cycle has focused on measuring net CO2 exchange of particular species or communities under laboratory conditions (for example, Grote and others 2010), or on estimating net CO2 exchange and/or soil respiration in the field over short periods (for example, Maestre and Cortina 2003; Thomas and others 2008; Wilske and others 2008). Although this research has provided valuable insights on the effects of BSCs on C fluxes, the annual extrapolations and predictive models obtained using short-term measurements must be considered with caution given the high spatio-temporal variability in both environmental conditions and BSC distribution typically found in drylands (Lázaro and others 2008; Maestre and Cortina 2002, 2003). Accounting for the spatial heterogeneity in the distribution of BSC-forming organisms is particularly important to reduce the bias of ecosystem-level estimations of soil respiration (Maestre and Cortina 2003). In addition, and given the prevailing effects of variables such as temperature and moisture on the photosynthetic performance of BSC-forming organisms (Lange 2003), investigating how soil respiration in BSC-dominated areas responds to changes in temperature and rainfall is crucial to accurately predict how climate change will impact CO2 fluxes in drylands (Shen and others 2009).
Despite the recognized role of BSCs in the C cycle, and their prevalence in drylands, the effects of BSCs on the spatio-temporal heterogeneity of soil respiration over a multi-year period have not, to our knowledge, been evaluated yet. We aimed to do so in a semiarid Mediterranean steppe dominated by Stipa tenacissima L. These steppes are one of the most important vegetation types in the driest areas of the Mediterranean Basin (see Maestre and others 2009 for an account of their natural history), and their vegetation patterns and ecosystem processes often resemble that of the “tiger-bush” vegetation described in arid and semiarid regions worldwide (Puigdefábregas and others 1999; Valentin and others 1999). The specific objectives of this study were to: (i) quantify the effects of perennial plants and the abundance of BSC patches (measured through changes in cover) on the spatio-temporal heterogeneity of soil respiration; (ii) investigate the relative roles of soil temperature and soil moisture as drivers of this flux; (iii) evaluate the effects of plants and BSCs on the sensitivity of soil respiration to changes in these environmental variables, and (iv) assess the relative contributions of plant- and BSC-dominated areas to the annual amount of C released by soil respiration at the ecosystem level.
Materials and Methods
Site Description
This research was conducted in the Aranjuez Experimental Station, in the center of the Iberian Peninsula (40°02′N–3°37′W; 590 m a.s.l.; 8° slope facing SE). The climate is Mediterranean semiarid, with a 30-year average rainfall and temperature of 388 mm and 13.8°C, respectively, and a pronounced summer drought lasting from June to September. Perennial plant cover is below 30% and is dominated by Stipa (18% of total cover) and the N-fixing shrub Retama sphaerocarpa (L.) Boiss (6% of total cover). The open areas between perennial plants are colonized by well-developed BSCs dominated by lichens such as Diploschistes diacapsis (Ach.) Lumbsch, Squamarina lentigera (Weber) Poelt, Fulgensia subbracteata (Nyl.) Poelt, Toninia sedifolia (Scop.) Timdal, and Psora decipiens (Hedw.) Hoffm. (see Castillo-Monroy and others 2010 for a full species checklist). Bare soil and BSC-dominated areas cover 28 and 32% of the study site, respectively. The soil is derived from gypsum outcrops, which cover 14% of the total surface, and it is classified as Xeric Haplogypsid (Soil Survey Staff, 1994). It is characterized by a fine texture, a high gypsum content, and pH, organic C and total N values ranging between 7.2 and 7.7%, 1 and 3.2%, and 0.2 and 0.4%, respectively; both extremes corresponding to bare ground areas and those under Retama canopies, respectively (0–10 cm; see Castillo-Monroy and others 2010 for details).
Experimental Design
We selected the six most frequent soil cover types (hereafter called microsites) at the study site (Figure 1); Stipa tussocks (ST), Retama shrubs (RS), and open areas with very low (<5%; hereafter BS), low (5–25%; hereafter LC), medium (25–75%; hereafter MC) and high (>75%; hereafter HC) cover of well-developed and lichen-dominated BSCs. ST microsites were selected at the north side of Stipa canopies and are characterized by permanent shade conditions, high litter accumulation, and presence of mosses on the soil surface (mostly Pleurochaete squarrosa [Brid.] Lindb. and Tortula revolvens [Schimp.] G. Roth). RS microsites were placed under the canopy of Retama and are characterized by moderate shade conditions, high litter accumulation and cover of annual plants (particularly during spring), and the occasional presence of mosses. The different BSC-dominated microsites are located in open areas, under full sunlight. Bare soil corresponds to areas where there is neither BSCs (<5%) nor vascular plants.
Measurements of soil respiration, soil moisture, and soil temperature were taken according to a stratified random design. On September 2006, we randomly selected 15 replicated plots (50 × 50 cm) for ST and RS microsites, and 12 for the other microsites (total n = 78). In each plot, we placed a PVC collar (length 10 cm, internal diameter 20 cm) inserted 7 cm into the soil. During the establishment of PVC collars, each plot falling within distances less than 1.5 m of an existing replicate was discarded and reallocated to ensure a minimum distance between replicates of 1.5 m.
Field Measurements
Soil respiration was measured in situ with a portable LI-8100 Automated Soil CO2 Flux System (LI-COR, Lincoln, Nebraska, USA). Measurements were performed monthly between November 2006 (2 months after the collars were inserted to avoid any bias promoted by soil alteration during the placement of collars) and June 2010. To avoid strong diurnal fluctuations, measurements were made between 10.00 and 14.00 h (local time, GTM + 1). This period was considered to give rates of soil respiration that are representative of the average daily value in grasslands (Mielnick and Dugas 2000; Maestre and Cortina 2003; Rey and others 2011). In every survey, half of the replicates were measured in 1 day and the other half were measured on the next day (replicates were measured in a random order each sampling day). We were unable to take measurements between January and June 2008 because of logistic problems (our LI-8100 had to be repaired and calibrated).
In parallel to the measurements of soil respiration, we measured soil temperature with protected diodes buried at 2 cm depth and soil volumetric water content using time-domain reflectometry (TDR; Topp and Davis 1985). For the later measurements, we installed two 5 cm length TDR probes vertically into the soil. We also set up sensors (EC-5, Decagon Devices Inc., Pullman, USA) to continuously monitor soil moisture at 0–5 cm depth in five microsites (ST, RS, BS, MC, and HC), with three replicates per microsite randomly selected. Additional sensors (HOBO® TMC20, Onset Corp., Pocasset, USA) were also installed to continuously monitor soil temperature at 2 cm depth in randomly selected BS and HC microsites. Air temperature and relative humidity were continuously monitored in three microsites (ST, RS, BS) using HOBO® U23 sensors (Onset Corp.). Rainfall and radiation were also monitored using an on-site meteorological station (Onset Corp.). All the continuous measurements were taken using a 15-min interval.
Modeling Soil Respiration
We modeled the dependency of soil respiration (R s) on soil temperature (T) and moisture (W) according to the following relationship:
and
where R s is the measured soil respiration (μmol m−2 s−1), R 0 is the basal respiration at 0°C (μmol m−2 s−1), and T is soil temperature (°C) at 0–2 cm. R 0 and β are estimated parameters. This relationship was fitted separately to all the microsites. The Q 10 value, defined as the increment in soil respiration rate when temperature is increased by 10°C (Luo and Zhou 2006), was used to describe the sensitivity of soil respiration to temperature. For each microsite, a Q 10 value was computed from the monthly measurements of soil respiration and soil temperature. Q 10 is related to the temperature coefficient (β), as follows:
The relationship between R s and soil moisture (W) was determined by fitting a non-linear relationship of the form:
where W is the soil volumetric water content at 0–5 cm depth (%) and Y o, a, b are fitted parameters.
Based on soil temperature (equation (2)) and soil moisture (equation (4)) as driving variables, we developed an empirical model to estimate the annual soil respiration at the ecosystem level. Given the large number of data points gathered throughout the study, and to reduce the noise in the data set used for modeling purposes, we first sorted all the data according to soil temperature and then averaged all the data falling between two consecutive degrees (that is, we obtained a single soil respiration, soil moisture, and soil temperature value from all the data falling between 0 and 1°C, another from all the data falling between 1 and 2°C, and so on). These averages were obtained for each microsite separately and were used to model the relationships between soil respiration, temperature, and moisture. Soil respiration rate was controlled only by soil temperature, according to equation (2), when soil moisture was above 25 and 11% in BS and the other microsites, respectively (see “Effects of soil temperature and soil moisture on soil respiration dynamics” section below). When soil moisture was below these thresholds, which largely occurred in summer (see “Effects of soil temperature and soil moisture on soil respiration dynamics” section below), soil respiration was controlled by soil moisture according to equation (4).
We used temperature and moisture data from our continuous monitoring sensors to get annual estimates of soil respiration. These data were first calibrated with the data obtained from our monthly measurements of soil moisture and temperature (measured with TDR probes and protected diodes, respectively). Soil temperature measured with protected diodes in ST and RS microsites was successfully calibrated with air temperature continuously monitored by U23 sensors, whereas for BS and HC microsites we used soil temperatures measured by TMC20 sensors (see Appendix 1 in Supplementary Material). Soil moisture measured with TDR probes was strongly related to the values measured by EC-5 sensors in all the microsites (Appendix 1). After this calibration, we obtained from the continuous monitoring sensors daily averages of temperature and moisture from the beginning to the end of experiment. Using these data, and equations (2) and (4) according to the soil temperature and soil moisture thresholds mentioned above, we obtained daily estimates of soil respiration fluxes for the four main sources of spatial heterogeneity present in our study area (BS, ST, RS, and HC microsites). Once we get daily soil respiration rates at each microsite, we extrapolated these values to the whole ecosystem by multiplying them by the relative cover of each microsite. Daily soil respiration rates were summed to obtain annual fluxes, and these estimates were used to calculate the amount of C released by soil respiration during the whole year, and during the main wet (October–May) and dry (June–September) seasons. It must be noted that for given periods of the year, particularly when soil respiration responds strongly to temperature (see “Effects of soil temperature and soil moisture on soil respiration dynamics” section below), the daily value of soil respiration may be overestimated by assuming that midday values are representative of average daily respiration rates. However, and given that during a good part of the year soil respiration is strongly limited by soil moisture (see “Effects of soil temperature and soil moisture on soil respiration dynamics” section below), and that we are using estimations based on daily averages of soil moisture and temperature data (and not only from the middle part of the day), we feel that the annual fluxes estimated are probably a good estimate and that they are not strongly biased or overestimated.
Statistical Analyses
To examine whether soil respiration, soil temperature, and soil moisture differed between microsites, we used a two-way (Microsite and Time) ANOVA, with repeated measures of one of the factors (Time). When significant microsite effects were found (P < 0.05), the Tukey HSD post hoc test was employed to evaluate differences between microsites. Prior to these analyses, data were tested for assumptions of normality and homogeneity of variances and were log-transformed when necessary. Regression analyses were used to examine the relationships between soil respiration and the different environmental variables as explained above. To compare the Q 10 values between microsites, we calculated the standard error of the parameter β and its confidence intervals, assuming that differences between Q 10 values are significant when these intervals do not overlap (Zar 1999). All the ANOVA and regression analyses were performed using the SPSS 15.0 statistical software (SPSS Inc., Chicago, Illinois, USA).
Results
Spatio-Temporal Variation in Soil Temperature, Soil Moisture and Soil Respiration
Both soil temperature and soil moisture varied markedly with season and year (Figure 2A, B). Seasonal changes in soil temperature followed the same overall pattern in the different microsites sampled, albeit slight differences between some of them were found (repeated measures [RM] ANOVA; F Time × Microsite = 3.54, df = 170, 2448, P < 0.001). Over the studied period, soil temperature was significantly lower in the microsites dominated by vascular plants than in the other microsites (RM ANOVA; F Microsite = 20.67, df = 5,72, P < 0.001; Figure 2A). Temporal variations in soil moisture showed some differences between microsites, particularly during periods of high water availability after major rainfall events (Figure 2B; Appendix 2 in Supplementary Material; RM ANOVA; F Time × Microsite = 5.57, df = 150, 1980, P < 0.001). Throughout the study period, soil moisture was consistently higher in the BS than in the other microsites, whereas the lowest values were found under the canopy of vascular plants (Figure 2B, RM ANOVA; F Microsite = 24.53, df = 5, 66, P < 0.001).
Spatio-temporal variation of soil temperature (2 cm depth, A), volumetric soil moisture (0–5 cm depth, B), and soil respiration rate (C) between November 2006 and May 2010 in the study area. Data represent means ± SE (n = 12–15). Shared letters after the key to symbol indicate no significant differences among microsites (repeated measures ANOVA, Tukey HSD test, P < 0.05). ST—Stipa tenacissima tussocks; RS—Retama sphaerocarpa shrubs; BS—bare soil; LC—low-biological soil crust (BSC) cover; MC—medium BSC cover; HC—high BSC cover.
Overall, soil respiration in all microsites varied during the year according to changes in soil temperature during the winter and early spring, and to variations in soil moisture during the rest of the year (Figure 2C). However, differences in the magnitude of the responses to changes in soil temperature and soil moisture were found between microsites (RM ANOVA, F Time × Microsite = 2.61, df = 160, 2144, P < 0.001). Over the whole studied period, soil respiration rates in the RS and BS microsites were significantly higher and lower, respectively, than in any other microsite (RM ANOVA, F Microsite = 24.18, df = 5,67, P < 0.001). Soil respiration did not differ between the BSC-dominated microsites (LC, MC and HC), but was significantly higher and lower than that found in BS and RS/ST microsites, respectively.
Effects of Soil Temperature and Soil Moisture on Soil Respiration Dynamics
The average Q 10 value found in our study was 2.2, albeit important differences between microsites were detected. Q 10 values were higher in the ST and RS microsites than in the rest of microsites (Figure 3). The Q 10 value obtained at the BS was the lowest of all the microsites sampled. This parameter showed a trend to increase with increases in BSC cover, Q 10 values of MC and HC microsites being significantly higher than those observed in LC and BS microsites (Figure 3).
Relationships between soil respiration rate and soil temperature when soil moisture was above 25 and 11% in bare soil areas and the rest of microsites, respectively. Values in parentheses indicate the confidence intervals of Q 10. Differences in the number of data points used in each graph are due to variations in the ranges of soil temperature found between microsites. Note also the differences in scale of the x axes.
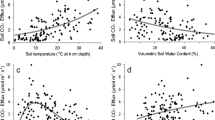
Soil respiration was exponentially related to soil temperature when soil moisture was higher than 11% in microsites dominated by vascular plants or BSCs and 25% in BS microsites (Figure 3). Below these soil moisture levels, soil respiration was not related to soil temperature and was driven by variations in soil moisture alone according to a quadratic relationship (Figure 4). The models fitted accounted for a substantial part of the variation in soil respiration rates (more than 80% in ST, RS, and HC microsites, and between 50 and 80% in the rest of microsites; Figures 3, 4).
Relationships between soil respiration rate and moisture when soil temperature was above 18, 12, and 25°C in biological soil crust (BSC), vascular plants, and bare soil microsites, respectively. Differences in the number of data points used in each graph are due to changes in the ranges of soil moisture found between microsites. Note also the differences in scale of the x and y axes. SM—soil moisture.
Microsite Contribution to Annual Soil Respiration Fluxes
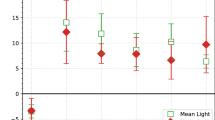
Using the threshold values of soil moisture corresponding to each microsite, we developed an empirical model using soil temperature and soil moisture to predict annual soil respiration rates. This simple model successfully predicted measured soil respiration rates, explaining over 85% of their temporal variation (Appendix 3 in Supplementary Material). Using this model, we obtained an approximate estimate of the total amount of C released at the Aranjuez site via soil respiration of 859.3 g C m−2 over the 3.5 years studied (Figure 5). This amount changed during this period and varied from 240.4 g C m−2 in 2007 to 322.6 g C m−2 in 2009. The amount of C released with soil respiration in wet periods was gradually higher from 2007 to 2009, whereas that measured during dry periods was quite heterogeneous. The contribution of each microsite to the C released by soil respiration of the whole ecosystem was, on average, 36.9, 20.4, and 42.6% for microsites dominated by vascular plants (ST and RS), bare soil and BSCs, respectively. Microsites dominated by BSCs were the main contributor to this flux in both wet and dry seasons.
Relative contribution of Retama sphaerocarpa (RS), Stipa tenacissima (ST), bare soil (BS), and biological soil crust (BSC) microsites to the total amount of carbon released by soil respiration throughout the study period, both in dry (D) and wet (W) seasons. Results in 2006 represent only data from November and December; those from 2010 are calculated using data from January to May.
Discussion
Spatio-Temporal Heterogeneity of Soil Respiration
Highly contrasting inter-annual and seasonal patterns of soil respiration were observed in the study area. These observations are not new, because previous studies conducted in drylands have shown similar patterns (for example, Han and others 2007; Almagro and others 2009). However, and despite its recognized importance for ecosystem functioning, small-scale spatial variation of soil respiration beyond the vegetated patches-interspaces dichotomy has rarely been described in dryland ecosystems (but see Maestre and Cortina 2003). Such variation is expected because of the highly heterogeneous distribution of vegetation, soil resources, microorganisms, and other surface features relevant to soil respiration (Schlesinger and others 1996; Maestre and Cortina 2002; Maestre and others 2009). Our results highlight the role of BSC-dominated microsites as a key factor introducing such heterogeneity.
Soil respiration was higher in microsites dominated by vascular plants than in open and BSC-dominated areas. This result is in agreement with other studies carried out in dryland ecosystems throughout the world, which have found higher soil respiration beneath plant patches (Maestre and Cortina 2003; Han and others 2007; Sponseller 2007; Cable and others 2008; Almagro and others 2009; but see Rey and others 2011). Such differences are not surprising given the differences in root biomass (Davidson and others 1998), organic matter (Reynolds and others 2007, Maestre and others 2001), microbial abundance (for example, Kieft 1991; Gallardo and Schlesinger 1992), and biological activity (Stubbs and Pyke 2005) typically found between plant patches and interspace soils. Interestingly, soil respiration was higher in RS than in ST microsites, despite the lack of differences in soil temperature and soil moisture between them. In our study area, RS microsites have higher organic C, total N, and in situ NO3 − availability than ST microsites (Castillo-Monroy and others 2010). These differences in soil fertility, together with the larger amounts of roots (Puigdefábregas and others 1999) and mycorrhizal propagules (Azcón-Aguilar and others 2002) typically found under Retama canopies compared to Stipa, and the higher litter quality of the former (for example, Maestre and Cortina 2006; Querejeta and others 2003), can explain the results observed.
It is interesting to note the strong differences in soil respiration between open and BSC-dominated areas, despite both microsites being devoid of vascular plants. These results agree with what has been reported by a previous short-term study carried out in a Stipa steppe from SE Spain (Maestre and Cortina 2003). These differences cannot be explained by differences in environmental conditions between microsites, as soil temperature did not differ among them and soil moisture was lower in BSC-dominated microsites (which should have promoted lower respiration rates during dry periods, according to our empirical model). Over the study period, soil respiration did not differ between BSC-dominated microsites, regardless of the strong differences in lichen cover. These results suggest that respiration of the lichens per se is not the main driver of the differences in soil respiration observed between BSC and BS microsites. Hence, differences in soil fertility induced by BSCs (organic and total N were higher in areas with BSCs; Castillo-Monroy and others 2010) or changes in the microbial and microfaunal populations associated with BSC-forming organisms (Housman and others 2007; Darby and others 2010; Castillo-Monroy and others 2011), could be potential explanations for the results obtained. In this direction, the lack of significant differences in soil respiration between BSC microsites (LC, MC, and HC) throughout the study period could be explained by the lack of differences in soil fertility between them (variables like organic C and total N did not significantly differ between them; Castillo-Monroy and others 2010). However, our experimental design and measurements do not allow us to know the specific mechanisms underlying the results, which need to be explored in future studies.
Controls on Soil Respiration
The relative importance of soil temperature and soil moisture as drivers of soil respiration in dryland ecosystems has been well studied during the last decade (for example, Casals and others 2000; Frank and others 2002; Conant and others 2000; Almagro and others 2009). In our study, soil respiration patterns closely tracked soil temperature when soil moisture was not a limiting factor for plants and microorganisms (mainly during winter, and parts of spring and autumn), and followed changes in soil moisture during the warmest periods of the year, when soil moisture was lowest. Our results are consistent with those of Fernandez and others (2006), as they found that both factors were important drivers of soil respiration and that low soil moisture limited the response of soil respiration to increments in soil temperature, despite working in a cold desert with different environmental conditions (for example, coarse texture, lower precipitation and temperature averages). Similar results have been obtained by previous studies conduced in S. tenacissima steppes (Rey and others 2011) and other dryland ecosystems (Conant and others 2004; Almagro and others 2009; Thomas and others 2008; Thomas and Hoon 2010), suggesting that threshold responses to variations in temperature and moisture are common in these environments. Thus, approaches to predict changes in soil respiration based solely on a continuous and predictable response to soil temperature, still commonly employed by global ecosystem models (for example, Lloyd and Taylor 1994; Chen and Tian 2005), are unlikely to be accurate when applied in drylands.
Although most of the variability in soil respiration could be explained by soil moisture limitations in all microsites, these restrictions acted in differing ways depending on the microsite considered. To our knowledge, the large differences between microsites in the soil moisture thresholds at which temperature becomes the main driver of soil respiration (11 vs. 25% in vegetated or BSC-covered vs. bare soils, respectively) have not been reported before. Although our experimental design does not allow us to disentangle the particular mechanisms behind these results, they provide additional evidence of the role of BSCs as modulators of soil respiration responses to temperature and moisture.
The average Q 10 value found in our study (2.2) is similar to the Q 10 value found in other ecosystems (Raich and Schlesinger 1992; Rey and others 2002; Jarvis and others 2007). However, we found a large variability in Q 10 values (from 1.5 to 4.1) depending on the microsite considered. According to the Q 10 values found, soil respiration was more sensitive to changes in soil temperature in the RS than in any other microsite. The differences in Q 10 values found in BS and HC microsites were statistically significant with a clear trend toward increasing with increases in BSC cover (the Q 10 value in HC microsites was a 20% higher than that of BS microsites). These results suggest that microsites with high cover of BSC were more sensitive to changes in soil temperature than open areas. Grote and others (2010) suggested that temperature increases of 3–5°C are unlikely to affect gross photosynthesis both in cyanobacteria- and lichen-dominated BSCs from the Chihuahuan desert and the Colorado Plateau. Our results indicate that soil respiration rates of BSC-dominated areas are likely to increase under future climatic conditions. Even if photosynthetic rates of BSC constituents are not modified by the predicted increase in temperature, as suggested by Grote and others (2010), our results indicate that such changes will likely reduce the ability of BSC microsites to become a net carbon sink because of increased soil respiration.
Relative Contributions of BSCs and Vascular Plants to Total Soil Respiration at the Ecosystem Level
We found that BSC-dominated microsites were responsible for 42% of the total amount of C emitted to the atmosphere through soil respiration during the studied period, whereas microsites dominated by vascular plants (RS + ST) provided 36% of this amount. Even though the soil respiration rate of BSC-dominated microsites was much lower than those of areas covered with vascular plants, the dominance of BSCs at the study area made them the main contributor to the total C released. It is worth noting the large differences in the relative contribution to this flux between BSC and BS microsites despite a similar cover in our study area (32 vs. 28%). Our findings highlight the importance of considering the different sources of spatial heterogeneity in soil cover to accurately estimate soil respiration at the ecosystem level in drylands.
Although more attention is being paid to the role of BSCs as a modulator of soil respiration (Maestre and Cortina 2003; Thomas and others 2008; Thomas and Hoon 2010), most previous studies on the role of BSCs in the C cycle have focused on their photosynthetic capacity (see Lange 2003 for a review) and their importance as a net CO2 sink in drylands (for example, Zaady and others 2000; Wilske and others 2008; Wohlfhart and others 2008). Our results emphasize the importance of BSCs as a modulator of another key component of the C cycle in drylands, soil respiration, and indicate that estimates of the role of these organisms in the global C cycle, which are largely based on photosynthesis measurements (for example, Elbert and others 2009), may be taken with caution because of the large soil respiratory fluxes found in BSC-dominated areas. In this direction, previous studies have failed to detect net CO2 fixation in crusted soils dominated by cyanobacteria in sandy soils from the Kalahari Desert (Thomas and Hoon 2010), a response likely observed because the C uptake by photosynthetic organisms is masked by respiration from other heterotrophic BSC components. Ongoing measurements of net CO2 uptake at our study site are revealing that both BSC- and BS-dominated microsites can show net CO2 uptake during particular and short moments of the year (mostly during early morning when there is high air humidity or after low-intensity rainfall events; J. L. Quero and F. T. Maestre, unpublished data). These short periods of net uptake are not properly accounted for by our modeling approach, and thus our estimations of the total amount of soil C released by BSC-dominated and BS microsites may be slightly over-estimated at some moments of the year. However, and given that these events are short, and net respiration is found during most of the day (and most of the days) at our study area, we do not feel that our annual soil respiration totals are over estimated. The conditions under which BSCs behave as a net sink or source of C are largely unknown, as the isolation of net photosynthesis from the background soil respiration under field conditions is quite challenging. What our results suggest is that the future increase in temperature expected with ongoing climate change may exacerbate soil respiration in BSC-dominated microsites, a response that could shift these areas from a net C sink to a net C source. Given the dominance of these organisms, such a response could have profound consequences for the C budget of drylands ecosystems worldwide.
Concluding Remarks
Our results indicate that accounting for the spatial heterogeneity in soil respiration induced by BSCs is highly relevant to provide an accurate estimation of this key component of the C cycle at the ecosystem level. Estimations based only on vegetated and bare soils have been used to predict carbon budgets in temperate (Kim and others 1992) and semi-arid (Rey and others 2011) grasslands. However, the large differences found in soil respiration between BSC-dominated areas and those devoid of them suggest that such approaches can largely underestimate soil respiration in heterogeneous semiarid environments containing BSCs. Our findings also highlight that BSCs are the main contributor to the total C released by soil respiration and therefore must be taken into account when estimating C budgets at the ecosystem level. Given the extent of dryland ecosystems worldwide, and the large areas covered by BSCs, not only in drylands, but also in other temperate, polar, and alpine ecosystems (Belnap and Lange 2003), the explicit consideration of BSC-dominated microsites in future empirical and modeling studies may greatly contribute toward advancing our knowledge of the global C cycle and to better predict the effects of global environmental change on soil respiration.
References
Almagro M, López J, Querejeta JI, Martínez-Mena M. 2009. Temperature dependence of soil CO2 efflux is strongly modulated by seasonal patterns of moisture availability in a Mediterranean ecosystem. Soil Biol Biochem 41:594–605.
Azcón-Aguilar C, Palenzuela J, Roldan A, Bautista S, Vallejo VR, Barea JM. 2002. Analysis of the mycorrhizal potential in the rhizosphere of representative plant species from desertification-threatened Mediterranean shrublands. Appl Soil Ecol 21:1–9.
Belnap J. 2002. Nitrogen fixation in biological soil crusts from southeast Utah, USA. Biol Fertil Soils 35:128–35.
Belnap J. 2006. The potential roles of biological soil crusts in dryland hydrologic cycles. Hydrol Process 20:3159–78.
Belnap J, Lange OL. 2003. Biological soil crusts: structure, function and management. Berlin: Springer.
Bond-Lamberty B, Thomson A. 2010. A global database of soil respiration data. Biogeosciences 7:1915–26.
Bowker MA, Belnap J, Chaudhary VB, Johnson NC. 2008. Revisiting classic water erosion models in drylands: the strong impact of biological soil crust. Soil Biol Biochem 40:2308–16.
Cable JM, Ogle K, Williams DG, Weltzin J, Huxman TE. 2008. Soil texture drives responses of soil respiration to precipitation pulses in the Sonoran Desert: implications for climate change. Ecosystems 11:961–79.
Casals P, Romanyà J, Cortina J, Bottner P, Couteaux MM, Vallejo VR. 2000. CO2 efflux from a Mediterranean semi-arid forest soil. I. Seasonality and effect of stoniness. Biogeochemistry 48:261–81.
Castillo-Monroy AP, Maestre FT, Delgado-Baquerizo M, Gallardo A. 2010. Biological soil crusts modulate nitrogen availability in semi-arid ecosystems: insights from a Mediterranean grassland. Plant Soil 333:21–34.
Castillo-Monroy AP, Bowker MA, Maestre FT, Rodríguez-Echeverría S, Martínez I, Barraza-Zepeda CE, Escolar C. 2011. Relationships between biological soil crusts, bacterial diversity and abundance, and ecosystem functioning: insights from a semi-arid Mediterranean environment. J Veg Sci 22:165–74.
Chaudhary VB, Bowker MA, O’Dell TE, Grace JB, Redman AE, Rillig MC, Johnson NC. 2009. Untangling the biological contributions to soil stability in semiarid shrublands. Ecol Appl 19:110–22.
Chen H, Tian H-Q. 2005. Does a general temperature-dependent Q10 model of soil respiration exist at biome and global scale? J Integr Plant Biol 47:1288–302.
Conant RT, Klopatek JM, Klopatek CC. 2000. Environmental factors controlling soil respiration in three semiarid ecosystem. Soil Sci Soc Am J 65:383–90.
Conant RT, Dalla-Betta P, Klopatek CC, Klopatek JM. 2004. Controls on soil respiration in semiarid soils. Soil Biol Biochem 36:945–51.
Darby BJ, Neher DA, Belnap J. 2010. Impact of biological soil crusts and desert plants on soil microfaunal community composition. Plant Soil 328:421–31.
Davidson EA, Belk E, Boone RD. 1998. Soil water content and temperature as independent or confounded factor controlling soil respiration in a temperate mixed hardwood forest. Glob Change Biol 4:217–27.
Delgado-Baquerizo M, Castillo-Monroy AP, Maestre FT, Gallardo A. 2010. Plants and biological soil crusts modulate the dominance of N forms in a semi-arid grassland. Soil Biol Biochem 42:376–8.
Elbert W, Weber B, Büdel B, Andreae MO, Pöschl U. 2009. Microbiotic crusts on soil, rock and plant: neglected major players in the global cycles of carbon and nitrogen? Biogeosci Discuss 6:6983–7015.
Eldridge DJ, Bowker MA, Maestre FT, Alonso P, Mau RL, Papadopulos J, Escudero A. 2010. Interactive effects of three ecosystem engineers on infiltration in a semi-arid Mediterranean grassland. Ecosystems 13:499–510.
Fernandez DP, Neff JC, Belnap J, Reynolds RL. 2006. Soil respiration in the cold desert environment of the Colorado Plateau (USA): abiotic regulators and thresholds. Biogeochemistry 78:247–65.
Frank A, Liebig MA, Hanson JD. 2002. Soil carbon dioxide fluxes in northern semiarid grasslands. Soil Biol Biochem 34:1235–41.
Gallardo A, Schlesinger WH. 1992. Carbon and nitrogen limitations of soil microbial biomass in desert ecosystems. Biogeochemistry 18:1–17.
Grote EE, Belnap J, Housman DC, Sparks JP. 2010. Carbon exchange in biological soil crust communities under differential temperatures and soil water content: implications for global change. Glob Change Biol 16:2763–74.
Han G, Zhou G, Xu Z, Yang Y, Liu J, Shi K. 2007. Biotic and abiotic factors controlling the spatial and temporal variation of soil respiration in an agricultural ecosystem. Soil Biol Biochem 39:418–25.
Housman DC, Yeager CM, Darby BJ, Sanford RL Jr, Kuske CK, Neher DA, Belnap J. 2007. Heterogeneity of soil nutrients and subsurface biota in a dryland ecosystem. Soil Biol Biochem 39:2138–49.
Jackson RB, Banner JL, Jobbagy EG, Pockman WT, Wall DH. 2002. Ecosystem carbon loss with woody plant invasion of grasslands. Nature 418:623–6.
Jarvis P, Rey A, Petsikos C, Wingate L, Rayment M, Pereira J, Banza J, David J, Miglietta F, Borghetti M, Manca R, Valentini R. 2007. Drying and wetting of Mediterranean soil stimulate decomposition and carbon dioxide emission: the “Birch effect”. Tree Physiol 27:929–40.
Kieft TL. 1991. Soil microbiology in reclamation of arid and semi-arid land. In: Skujins J, Ed. Semiarid land and desert: soil resource and reclamation. New York: Marcel Dekker. p 209–57.
Kim J, Verma SB, Clements RJ. 1992. Carbon dioxide budget in a temperate grassland ecosystem. J Geogr Res 97:6057–63.
Körner Ch. 2000. Biosphere responses to CO2 enrichment. Ecol Appl 10:1590–619.
Lange OL. 2003. Photosynthesis of soil-crust biota as dependent on environmental factors. In: Belnap J, Lange OL, Eds. Biological soil crusts: structure, function, and management. Berlin: Springer. p 217–40.
Law BE, Kelliher FM, Baldocchi DD, Anthoni PM, Irvine J, Moore D, Van Tuyl S. 2001. Spatial and temporal variation in respiration in a young ponderosa pine forest during a summer drought. Agric For Meteorol 110:27–43.
Lázaro R, Canton Y, So-lé-Benet A, Bevan J, Alexander C, Sancho L, Puigdefábregas J. 2008. The influence of competition between lichen colonization and erosion on the evolution of soil surfaces in the Tabernes badlands (SE Spain) and its landscape effects. Geomorphology 102:252–66.
Lloyd J, Taylor JA. 1994. On the temperature dependence of soil respiration. Funct Ecol 8:315–23.
Luo Y, Zhou X. 2006. Soil respiration and the environment. Burlington: Academic Press.
Maestre FT, Cortina J. 2002. Spatial patterns of surface soil properties and vegetation in a Mediterranean semi-arid steppe. Plant Soil 241:279–91.
Maestre FT, Cortina J. 2003. Small-scale spatial variation in soil CO2 efflux in a Mediterranean semiarid steppe. Appl Soil Ecol 23:199–209.
Maestre FT, Cortina J. 2006. Ecosystem structure and soil surface conditions drive the variability in foliar δ13C and δ15N of Stipa tenacissima in semiarid Mediterranean steppes. Ecol Res 21:44–53.
Maestre FT, Bautista S, Cortina J, Bellot J. 2001. Potential of using facilitation by grasses to establish shrubs on a semiarid degraded steppe. Ecol Appl 11:1641–55.
Maestre FT, Escudero A, Martínez I, Guerrero C, Rubio A. 2005. Does spatial pattern matter to ecosystem functioning? Insights from biological soil crusts. Funct Ecol 19:566–73.
Maestre T, Bowker MA, Puche MD, Hinojosa MB, Martínez I, García-Palacios P, Castillo AP, Soliveres S, Luzuriaga AL, Sánchez AM, Carreira JA, Gallardo A, Escudero A. 2009. Shrub encroachment can reverse desertification in Mediterranean semiarid grasslands. Ecol Lett 12:930–41.
Maestre FT, Bowker MA, Escolar C, Soliveres S, Mouro S, García-Palacios P, Castillo AP, Martínez I, Escudero A. 2010. Do biotic interactions modulate ecosystem functioning along abiotic stress gradients? Insights from semi-arid Mediterranean plant and biological soil crust communities. Philos Trans R Soc B 365:2057–70.
Mielnick PC, Dugas WA. 2000. Soil CO2 flux in a tallgrass prairie. Soil Biol Biochem 32:221–8.
Pacala SW, Hurtt GC, Baker D, Peylin P, Houghton RA, Birdsey RA, Heath L, Sundquist ET, Stallard RF, Ciais P, Moorcrof P, Caspersen JP, Shevliakova E, Moore B, Kohlmaier G, Holland E, Gloor M, Harmon HE, Fan SM, Sarmiento JL, Goodale CL, Schimel D, Field CB. 2001. Consistent land- and atmosphere-based US carbon sink estimates. Science 292:2316–20.
Puigdefábregas J, Solé-Benet A, Gutierrez L, Del Barrio G, Boer M. 1999. Scales and processes of water and sediment redistribution in drylands: results from the Rambla Honda field site in Southeast Spain. Earth-Sci Rev 48:39–70.
Querejeta JI, Barea JM, Allen M, Caravaca F, Roldán A. 2003. Differential response of d13C and water use efficiency to arbuscular mycorrhizal infection in two aridland woody plant species. Oecologia 135:510–15.
Raich JW, Schlesinger WH. 1992. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus 44B:81–99.
Raich JW, Potter CS, Bhawagati D. 2002. Interannual variability in global soil respiration, 1980–1994. Glob Change Biol 8:800–12.
Rey A, Pegoraro E, Tedeschi V, De Parri I, Jarvis PG, Valentini R. 2002. Annual variation in soil respiration and its components in a coppice oak forest in Central Italy. Glob Change Biol 8:581–866.
Rey A, Pegoraro E, Oyonarte C, Were A, Escribano P, Raimundo J. 2011. Impact of land degradation on soil respiration in a steppe semiarid ecosystem. Soil Biol Biochem 43:393–403.
Reynolds JF, Stafford Smith M, Lambin EF, Turner BLII, Mortimore M, Batterbury SPJ, Downing TE, Dowlatabadi H, Fernandez RJ, Herrick JE, Huber-Sannvald E, Leemans R, Lynam T, Maestre FT, Ayarza M, Walker B. 2007. Global desertification: building a science for dryland development. Science 316:847–51.
Schlesinger WH, Raikes JA, Hartley AE, Cross AF. 1996. On the spatial pattern of soil nutrients in desert ecosystems. Ecology 77:364–74.
Shen W, Reynolds JF, Hui D. 2009. Responses of dryland soil respiration and soil carbon pool size to abrupt versus gradual and individual versus combined changes in soil temperature, precipitation, and atmospheric [CO2]: a simulation analysis. Glob Change Biol 15:2274–94.
Soil Survey Staff. 1994. Keys to soil taxonomy, 6th edn. Blacksburg: USDA Soil Conservation Service, Pocahontas Press.
Sponseller RA. 2007. Precipitation pulses and soil CO2 flux in Sonoran desert ecosystem. Glob Change Biol 13:426–36.
Stubbs MM, Pyke DA. 2005. Available nitrogen: a time-based study of manipulated resource islands. Plant Soil 270:123–33.
Thomas AD, Hoon SR. 2010. Carbon dioxide fluxes from biologically-crusted Kalahari Sands after simulated wetting. J Arid Environ 74:131–9.
Thomas AD, Hoon SR, Linton PE. 2008. Carbon dioxide fluxes from cyanobacteria crusted soils in the Kalahari. Appl Soil Ecol 39:254–63.
Topp GC, Davis JL. 1985. Measurement of soil water content using time-domain reflectometry (TDR): a field evaluation. Soil Sci Soc Am J 49:19–24.
Valentin C, d’Herbès JM, Poesen J. 1999. Soil and water components of banded vegetation pattern. Catena 37:1–24.
West NE, Stark JM, Johnson DW, Abrams MM, Wight JR, Heggem D, Peck S. 1994. Effects of climatic-change on the edaphic features of arid and semiarid lands of western north-America. Arid Soil Res Rehabil 8:307–75.
Wilske B, Burgheimer J, Karnieli A, Zaady E, Andreae MO, Yakir D, Kesselmeier J. 2008. The CO2 exchange of biological soil crusts in a semiarid grass-shrubland at the northern transition zone of the Negev desert, Israel. Biogeosciences 5:1411–23.
Wohlfahrt G, Fenstermaker LF, Arnone JAIII. 2008. Large annual net ecosystem CO2 uptake of a Mojave Desert ecosystem. Glob Change Biol 14:1475–87.
Xie JX, Li Y, Zhai CX, Lan Z. 2009. CO2 absorption by alkaline soil and its implication to the global carbon cycle. Environ Geol 56:953–61.
Zaady E, Kuhm U, Wilske B, Sandoval-Soto L, Kesselmeier J. 2000. Patterns of CO2 exchange in biological soil crusts of successional age. Soil Biol Biochem 32:959–66.
Zar JH. 1999. Biostatistical analysis. 4th edn. New Jersey: Prentice Hall.
Zhang LH, Chen YN, Zhao RF, Li WH. 2010. Significance of temperature and soil water content on soil respiration in three desert ecosystems in Northwest China. J Arid Environ 74:1200–11.
Acknowledgements
We thank C. Escolar, R. Mau, M. Carpio, E. Pigem, P. Alonso, C. Alcalá, J. Papadopoulos, P. Izquierdo, J. Gutiérrez, M. Méndez, R. Jiménez, A. Escudero, I. Martínez, and I. Conde for their help in laboratory and in the fieldwork, and R. Lázaro, J. Cortina, M. Goberna, A. Escudero, L. García-Sancho, J. Neff, and two anonymous referees for comments on a previous version of the manuscript. We thank the Instituto Madrileño de Investigación y Desarrollo Rural, Agrario y Alimentario (IMIDRA) for access to the Aranjuez Experimental Station (Finca de Sotomayor). APC, SS, and PGP were supported by Ph.D. fellowships from the INTERCAMBIO (BIOCON06/105) and EXPERTAL grants, funded by the Fundación BBVA and Fundación Biodiversidad/CINTRA S.A., respectively. This research was funded by an Early Career Project Grant from the British Ecological Society (ECPG 231/607), by the Spanish Ministry of Science and Innovation (grant CGL2008-00986-E/BOS), by the INTERCAMBIO grant, and by the European Research Council under the European Community’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement no. 242658 (BIOCOM).
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
FTM conceived the study; APCM, FTM, SS and PGP performed the research; APCM and AR analyzed the data; and APCM and FTM wrote the paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Castillo-Monroy, A.P., Maestre, F.T., Rey, A. et al. Biological Soil Crust Microsites Are the Main Contributor to Soil Respiration in a Semiarid Ecosystem. Ecosystems 14, 835–847 (2011). https://doi.org/10.1007/s10021-011-9449-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-011-9449-3