Abstract
Ecosystem respiration (ER) is an important but poorly understood part of the carbon (C) budget of peatlands and is controlled primarily by the thermal and hydrologic regimes. To establish the relative importance of these two controls for a large ombrotrophic bog near Ottawa, Canada, we analyzed ER from measurements of nighttime net ecosystem exchange of carbon dioxide (CO2) determined by eddy covariance technique. Measurements were made from May to October over five years, 1998 to 2002. Ecosystem respiration ranged from less than 1 μmol CO2 m−2 s−1 in spring (May) and fall (late October) to 2–4 μmol CO2 m−2 s−1 during mid-summer (July-August). As anticipated, there was a strong relationship between ER and peat temperatures (r2 = 0.62). Q10 between 5° to 15°C varied from 2.2 to 4.2 depending upon the choice of depth where temperature was measured and location within a hummock or hollow. There was only a weak relationship between ER and water-table depth (r2 = 0.11). A laboratory incubation of peat cores at different moisture contents showed that CO2 production was reduced by drying in the surface samples, but there was little decrease in production due to drying from below a depth of 30 cm. We postulate that the weak correlation between ER and water table position in this peatland is primarily a function of the bog being relatively dry, with water table varying between 30 and 75 cm below the hummock tops. The dryness gives rise to a complex ER response to water table involving i) compensations between production of CO2 in the upper and lower peat profile as the water table falls and ii) the importance of autotrophic respiration, which is relatively independent of water-table position.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Northern peatlands occupy about 3.5 million km2 and contain up to one-third of the world’s soil carbon (C) pool (Gorham 1991). These peatlands have accumulated C through an imbalance between plant uptake of atmospheric CO2 by photosynthesis and the release of CO2 to the atmosphere through plant respiration and the decomposition of organic matter. The C budget of these systems under a changing climate will depend on the relative response of plant productivity and the rate at which organic matter decomposes, with thermal and hydrologic regimes being of critical importance.
Ecosystem respiration (ER) is the emission of carbon dioxide to the atmosphere from vegetation (autotrophic) and soil (heterotrophic) activities. Compared to photosynthetic uptake, ER is a poorly understood and modeled part of the ecosystem C budget (Giardina and Ryan 2000; Grace and Rayment 2000; Melillo and others 2002). Although thermal and hydrologic regimes should play a role in determining ER in all ecosystems (Reichstein and others 2003), there are conflicting conclusions about the relative importance of these two environmental controls in peatlands. Although most studies find temperature to be an important control on peatland ER, the influence of soil moisture or water-table depth (WTD) on ER is less clear. Periodic chamber-based measurements of small areas have shown that both peat temperature and WTD are important controls on ER, with their relative importance being dependent on the vegetation, mircrotopography and temperature range. Bridgham and Richardson (1992) made monthly measurements of soil CO2 emission in three southern peatlands with clipped vegetation in the chambers and showed that temperature was more important than WTD, although the latter was significantly correlated to soil CO2 emission. In Finland, Silvola and others (1996a) determined soil CO2 emission from 26 sites and noted that temperature played an important role in controlling emission rates at many sites. They also found that when standardized for temperature, a fall in water-table position resulted in an increase in emission, but that the relative increase was reduced when the water table fell below 30 cm. For a number of boreal peatland types Bubier and others (1998) concluded that trophic status, above ground net primary productivity, and soil temperature were more important than water table in determining ER. Although water table was of minor importance in predicting daily ER, it became more important in predicting seasonal average ER at these sites. In an experimental manipulation of peat temperature and WTD in a bog and a fen, Updegraff and others (2001) showed that ER responded to temperature increases but not to a variation in water-table position of 20 cm. At six sites in the Mer Bleue bog over two summers, Bubier and others (2003a) found that WTD was strongly appelated with ER during a dry summer, but that temperature was more important during a wet summer.
Laboratory incubations are more consistent in their findings than field experiments. In such studies, peat samples or mesocosms of reconstituted peat are subjected to controlled and manipulated temperature and moisture, and the rates of CO2 production are monitored. Moisture is normally treated in terms of flooded and drained (water table held at depth below the surface) conditions. There is general agreement that CO2 emissions increase with increasing temperature and are higher under drained than flooded conditions (for example, see Bridgham and Richardson 1992; Moore and Dalva 1993; Funk and others 1994; Moore and Dalva 1997; Scanlon and Moore 2000; Waddington and others 2001).
Chamber and laboratory studies are limited because of infrequent measurements over short sampling periods and small areas, disturbance of the ecosystem, and in many cases only soil CO2 emissions are measured and not ER. Eddy covariance measurements from towers provide an alternative, allowing an assessment of CO2 flux under undisturbed conditions with intact vegetation and at larger spatial scales – that is, footprints of several hundreds to thousands of square meters. Despite some issues surrounding sampling under stable atmospheric conditions, carefully analyzed nighttime eddy covariance measurements can be used to estimate ER (for example, see Hollinger and others 1994; Law and others 1999; Valentini and others 2000). Eddy covariance measurements, when collected over a long period, can capture fluxes from a large combination of environmental controls and antecedent conditions and at a larger spatial scale that are difficult if not impossible to obtain in manipulation or chamber-based studies. Very few peatland studies employing the eddy covariance technique have analyzed ER measurements (see Discussion).
In this paper, we investigate ER during the growing season (May through October) at a large ombrotrophic bog as determined by nighttime net ecosystem CO2 exchange measurements from an eddy covariance tower. Data from five years were available, providing a wide range of environmental conditions and antecedent controls influencing ER. Specifically, we examine relationships between ER and the peat thermal regime and water-table position. These results, combined with laboratory incubation of intact peat samples from the bog under varying moisture content, show that the lowering of the water table accompanied by a drying of the surface layers results in the conclusion that ER is independent of water-table position in this relatively dry bog.
METHODS
Site
The measurements were made at Mer Bleue, a large (2800 ha), low-shrub ombrotrophic bog located just east of Ottawa, Ontario, Canada (45.40°N lat., 75.50°W long.). The climate of this area is cool continental, with a mean annual temperature of 5.8°C and mean annual precipitation of 910 mm. Average May to October temperature and precipitation are 15.5°C and 491 mm respectively. The dominant vegetation types are evergreen shrubs (Chamaedaphne calyculata, Ledum groenlandicum, Kalmia angustifolium), a deciduous shrub (Vaccinium myrtilloides) and a continuous ground cover of Sphagnum mosses (S. capillifolium, S. fuscum, S. magellanicum). In 1998, total aboveground and belowground biomass averaged 356 ± 100 and 1820 ± 660 g m−2 for vascular species and 144 ± 30 g m−2 for the Sphagnum capitula (Moore and others 2002). See Moore and others (2002) and Bubier and others (2003a) for further details on ecological characteristics of the site. The peat thickness exceeds 5 m in the central portion of the bog. The surface has a typical hummock-hollow micro-topography.
Ecosystem Respiration Measurements
The overall CO2 exchange and eddy covariance measurements employed at Mer Bleue have been discussed in detail by Lafleur and others (2001, 2003) and modeled by Frolking and others (2002). Here we give a brief description of the instruments and data processing used in support of the current analysis. A 3-dimensional sonic anemometer (R2 Solent, Gill Instruments, UK) and closed-path infrared gas analyzer (IRGA, LI-6262, Li-COR, NE, USA) were used to measure rapid (10 Hz) fluctuations in vertical wind speed and CO2 concentration. Eddy fluxes of CO2 were computed from 30-min covariances of these quantities after accounting for the travel time for the sample air between the intake (located near the anemometer) and the IRGA located in a heated shed at the base of the instrument tower. Air was drawn down the sampling tube and through the IRGA at a rate of 6.5–8.5 L min−1. The sonic anemometer and intake for CO2 sampling were mounted at 3.2 m above the bog surface. Post processing of the eddy flux data included corrections for changes in air density due to water vapour fluctuations.
Continuous measurements of several environmental variables were also conducted during the study. Incident photosynthetically active radiation (PAR) was measured with a quantum sensor (model QZ190, LI-COR, NE, USA). Air temperature and humidity were measured at 2.0 m with a probe (model HMP35C, Campbell Scientific, UT, USA). Wind speed at 2.0 m was measured with a cup anemometer (model 20120, R.M. Young, MI, USA). Two arrays of copper-constantan thermocouples were installed to measure soil temperatures at depths of 1, 5, 10, 20, 40, 60, 80, 150, and 250 cm. One array was placed in a hummock and one in a hollow. Precipitation was measured on site with a tipping bucket gauge (model 525 m, Campbell Scientific) during the snow-free period only. Winter precipitation was obtained from a near-by weather station. Water level was measured in two wells, one in a hollow and one in a hummock, with a float and counterweight attached to a potentiometer. In addition, frequent manual measurements were made on both wells. All water level measurements were referenced to the mean height of the hummock surfaces. We also measured soil moisture content with a profile of TDR probes (model CS615, Campbell Sci.). We could not establish a satisfactory calibration for peat soil, but the TDR probes do give an indication of the relative magnitude of changes in soil moisture for a given change in water table at a specific depth. Signals from all of the sensors were monitored on a CR7X and a CR10X data logger every 5 s and averaged every 30 min.
Long-term records of precipitation and air temperature are not available for the Mer Bleue site. To determine the variance of the five years of Mer Bleue data against a longer term record we used precipitation and air temperature recorded at the Ottawa International Airport, 10 km southwest of Mer Bleue.
Flux Data Handling
Flux data were subjected to a series of screening procedures. In the first pass, we discarded data during periods of instrument malfunction (such as during heavy rain) or out of range values. The second screening related to problems arising from eddy covariance theory. Nighttime eddy covariance data continue to be the subject of considerable debate (Aubinet and others 2000; Baldocchi and others 2001). Low wind speeds, thermal stratification of the atmosphere, and incomplete mixing that often occur at night can generally result in inaccurate flux measurements. A common practice used to filter ‘bad’ data is to plot flux data against friction velocity (u*) and then assess a u* cut-off limit below which data are rejected (Goulden and others 1996, 1997). In an earlier paper we examined this problem for the Mer Bleue flux system and determined that the critical value u* was 0.1 m s−1 (Lafleur and others 2001). Thus, all CO2 fluxes were discarded when u* was less than 0.1 m s−1. Data loss from all sources accounted for between 33% and 44% of all possible values (on an annual basis).
ER was determined from nighttime flux data obtained during May to October for the years from 1998 to 2002, inclusive. Nighttime was defined as the period when incident PAR = 0 μmol m−2 s−1. Each night was defined as the dark period centered on 2400 h EST. For all nights with at least six good 30-min flux measurements mean ER was computed as the average of all available measurements. Nighttime mean soil and air temperatures and water-table depths were also computed.
Laboratory Peat Respiration Measurements
The influence of moisture content on CO2 production from peat samples was examined by incubating (at 20°C) replicate, small (5 cm height, 5 cm diameter), intact cores of peat collected from depths of 0–5, 10–15, 20–25, 30–35 and 40–45 cm from a hummock at Mer Bleue. The cores were allowed to dry over 10 days, rewetted to field capacity over 1 h and the drying was repeated for 3 drying cycles over 30 days. Drying occurred from the top and sides of the cores to reduce heterogeneity of water content within the core. CO2 production was determined from changes in the headspace concentration over a 1 h period, by sampling every 15 min. CO2 concentrations were analyzed using a Shimadzu Mini II gas chromatograph equipped with a methanizer. Volumetric moisture content of the cores was determined by changes in mass of the core and its saturated volume.
RESULTS
Growing season (May to October) air temperatures were warmer than the long-term average by 1.3 to 1.8°C in 1998, 2001 and 2002. Air temperatures were near average in 1999 and 2000 (Table 1). Growing season precipitation totals were highly variable, ranging from 60 mm below average in 1998 to 65 mm above average in 2002. Mean growing season water table was near −48 cm in all years, except 2000 when it was −34.8 cm (Table 1).
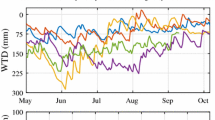
ER followed a roughly symmetrical trend around a broad mid-summer peak in each year, although with high day-to-day variability (Figure 1a). Values ranged from less than 1.0 μmol CO2 m−2 s−1 in the spring (early-May) and autumn (late-October) to 2.0 to 4.5 μmol CO2 m−2 s−1 in mid-summer (July). The 5 cm hummock soil temperatures followed a similar temporal trend as ER, ranging from 0 to 10°C in the spring, to peak values of 12 to 17°C in July and falling to 3 to 7°C in October (Figure 1b). The same pattern of soil temperatures occurred at depths of −10 and −20 cm but the seasonal amplitude was reduced. Water table was variable in all years (Figure 1c). Based on previous studies the seasonal pattern in water table is driven by the distribution of precipitation relative to the loss of water by evapotranspiration (Fraser and other 2001). In general, water tables were highest in spring and autumn with periods of drawdown occurring in summer. The year with the shallowest (least negative) WTD and the smallest daily variation was 2000, as a result of consistent rainfall in all months. Reduced July/August precipitation in both 2001 and 2002 (40% and 51% below the long-term average) caused prolonged water table drawdowns to develop by mid-summer, lasting approximately 40 and 65 days respectively. The early summer decline of the water table each year was at a rate of about −0.4 cm d−1 and the rise in the autumn was at a rate of about 3 cm d−1. When data for all years were pooled, WTD and peat hummock temperature had a weak inverse correlation (r2 = 0.26, p < 0.001). Correlation coefficients for individual years ranged from −0.72 (1999) to 0.22 (2000).
Seasonal trends in A) ecosystem respiration, ER, measured by nighttime eddy covariance measurements, B) nighttime temperature at 5 cm below the hummock surface, and C) water-table depth, WTD, for the period May–October, 1998 to 2002. ER points are mean nighttime fluxes on days with more than six 30-min periods of good data. Soil temperature is nighttime average hummock temperature at −5 cm depth. Daily mean WTD is relative to the hummock surface.
The relationship between the position of the water table and relative water content of the peat above the water table is not constant with depth. Peat 28 cm below the hummock surface dewaters rapidly from about 80 to about 10% once WTD drops below about −33 cm, then decreases marginally with further falls in water table to 70 cm (Figure 2a). In contrast, more decomposed peat 48 cm below the hummock surface loses water very gradually as WTD falls below −50 cm and is still quite wet (50–60% water content) even when the water table is at −70 cm (Figure 2b).
ER was strongly correlated to air and soil temperatures (Table 2, Figures 3a–c). Employing a first order exponential model, explained variance in ER was similar using the air and upper soil profile temperatures (r2 = 0.61 and 0.62 respectively). Explained variance only decreased appreciably when the deeper hollow temperatures were used. Values of the Q10 coefficient generally increased with depth into the soil, and were larger for the peat hollow than for the hummock (Table 2). There was a strong inverse relationship (r2 = 0.81) between the calculated Q10 coefficient and the range of temperature measured at each location (not shown).
Relationship between ER and selected environmental temperatures: A) hummock soil temperature at 5 cm, B) air temperature (0.5 m height), and C) hummock soil temperature at 20 cm. Points are nighttime averages for all years combined. Equation shown is the best-fit exponential relationship. D) Relationship between natural logarithm of ER, ln(ER), with ER in energy units (W ha−1), against inverse temperature (1000/T), T is air temperature at 50 cm height in K. Equation shown is the best-fit linear relationship.
ER data were sorted into shallow (>−40 cm) and deep (<−60 cm) water table groups and exponential relationships with hummock 5 cm temperature were computed (not shown). The goodness of fit was poorer for the deep water table data (r2 = 0.48) than for the shallow (r2 = 0.68), possibly because of the narrow range of temperatures when the water table was deeper. The resulting Q10 quotients were 2.58 (shallow) and 2.02 (deep).
There was a weak (r2 = 0.11), but statistically significant (p < 0.05), relationship between ER and water-table position, with an increase in ER as the water table falls (Figure 4a). When data were sorted into individual years, some years produced significantly better relationships (that is, r21998 = 0.27, r21999 = 0.36, r22001 = 0.35) and others were poorer and insignificant (that is, r22000 = 0.06, r22002 = 0.09). As with the pooled data set, the slope of these relationships was negative except for 2000, which was positive but not statistically significant. It is notable that in 2000 the water table was the highest for all years and showed the least variation over the season, whereas in 2002 the water table was the lowest of the five years. There was no apparent relationship between WTD and the residuals from the ER versus T model (Figure 4b).
The laboratory incubation of intact peat cores with varying moisture content showed that CO2 production rates declined with reduced soil moisture contents (Figure 5). The uppermost samples of peat (0 to 5 cm) had the largest CO2 production rates, on a per mass basis, and the greatest decline as the moisture content fell. There was also an overall decrease in CO2 production rate through the three drying cycles. CO2 production rates were smallest in the lower samples (from 20–25 to 40–45 cm), decreased little as the volumetric moisture content was reduced from 60 to 20% and showed no difference among the three drying cycles.
Relationship between CO2 production during laboratory incubation of intact peat cores, collected from 0 to 45 cm depth at Mer Bleue, as a function of volumetric water content. Three drying cycles for the 0 to 5 cm sample are shown separately; data from the three drying cycles of the deeper samples have been combined into one set. Incubation temperature was 20°C. Lines represent the linear regressions between CO2 production and volumetric water content. Note that these incubation conditions represent drying from field capacity, but not conditions between field capacity and saturation.
DISCUSSION
The strong temperature dependence of ER at the Mer Bleue bog is consistent with findings from field studies that have examined respiration at the plot scale with chambers (Bubier and others 1998; Silvola and others 1996a; Bubier and others 2003a, 2003b). However, we found that near-surface temperatures are better predictors of ER than deeper soil temperatures. Q10 values derived for Mer Bleue ranged from 2.24 to 4.18 depending on the depths of the temperature measurements used in the analyses. Larger Q10 values were associated with locations with a smaller temperature range, which occur deeper in the profile. All values fell within the considerable range in Q10 for peatlands reported in the literature, 1.8–6.1 (Svensson 1980; Chapman and Thurlow 1996; Silvola and others 1996a). We conclude that at least some of the variation in Q10 reported in the literature is likely due to the lack of standardization of the depth at which peat temperature is measured.
Our results suggest that ER was not strongly dependent upon WTD at Mer Bleue bog, despite a considerable range in WTD (−30 to −75 cm). As discussed above most laboratory manipulations on peat soil columns tend to support the notion of a strong dependence of CO2 emission on WTD, whereas field studies with chambers both support (for example, see Silvola and others 1996a; Oechel and others 1998; Bubier and others 2003b) and refute (for example, Bubier and others 1998; Updegraff and others 2001) the argument that WTD strongly influences ER. Previous tower studies employing eddy covariance at peatland sites shed little light on this issue. Most studies offer no detailed analysis of nighttime data and instead model ER (for example, Shurpali and others 1995; Aurela and others 1998, 2001; Soegaard and Nordstoem 1999). A few studies present temperature-nighttime flux relationships, but do not consider the influence of WTD (for example, Neumann and others 1994; Nieveen and others 1998; Arneth and others 2002). One exception is Vourlitis and Oechel (1999), who provide a response surface for ER as a function of temperature and water table in arctic tussock tundra. The lack of ER analysis in previous eddy covariance studies seems to arise from two factors. First, some peatland systems were quite wet (that is, WTD > −10 cm) and WTD does not vary enough to produce an effect on ER (Suyker and others 1997; Nieveen and others 1998; Nordstroem and others 2001). Second, WTD information was only anecdotal and could not be formally treated (Aurela and others 2001; Arneth and others 2002). We suggest that future eddy covariance measurements can provide valuable information on peatland ER, if combined with an appropriate suite of environmental variables.
At the Mer Bleue bog Bubier and others (2003a) measured daytime ER using opaque chambers from June to August 2000 and 2001 at hummock and hollow microhabitats, representing the vegetation and hydrology within the footprint of the flux tower. They found that the bog hummock showed no statistical difference in respiration between the two years, whereas the hollow had significantly greater respiration in the drier year (2001). Linear relationships between ER and WTD were poor (r2 ≤ 0.12) for both microsites in the wet year (2000), but improved markedly (r2 = 0.62 for hummock; r2 = 0.39 for hollow) in the drier year. Combining both years resulted in intermediate values of r2. These values are slightly higher, but in broad agreement with those found in the present study (r2 = 0.06 in 2000, r2 = 0.35 in 2001). Employing multiple regression with various environmental temperatures and water table, Bubier and others (2003a) found that temperature was a stronger determinant of ER than WTD for both hummock and hollow sites.
The lack of a strong ER response to WTD in peatland environments has been reported in other studies (Updegraff and others 2001), but these somewhat counter-intuitive findings are in want of an explanation. We offer the following speculation for the apparent lack of a strong dependency of ER on water table at the Mer Bleue low-shrub bog:
-
1)
Mer Bleue is an inherently dry peatland ecosystem. The 5-year mean growing season WTD was −45 cm below the peat surface of hummocks. Other peatlands studied for CO2 or CH4 exchanges are generally wetter, although Mer Bleue does not appear to be atypical based on a broad survey of peatland water tables in Ontario (for example, Riley 1983). Soil moisture characteristic curves for the surface layers of most peatlands indicate that small changes in water-table elevation within a narrow range can result in large changes in soil moisture and hence air filled porosity; peat water content is relatively insensitive to changing water-table depth outside of this narrow range. This sensitivity was also present at Mer Bleue (Figure 2a). Because WTD was usually below −30 cm (Figure 1c) the surface peat water content was generally low and quite constant. In contrast, the peat water contents are much greater at depth and are less sensitive to changes in WTD (Figure 2b), due to 10 to 1000 fold decrease in hydraulic conductivity and specific yields in the more humified, deeper peat (Letts and others 2000). Our laboratory experiments suggest that CO2 production is greatest and most sensitive to moisture changes in the uppermost portions of the peat profile (Figure 5). Other studies of peatland soils have found similar results (Updegraff and others 1995; Waddington and others 2001). However, because the soil moisture content was relatively invariant in the upper layers, little change in heterotrophic respiration would be expected to result from observed changes in water-table depth.
-
2)
Respiration is dependent upon oxygen availability; thus to increase substantially heterotrophic respiration in the deeper peat layers there would have to be significant oxygen diffusion into those layers. The change of soil moisture at −48 cm indicates that there is only a small increase in air-filled porosity except when the water table is extremely deep, for example, less than −60 cm. Although we are unaware of any studies of oxygen diffusion in peat soils relative to moisture content, in mineral soils at saturation ratios above 0.6 (that is, volume of water/volume of pores), soil respiration becomes oxygen limited (Linn and Doran 1984; Skopp and others 1990). At −28 cm the peat of Mer Bleue seldom reaches saturation ratios of 0.6, but at −48 cm the saturation ratio exceeds 0.6 much of the time. Studies of gas diffusion in soils show that increasing the saturation ratio from 0.6 to 0.9, the range observed at −48 cm, results in a three order of magnitude decrease (10−1 to 10−4 m2/3) in normalized gas permeability (Nazaroff 1992). Thus even with a deep water table (for example, <−60 cm), unsaturated peat in the 40–60 cm depth range is probably not well-oxygenated.
-
3)
A number of studies have argued that the substrate quality in peatlands, upon which decomposition and CO2 production are highly dependent, varies inversely with depth (Updegraff and others 1995; Christensen and others 1999; Waddington and others 2001). Fresh litter and the partially decomposed plant material of the uppermost peat decompose at a faster rate than older peat material lower in the profile. In the upper most peat layers moisture increases respiration, as in the case of our incubation experiments (Figure 5), presumably because of the ready supply of fresh substrate and or nutrient (Skopp and others 1990). The Peat Decomposition Model (Frolking and others 2001), which successfully reproduces peat accumulation over periods of several thousand years, predicts an order of magnitude drop in decomposability due to increasing recalcitrance from the surface to a depth of −40 to −50 cm. Even if this deeper peat is exposed to increasingly oxic conditions, the highly recalcitrant nature of the peat is such that there is little available substrate to decompose. In simulations of the carbon exchanges of Mer Bleue, the Peatland Carbon Simulator (Frolking and others 2002) predicted that there is very little contribution to ER from the peat layers below −35 cm, even when the water table is quite deep.
-
4)
The relationship between ER and water table is not simply dependent on heterotrophic respiration, but also on the response of the living plant respiration to changes in moisture and WTD. The exact proportion of ER attributable to plant respiration at Mer Bleue is unknown, but estimated at about 50% of total ecosystem respiration (Moore and others 2002). Elsewhere it has been estimated that plant roots contributed 35–45% of total soil respiration at a central Finland peatland (Silvola and others 1996b) and a recent model of peatland C exchange estimated autotrophic respiration to be about 50% of total respiration (Frolking and others 2002). This proportioning between autotrophic and heterotrophic respiration is unlikely to be constant, but may vary more with autotrophic production, which would be much less sensitive to changes in water table than to temperature at the leaf surface. Hence a stronger relationship with air temperature and/or the upper most soil temperature is probable. If so, such behavior would contribute to the similar results found for ER versus temperature relationships for the upper soil and air temperatures, as well as the similarity of the relationships for shallow and deep water tables.
The points discussed above suggest some important differences between peatland and forest ecosystems. The extent to which these differences are reflected in terms of ER may be evident in the recent model by Enquist and others (2003). These authors proposed a generalized model of ER based on an analysis of natural logarithm of ER against inverse temperature. The model is based on metabolic kinetics and, as such, ER fluxes usually reported in units of μmol CO2 m−2 s−1 are converted to metabolic energy units of W ha−1 by multiplying by 0.272 J μmol−1 of CO2 and scaling to a hectare (see Methods in Enquist and others 2003). Applying the model to data from Mer Bleue produced an inverse linear relationship between ln(ER) and inverse temperature (Figure 3d), as did the forest ecosystems used in the Enquist analysis. However, the slope (−6.50 W ha−1 K−1) and intercept (30.89 W ha−1) of the Mer Bleue relationship are smaller than those found for the upland ecosystems. As a consequence, the Mer Bleue peatland becomes an apparent outlier (under-predicted) when the predicted ER at 10°, 15° and 20°C is compared to values from other sites plotted against latitude (see Figure 2b in Enquist and others 2003). This may represent limitations in resource supply at Mer Bleue, such as poor nutrient availability and/or the behavior of an ecosystem with a large anaerobic sub-environment.
CONCLUSION
Growing season ecosystem respiration in a dry ombrotrophic bog was strongly correlated with peat temperature and very weakly correlated with water-table depth. As explained by the four points given above, we believe that the relationship between ER and WTD is the result of complex but predictable interactions among WTD, vertical profiles of peat water content in the unsaturated zone above the water table, and vertical profiles of peat decomposability. A natural hypothesis derived from this reasoning would be that wetter peatlands should have a stronger ER sensitivity to WTD than observed in this study (provided WTD falls below the peat surface). A second hypothesis is that peatland ER will not follow a generalized model of ER such as proposed by Enquist and others (2003). Eddy covariance measurements of ER would be a useful platform for testing both these hypotheses.
Currently, much of our current understanding of ER comes from forest environments, as evidenced by two recent attempts to produce generalized models of ER (or soil respiration) from existing data sets (Enquist and others 2003; Reichstein and others 2003). Peatland ecosystems have received much less attention than upland ecosystems; thus no comparable modeling synthesis exists. Predicting how the C balance of peatlands will respond to anticipated climatic change requires a process-level understanding of how C cycles through peatlands, mapping of the spatial distribution of relevant peatland characteristics, and the ability to predict how climatic change will impact peatland hydrology and water-table depth.
References
Arneth A, Kurbatova J, Kolle O, Shibistrova OB, Lloyd J, Schulze E-D. 2002. Comparative ecosystem-atmosphere exchange of energy and mass in a European Russian and a central Siberian bog II. Interseasonal and interannual variability of CO2 fluxes. Tellus B 54:514–30
Aubinet M and others. 2000. Estimates of annual net carbon and water exchange of European forests: The EUROFLUX methodology. Adv Ecol Res 30:113–75
Aurela M, Tuovinen J-P, Laurila T. 1998. Carbon dioxide exchange in a subarctic peatland ecosystem in northern Europe measured by eddy covariance technique. J Geophys Res 103:11289–301
Aurela M, Laurila T, Tuovinen J-P. 2001. Seasonal CO2 balances of a subarctic mire. J Geophys Res 106:1623–37
Baldocchi DD, others. 2001. Fluxnet: A new tool to study the temporal and spatial variability of ecosystem-scale carbon dioxide, water vapor and energy flux densities. Bull Amer Meteorol Soc 82:2415–34
Bridgham SD, Richardson CJ. 1992. Mechanisms controlling soil respiration (CO2 and CH4) in southern peatlands. Soil Biol Biochem 24:1089–99
Bubier JL, Crill PM, Moore TR, Savage K, Varner RK. 1998. Seasonal patterns and controls on net ecosystem CO2 exchange in a boreal peatland complex. Global Biogeochem Cycles 12:703–14
Bubier JL, Bhatia G, Moore TR, Roulet NT, Lafleur PM. 2003a. Spatial and temporal variability in growing season net ecosystem carbon dioxide exchange at a large peatland in Ontario, Canada. Ecosystems 6:353–67
Bubier J, Crill P, Mosedale A, Frolking S, Linder E. 2003b. Peatland responses to varying interannual moisture conditions as measured by automatic CO2 chambers. Global Biogeochem Cycles 17:doi:10.1029/2002GB0019
Chapman SJ, Thurlow M. 1996. The influence of climate on CO2 and CH4 emissions from organic soils. Agric For Meteorol 79:205–17
Christensen TR, Jonasson S, Callaghan TV, Harstrom M. 1999. On potential CO2 release from tundra soils in a changing climate. Appl Soil Ecol 11:127–34
Enquist BJ, Economo EP, Huxman TE, Allen AP, Ignace DD, Gillooly JF. 2003. Scaling metabolisms from organisms to ecosystems. Nature 423:639–42
Fraser CJD, Roulet NT, Lafleur PM. 2001. Groundwater flow patterns in a large peatland. J Hydrol 246:142–54
Frolking S, Roulet NT, Moore TR, Richard PJH, Lavoie M, Muller SD. 2001. Modelling northern peatland decomposition and peat accumulation. Ecosystems 4:479–98
Frolking S, Roulet NT, Moore TR, Lafleur PM, Bubier JL, Crill PM. 2002. Modeling the seasonal to annual carbon balance of Mer Bleue bog, Ontario, Canada. Global Biogeochem Cycles 16:doi:10.1029/2001GB1457
Funk DW, Pullman ER, Peterman KM, Crill, Billings WD. 1994. Influence of water table on carbon dioxide, carbon monoxide, and methane fluxes from taiga bog microcosms. Global Biogeochem Cycles 8:271–78
Giardina CP, Ryan MG. 2000. Evidence that decomposition rates of organic carbon in mineral soil do not vary with temperature. Nature 404:858–61
Gorham E. 1991. Northern peatlands: Role in the carbon balance and probable responses to climatic warming. Ecol Appl 1:182–95
Goulden ML, Munger JW, Fan SM, Daube BC, Wofsy SC. 1996. Measurement of carbon storage by longterm eddy correlation: Methods and a critical assessment of accuracy. Global Change Biol 2:169–82
Goulden ML, Daube BC, Fan S-M, Sutton DJ, Bazzaz A, Munger JW, Wofsy SC. 1997. Physiological response of black spruce forest to weather. J Geophys Res 102:28987–96
Grace J, Rayment M. 2000. Respiration in the balance. Nature 404:819–20
Hollinger DY, Kelliher FM, Byers JN, Hunt JE, McSeveny TM, Weir PL. 1994. Carbon dioxide exchange between an undisturbed old-growth temperate forest and the atmosphere Ecology 75:134–50
Lafleur PM, Roulet NT, Admiral SW. 2001. Annual cycle of CO2 exchange at a bog peatland. J Geophys Res 106:3071–81
Lafleur PM, Roulet NT, Bubier JL, Frolking S, Moore TR. 2003. Interannual variability in the peatland-atmosphere carbon dioxide exchange at an ombrotrophic bog. Global Biogeochem Cycles 17:doi:10.1029/2002GB001983
Law BE, Ryan MG, Anthoni PM. 1999. Seasonal and annual respiration of a ponderosa pine ecosystem. Global Change Biol 5:169–82
Letts MG, Roulet NT, Comer NT, Skarupa MR, Verseghy DL. 2000. Parameterization of peatland hydraulic properties for the Canadian Land Surface Scheme. Atmosphere-Ocean 38:141–60
Linn DM, Doran JW. 1984. Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and non-tilled soils. Soil Sci Soc Am J 48:1267–72
Melillo JM, Steudler PA, Aber JD, Newkirk K, Lux H, Bowles FP, Catricala C, Magill A, Aherns T, Morrisseau S. 2002. Soil warming and carbon-cycle feedbacks to the climate system. Science 298:2173–76
Moore TR, Dalva M. 1993. The influence of temperature and water table position on carbon dioxide and methane emissions from laboratory columns of peatland soils. J Soil Sci 44:651–64
Moore TR, Dalva M. 1997. CH4 and CO2 exchange potentials of peat soils in aerobic and anaerobic laboratory incubations. Soil Biol Biochem 29:1157–64
Moore T, Bubier J, Lafleur P, Frolking S, Roulet N. 2002. Plant biomass, production and CO2 exchange in an ombrotrophic bog. J Ecol 90:25–36
Nazaroff WW. 1992. Radon transport from soil to air. Rev Geophys 30:137–60
Neumann HH, den Hartog G, King KK, Chipanshi AC. 1994. Carbon dioxide fluxes over a raised open bog at the Kinosheo Lake tower site during the Northern Wetlands Study (NOWES). J Geophys Res 99:1529–38
Nieven JP, Jacobs CMJ, Jacobs AFG. 1998. Diurnal and seasonal variation of carbon dioxide exchange from a former true raised bog. Global Change Biol 4:823–50
Nordstroem C, Soegaard H, Christensen TR, Friborg T, Hansen BU. 2001. Seasonal carbon dioxide balance and respiration of a high-arctic fen ecosystem in NE-Greenland. Theor Appl Climatol 70:149–66
Oechel WC, Voulitis GL, Hastings SJ, Ault RP Jr, Bryant P. 1998. The effect of water table manipulation and elevated temperature on the CO2 flux of wet sedge tundra ecosystems. Global Change Biol 4:77–90
Reichstein M and others. 2003. Modeling temporal and large-scale spatial variability of soil respiration from soil water availability, temperature and vegetation productivity indices. Global Biogeochem Cycles 17:doi:10.1029/2003GB002035
Riley J. 1983. Peatland Inventory Project. Geological Survey, Ontario Ministry of Natural Resources, Miscellaneous Paper 119, Toronto, Ontario, Canada
Scanlon D, Moore TR. 2000. CO2 production from peatland soil profiles: the influence of temperature, oxic/anoxic conditions and substrate. Soil Sci 165:153–60
Shurpali NJ, Verma SB, Kim J, Arkebauer TJ. 1995. Carbon dioxide exchange in a peatland ecosystem. J Geophys Res 100:14319–26
Silvola J, Alm J, Ahlholm U, Nykänen H, Martikainen PJ. 1996a. CO2 fluxes from peat in boreal mires under varying temperature and moisture conditions. J Ecol 84:219–28
Silvola J, Alm J, Ahlholm U, Nykänen H, Martikainen PJ. 1996b. The contribution of plant roots to CO2 fluxes from organic soils. Biol Fert Soils 23:126–31
Skopp J, Jawson MD, Doran JW. 1990. Steady-state aerobic microbial activity as a function of soil water content. Soil Sci Soc Am J 54:1619–25
Soegaard H, Nordstroem C. 1999. Carbon dioxide exchange in a high-arctic fen estimated by eddy covariance measurements and modelling. Global Change Biol 5:547–62
Suyker AE, Verma SB, Arkebauer TJ. 1997. Season-long measurement of carbon dioxide exchange in a boreal fen. J Geophys Res 102:29021–28
Svensson BH. 1980. Carbon dioxide and methane fluxes from the ombrotrophic parts of a subarctic mire. Ecol Bull Stockholm 30:235–50
Updegraff K, Pastor J, Bridgham SD, Johnston CA. 1995. Environmental and substrate controls over carbon and nitrogen mineralization in northern wetlands. Ecol Appl 5:151–63
Updegraff K, Bridgham SD, Pastor J, Weishampel P, Harth C. 2001. Response of CO2 and CH4 emissions in peatlands to warming and water table manipulation. Ecol Appl 11:311–26
Valentini R, Matteucci G, Dolman AJ, Schulze E-D, Rebmann C, others. 2000. Respiration as the main determinant of carbon balance in European forests. Nature 404:861–65
Vourlitis GL, Oechel WC. 1999. Eddy covariance measurements of net CO2 and energy fluxes of an Alaskan tussock tundra. Ecology 80:686–701
Waddington JM, Rotenberg PA, Warren FJ. 2001. Peat CO2 production in a natural and cutover peatland: Implications for restoration. Biogeochemistry 54:115–30
Acknowledgements
This project has been supported by funds from the NSERC Strategic Grant Program, NSERC Discovery Grants Program, the NSERC/CFCAS/BIOCAP Canada Fluxnet Canada Research Network, and the NASA Terrestrial Ecology and EOS Interdisciplinary Science Programs (to SF). We thank the National Capital Commission for permission to use Mer Bleue and Gershon Rother for his assistance over the last five years. We thank Adina Gillespie for laboratory assistance and Jill Bubier for insightful comments and suggestions. The authors are grateful to two anonymous reviewers and Ecosystems subject editor Dr. G. Shaver for their helpful suggestions on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lafleur, P., Moore, T., Roulet, N. et al. Ecosystem Respiration in a Cool Temperate Bog Depends on Peat Temperature But Not Water Table. Ecosystems 8, 619–629 (2005). https://doi.org/10.1007/s10021-003-0131-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-003-0131-2