Abstract
An easily prepared biosensor based on reduced graphene oxide (rGO) and glucose oxidase (GOx) enzyme was developed to monitor the enzymatic hydrolysis process of the second-generation (2G) ethanol process from green coconut biomass. The rGO-GOx biocomposite that modified a glassy carbon (GC) electrode was characterized by morphological, electrochemical and spectrophotometric techniques showing that the GOx enzyme was immobilized on the rGO. The parameters for glucose determination were optimized by square wave voltammetry (SWV). The developed biosensor was applied for the determination of glucose during the enzymatic hydrolysis step, showing that the process can be stopped with 12 h of reaction. Thus, an important achievement of this analysis is the reduced time to get a valuable result for the test, saving time and reducing the cost of the 2G ethanol process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Brazil has a large cultivable land area, being one of the countries with the highest amount of biomass, being the fourth largest producer of coconut in the world. The green coconut (Cocus nucifera L.) has a high biomass content; 80 to 95% of the fruit is disposable and not eatable, being responsible for 70% of the garbage generated in Brazilian’s beaches [1, 2]. Coconut lignocellulosic biomass has drawn the attention of researchers due to its application in technologies for renewable fuel production. Coconut fiber is one of the most abundant residues of lignocellulosic material; therefore, the coconut husk presents itself as a biomass that can be used for the production of second-generation ethanol (2G ethanol), and moreover, it is a low-cost raw material [3].
First-generation ethanol (1G ethanol) is derived from the fermentation of sugars from sugarcane or starch, which are also sources of food, while 2G ethanol is derived from the fermentation of sugars obtained from lignocellulosic biomass, reusing residues [4]. In general, the raw materials used in the production of 2G ethanol are agricultural, industrial and urban waste and wood originating from cultivated forests. In this context, coconut fiber is a waste generated in high quantity and presents as a potential biomass for the production of 2G ethanol [5].
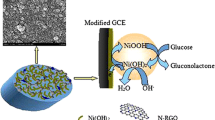
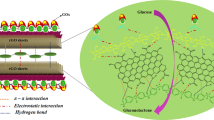
Monitoring glucose levels during alcoholic fermentation is one of the most important key factors to achieve successful ethanol production. The excess of the substrate can inhibit the enzymatic hydrolysis by glucose oxidase enzyme (GOx), which is an oxirreductase enzyme that catalyzes the oxidation of β-D-glucose using molecular oxygen as an electron acceptor, generating hydrogen peroxide (H2O2) which can be quantified by electrochemical techniques according to eq. 1 and 2 [6].
The amount of glucose during fermentation process to generate alcohol is a crucial step. Chromatographic, spectrophotometric, colorimetric and enzyme-based protocols have been well established for monitoring glucose. However, these techniques offer some limitations and expensive materials, such as clean-up steps, high quality solvents, solid phase extraction and trained staff. To overcome such limitations, electrochemical biosensors based on glucose oxidase have been extensively investigated due to its low-cost, easy preparation and reproducibility [7]. Due to its outstanding properties, graphene and graphene-like materials are coming to the forefront of electrochemical biosensors research. High electronic motility (about 200,000 cm2 V−1 s−1), good biocompatibility and large specific surface area make these materials interesting choices for enzyme immobilization. Reduced graphene oxide (rGO) is a graphene-like material that has been extensively used in the preparation of electrochemical biosensors. Several reports demonstrate the easy enzyme immobilization of different enzymes, such as laccase [8], acetylcholinesterase [9] and GOx [10, 11] by physical entrapment in rGO structural defects or direct covalent bond in residual carbonyl groups [12,13,14,15,16].
Samphao et al. [7] investigated a GOx-based biosensor using a core shell Fe3O4@Au nanoparticles for detection of glucose with a LOD of 0.1 mmol L−1, being successfully applied for honey wine monitoring. Pakapongpan et al. [17] developed a biosensor using rGO modified with Fe3O4 nanoparticles for GOx immobilization; the proposed biosensor detected glucose levels ranging 0.05 to 1 mmol L−1 with a LOD of 0.1 μmol L−1. There are several reports about glucose monitoring, precisely those aiming for food quality control. However, monitoring glucose levels in fermentation process for fuel production is a novelty approach for enzymatic biosensors.
In that way, this paper presents for the first time an enzymatic biosensor based on GOx immobilized on rGO substrate, for detection of glucose during the enzymatic hydrolysis of 2G ethanol production from green coconut biomass. The electrochemical behavior of the proposed biosensor as well as the viability for process monitoring is discussed in detail.
Methodology
Chemicals and solutions
All solutions were prepared with water purified from a Millipore ultrapure water system with resistivity ≥ 18 MΩ cm (Millipore). All reagents used in this study were of analytical grade and were used without further purification. Graphene oxide, glucose oxidase from Aspergillus niger Type X-S, lyophilized powder, 100,000–250,000 units/g solid (without added oxygen) and glucose were purchased from Sigma-Aldrich (Germany).
Apparatus
Cyclic voltammetry (CV) and square-wave voltammetry (SWV) experiments were performed using a model PGSTAT128N Autolab electrochemical system (Metrohm) equipped with NOVA 2.0 software (Metrohm). The cell was assembled conventionally with a three-electrode electrochemical system: bare GC, GC/rGO or GC/rGO-GOx as the working electrode (diameter: 3 mm); Ag/AgCl/KCl (3.0 mol L−1) as the reference electrode; and a Pt plate as the auxiliary electrode. All experiments were carried out at a controlled temperature (25 ± 1 °C).
Electrochemical experiments
Electrochemical characterization of the GC/rGO/GOx electrode was performed using CV in 0.2 mol L−1 phosphate buffer solution (PBS) pH 7.0 with a scan rate of 50 mV s−1 and in 0.1 mol L−1 KCl solution (pH 7.4) containing 5 mmol L−1 [Fe(CN)6]3−/4− with a scan rate of 50 mV s−1. The EIS spectra were scanned from 107 to 10−2 Hz frequency range and 10 mV amplitude, with 20 data points per frequency decade. The impedance spectra was recorded in open circuit potential (OCP) conditions in 0.1 mol L−1 KCl solution (pH 7.4) containing 5 mmol L−1 [Fe(CN)6]3−/4−. Fitting and calculation to equivalent electrical circuit, Rs, CPE, α and Rct values were performed using electrochemical circle fit tool in Nova 2.0 software. The surface morphology of each material was characterized by scanning electron microscopy (SEM) and the images were recorded using a model Quanta 200 (FEI Company, Hillsboro, USA). The ultraviolet-visible (UV-Vis) spectroscopy characterization was performed in a spectrophotometer BEL Photonics UV-M51 model.
Fabrication of GC/rGO-GOx biosensor
Reduced graphene oxide was synthesized by a simple two-step chemical reduction using SDS and NaBH4, as described elsewhere [8, 18, 19]. Prior to electrode modification, the GC was polished with an alumina slurry solution (0.05 μm). Then, the electrodes were sonicated for 5 min in ethanol and 5 min in ultrapure water. A suspension containing 25 μg/mL of rGO and 3 mg/mL of glucose oxidase was prepared. A 10-μL aliquot of the rGO-GOx composite dispersion was dropped onto the GC electrode surface and dried at approximately 4 °C in the refrigerator for 2 h until an adsorbed film was observed in the electroactive area.
Second-generation ethanol production
Collection and processing of Cocus nucifera L.
The Cocus nucifera L. husk was collected manually in the premises of the Sao Paulo State University/School of Agricultural Science (UNESP/FCA). The husks were rinsed with water, then manually cut into small pieces that were oven dried (Imarvil) at 60 °C for 7 days. After drying, the coconut was ground in a Willey-type knife mill (Marconi) with a 20 mesh sieve (0.841 mm).
Biomass pretreatment
The pretreatment was conducted in combination of mechanical and chemical steps. For this purpose, 75 g of biomass was placed in two reaction flasks, each receiving 375 mL of NaOH (17.5% v/v). The mechanical stirrer was inserted into the reaction medium and operated for 20 min at a speed of 1500 rpm. The biomass was vigorously rinsed with tap water in a 200 mesh sieve (0.074 mm) for the removal of any chemical residue.
Enzymatic hydrolysis
For the enzymatic hydrolysis step, 25 Erlenmeyers were placed each with 2.5 g of biomass (dry weight) and 100 mL of deionized water. Prior to hydrolysis reaction, the pH of the reaction was measured and corrected to values between 4.8 and 5.0 (pH-meter Lucadema, model: mPA210), with citric acid (C6H8O7) or sodium hydroxide (NaOH) solutions, both prepared at 1.0 M concentration. In order to minimize contamination, a sterilization step was applied by autoclaving at 121 °C for 15 min at a pressure of 1.1 kgf/cm2. With bioreactors at room temperature, 1.5 mL of commercial Novozymes enzyme (CellicCTec3) was inoculated into the reaction medium under sterile conditions. The procedure was followed by incubation of the bioreactors in a shaker incubator (SOLAB, model: SL-222) at 50 °C, with rotation of 150 rpm. Different reaction times were studied to assure maximum yield, as following: 3, 6, 12, 24 and 48 h. After incubation process, the bioreactors were placed in a thermostatic water bath (Ethik Technology, model: 500-1D) at 100 °C for 10 min to inactivate the enzymes. Finally, the hydrolyzed solutions were filtered in a vacuum pump (Biothec) using 12-mm diameter filters, and the liquids were kept at − 20 °C.
Glucose analysis
The developed biosensor was applied for the determination of glucose during the enzymatic hydrolysis step of 2G ethanol production. For this purpose, the reactions times of 3, 6, 12, 24 and 48 h of the hydrolysis reaction were analyzed in triplicate using the standard addition method.
Results and discussion
Morphological, electrochemical and spectroscopical characterization of the biomaterial
Scanning electron microscopy (SEM) experiments were used for the characterization of the proposed biosensor and the micrographs are presented in Fig. 1. Figure 1a displays images of rGO, in which the material displays a wrinkled structure with plenty of corrugations. On the other hand, Fig. 1b shows the images of the rGO/GOx biocomposite, where it is possible to observe the immobilization of the glucose oxidase onto rGO due to its structural defects. These clusters can incorporate and immobilize enzymes, providing an excellent material for biosensing measurements since no cross-linking agent is needed [8], facilitating the preparation process of the biosensor and minimizing the blocking of the enzymes active site.
CV experiments in the presence of the [Fe(CN)6]3−/4− redox couple were also performed for the electrochemical characterization of the materials. Figure 2A shows the voltammetric profiles for the three different electrodes: (a) GC/GO, (b) CG/rGO and (c) GC/rGO/GOx in 0.2 mol L−1 PBS pH 7.4, 0.1 mol L−1 KCl containing 5.0 mmol L−1 of the redox couple [Fe(CN6)]3−/4− with a scan rate of 50 mV s−1. The voltammetric profiles show well-defined oxidation and reduction peaks for the GC/rGO (curve b) and GC/rGO-GOx (curve c) electrodes due to the Fe3+/Fe2+ redox couple in the potentials of + 0.36 V and + 0.10 V (curve b) and in the potentials of + 0.48 V and − 0.06 V (curve c), respectively. The GC electrode modified with GO (curve a) also showed oxidation and reduction peaks due to the Fe3+/Fe2+ redox couple in the potentials of + 0.29 V and + 0.13 V, respectively. However, the GC/rGO electrode (curve b) showed a 2.5-fold increase in the peak current compared to the GC/GO electrode (curve a). This increase is due to the presence of defects introduced in its structure, as well less oxygen atoms increase the electron transport. Compared to GC/rGO-GOx biosensor, the GC/rGO electrode showed a 1.2-fold increase in the peak current. Through the reduction in peak currents in the GC/rGO-GOx biosensor, it is possible to identify the immobilization of the enzyme, since it reduces the number of active sites that act on the redox couple electrochemical process [Fe(CN)6]3−/4−.
A CV experiments for the electrodes: (a) GC/GO, (b) CG/rGO and (c) GC/rGO-GOx in 0.2 mol L−1 PBS pH 7.4, 0.1 mol L−1 KCl containing 5.0 mmol L−1 of the redox couple [Fe(CN6)]3−/4− with a scan rate of 50 mV s−1. B Nyquist diagram for the electrodes: (a) GC/GO ( ), (b) GC/rGO (
), (b) GC/rGO ( ) and (c) GC/rGO-GOx (■) in the same solution described above. In detail: equivalent electric circuit
) and (c) GC/rGO-GOx (■) in the same solution described above. In detail: equivalent electric circuit
The electrochemical enhanced properties of GO and rGO were also verified using EIS in order to quantify the charge transfer resistance (Rct) values for the electrode process. Figure 2B displays the Nyquist plots for GC/GO, GC/rGO and GC/rGO-GOx electrodes in the presence of 5.0 mmol L−1 of the redox couple. Nyquist plots were used to analyze EIS data and presented with the equivalent circuit inset. The EIS plots exhibited Rs for GC/GO about 49.3 Ω, which was larger than GC/rGO (46.9 Ω). The fitted value of Rct obtained for GC/GO and GC/rGO was 1.52 kΩ and 2.84 kΩ, respectively. The value of Rs was 55.0 Ω for GC/rGO-GOx and the fitted value of Rct obtained was 3.20 kΩ, indicating a decrease in the electron transfer showing that the enzyme was immobilized. The lower value of Rct for rGO was also observed in previous publications, indicating the improvement in electron transfer of this material [20, 21]. This behavior is in agreement with CV experiments that rGO has a higher peak current response for the redox couple.
The UV-Vis spectroscopy was also used to characterize the materials. This is a physical technique used in the study of electronic transitions of molecules in colloidal solutions and biological molecules, but it can also be used in the quantitative analysis of diluted absorbent species concentration. In Fig. 3, the characterization of GO spectrum shows characteristic absorption bands at 230 nm and 300 nm. The absorption at 230 nm corresponds to the transitions π → π* of the aromatic rings bonds C=C, already at 300 nm the absorption corresponds to the transitions of type n → π* of the connections C=O. In the rGO spectrum, it is possible to observe a displacement of the absorption peak of 230 nm to 260 nm, which indicates that the crystalline structure of the oxidized graphene was partially reconstituted and the disappearance of the peak at 300 nm demonstrates that there was a deoxygenation of the graphene. In the rGO-GOx biocomposite spectrum, it is possible to observe peaks at 210 nm, 275 nm, 380 nm and 450 nm. These last two are related to the electronic transitions type π → π* along the three cycles of the isoaloxazine ring present in the FAD portion incorporated in each monomer of the enzyme, and the peak at 275 nm is related to the presence of the aromatic amino acid chain of GOx.
Cyclic voltammetry was applied on the evaluation of direct electrochemistry of the proposed biosensor. Figure 4 shows the voltammetric profiles for the two different electrodes: (a) GC/rGO and (b) GC/rGO-GOx in 0.2 mol L−1 nitrogen-saturated PBS pH 7.0 with scan rate of 50 mV s−1. The electrochemical characterization brings evidence that the enzyme was immobilized in the material of interest. No electrochemical process was observed in the voltammetric response using the GC/rGO electrode (curve a). However, the GC electrode modified with the rGO-GOx composite showed oxidation and reduction peaks at − 0.40 V and − 0.45 V, respectively. The rGO-GOx shows well-defined redox couple with a peak to peak separation (∆Ep) of 50 mV. The low ∆Ep value indicates a fast electron transfer process. These electrochemical processes are in agreement with other works described in the literature [10, 11].
Optimization of biosensor analysis and composition parameters
The effect of enzyme concentration on the GC/rGO surface was investigated for sensitive electrochemical detection of glucose by SWV, in 0.2 mol L−1 nitrogen-saturated PBS pH 7.0 as presented in Fig. 5. The amount of GOx was optimized in the range of 0.2 to 4.0 mg mL−1 and the SWV experiment was recorded in the potential range of + 1.0 to − 1.0 V, with amplitude of 25 mV and frequency of 10 Hz. The cathode peak current increased with the amount of enzyme glucose oxidase on the surface of the electrode up to the amount of 3.0 mg/mL, reaching the maximum current in that value. Higher concentrations of GOx decreased the cathode peak current response, due to reduction in the conductive area of the biosensor. Therefore, according to these results, a concentration of 3.0 mg mL−1 of glucose oxidase was used in the preparation of the biosensor.
Furthermore, the effect of pH and SWV parameters was also investigated. The pH study using the GC/rGO-GOx biosensor was performed by SWV in 0.2 mol L−1 PBS varying the pH at 6.0, 6.5, 7.0, 7.5 and 8.0 to determine the best cathode peak response (Ipc). This experiment showed that the cathode peak current presents a maximum value at pH 7.0, decreasing to the higher pH values. Therefore, according to these results, a 0.2 mol L−1 PBS pH 7.0 was used in analysis of glucose. The parameters of the SWV analysis, such as pulse amplitude and frequency, were studied. For pulse amplitude optimization, the values of 1, 5, 10, 15, 20, and 30 mV were analyzed and the best cathode peak was obtained at 30 mV. For the optimization of the frequency, the values of 1, 5, 10, 15, 20, 25, 30, 50, 70, and 100 Hz were analyzed. For frequencies above 25 Hz and below 20 Hz, a high signal distortion was observed, and therefore was disregarded. The best cathode peak with a lower signal distortion was 10 Hz. Therefore, the pulse amplitude and frequency parameters used in SWV were set at 30 mV and 10 Hz, respectively.
Analytical characteristics and determination of glucose content during 2G ethanol production
SWV experiments were carried out in triplicate using the optimized experimental parameters described above to obtain an analytical curve for determination of glucose with the GC/rGO-GOx biosensor. The analytical response shown in Fig. 6 has a linear response in the range 0.5 to 3.0 mmol L−1, in agreement with the following equation:
with a correlation coefficient of 0.96 (n = 7). The limit of detection (LOD) obtained was 0.51 mmol L−1, which was determined using a 3σ/slope ratio, where σ is the standard deviation of the mean value for 10 voltammograms of the blank. Wang et al. [10] found a similar LOD of 0.7 mmol L−1 using a sensor based on graphene and CdS nanocrystals. The proposed electrode was evaluated using CV experiments in the range of 2.0–16 mmol L−1 of glucose in order to develop a novel immobilization matrix for enzymes. In addition, an electrode decorated with chitosan/multi-walled carbon nanotubes/graphene quantum dots-gold nanoparticles was investigated on the determination of glucose in the range of 0.1–5000 μmol L−1, with a LOD of 64 nmol L−1 [22].
After obtaining the analytical parameters, the proposed biosensor was evaluated on the determination of glucose concentration during the hydrolysis step of 2G ethanol production. As previously discussed, five reactions times were analyzed in triplicate using the SWV optimized parameters. In addition, the samples of glucose were diluted previous to determination, using an aliquot of 500 μL of the sample in 20 mL of PBS solution. Table 1 summarizes the glucose content of the samples analyzed using the GC/rGO-GOx biosensor. From Table 1, we can observe that the glucose concentration was practically double when the hydrolysis time is increased from 3 to 6 h. In the 12 h time, the glucose concentration was 20% higher compared to the 6 h time. When analyzing longer reaction times of enzymatic hydrolysis, we can observe that the glucose concentration values are practically constant. According to the Student’s t test, there were no significant differences between the population means at reaction time of 12, 24 and 48 h, at the 95% confidence. Therefore, the enzymatic hydrolysis time could be interrupted in 12 h of reaction. Thus, an important achievement of this analysis is the reduced time to get a valuable result for the test, saving time and reducing the cost, stopping the process at 12 h time.
Conclusions
The GC/rGO-GOx biosensor was successfully characterized and optimized in order to improve the electrochemical quantification signal of glucose. The 2G ethanol process control was optimized by the use of the biosensor reducing the enzymatic hydrolysis time and the overall cost of the process. Therefore, the developed biosensor can be an efficient tool for the electrochemical determination of glucose during the production of 2G ethanol.
References
Ferreira-Leitao V, Gottschalk LMF, Ferrara MA et al (2010) Biomass residues in Brazil: availability and potential uses. Waste Biomass Valorization 1(1):65–76. https://doi.org/10.1007/s12649-010-9008-8
da Silva AC (2014) Reaproveitamento Da Casca De Coco Verde. Rev Monogr Ambient 13:1–38. https://doi.org/10.5902/2236130815186
Carrijo OA, de Liz RS, Makishima N (2002) Fibra da casca do coco verde como substrato agrícola. Hortic Bras 20:533–535. https://doi.org/10.1590/S0102-05362002000400003
Fu Z, Holtzapple MT (2010) Consolidated bioprocessing of sugarcane bagasse and chicken manure to ammonium carboxylates by a mixed culture of marine microorganisms. Bioresour Technol 101:2825–2836. https://doi.org/10.1016/J.BIORTECH.2009.11.104
Balat M, Balat H, Öz C (2008) Progress in bioethanol processing. Prog Energy Combust Sci 34:551–573. https://doi.org/10.1016/J.PECS.2007.11.001
Wohlfahrt G, Trivić S, Zeremski J, Pericin D, Leskovac V (2004) The chemical mechanism of action of glucose oxidase from Aspergillus Niger. Mol Cell Biochem 260(1-2):69–83. https://doi.org/10.1023/B:MCBI.0000026056.75937.98
Samphao A, Butmee P, Saejueng P et al (2018) Monitoring of glucose and ethanol during wine fermentation by bienzymatic biosensor. J Electroanal Chem 816:179–188. https://doi.org/10.1016/J.JELECHEM.2018.03.052
Kohori NA, da Silva MKL, Cesarino I (2018) Evaluation of graphene oxide and reduced graphene oxide in the immobilization of laccase enzyme and its application in the determination of dopamine. J Solid State Electrochem 22(1):141–148. https://doi.org/10.1007/s10008-017-3738-5
da Silva MKL, Vanzela HC, Defavari LM, Cesarino I (2018) Determination of carbamate pesticide in food using a biosensor based on reduced graphene oxide and acetylcholinesterase enzyme. Sensors Actuators B Chem 277:555–561. https://doi.org/10.1016/j.snb.2018.09.051
Wang K, Liu Q, Guan Q-M, Wu J, Li HN, Yan JJ (2011) Enhanced direct electrochemistry of glucose oxidase and biosensing for glucose via synergy effect of graphene and CdS nanocrystals. Biosens Bioelectron 26(5):2252–2257. https://doi.org/10.1016/J.BIOS.2010.09.043
Unnikrishnan B, Palanisamy S, Chen S-M (2013) A simple electrochemical approach to fabricate a glucose biosensor based on graphene–glucose oxidase biocomposite. Biosens Bioelectron 39(1):70–75. https://doi.org/10.1016/J.BIOS.2012.06.045
Paulus GLC, Nelson JT, Lee KY, Wang QH, Reuel NF, Grassbaugh BR, Kruss S, Landry MP, Kang JW, Vander Ende E, Zhang J, Mu B, Dasari RR, Opel CF, Wittrup KD, Strano MS (2015) A graphene-based physiometer array for the analysis of single biological cells. Sci Rep 4(1):6865–6811. https://doi.org/10.1038/srep06865
Raccichini R, Varzi A, Passerini S, Scrosati B (2015) The role of graphene for electrochemical energy storage. Nat Mater 14(3):271–279. https://doi.org/10.1038/nmat4170
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6(3):183–191. https://doi.org/10.1038/nmat1849
Wang QH, Jin Z, Kim KK, Hilmer AJ, Paulus GL, Shih CJ, Ham MH, Sanchez-Yamagishi JD, Watanabe K, Taniguchi T, Kong J, Jarillo-Herrero P, Strano MS (2012) Understanding and controlling the substrate effect on graphene electron-transfer chemistry via reactivity imprint lithography. Nat Chem 4(9):724–732. https://doi.org/10.1038/nchem.1421
Bello A, Fabiane M, Dodoo-Arhin D et al (2014) Silver nanoparticles decorated on a three-dimensional graphene scaffold for electrochemical applications. J Phys Chem Solids 75:109–114. https://doi.org/10.1016/J.JPCS.2013.09.006
Pakapongpan S, Poo-arporn RP (2017) Self-assembly of glucose oxidase on reduced graphene oxide-magnetic nanoparticles nanocomposite-based direct electrochemistry for reagentless glucose biosensor. Mater Sci Eng C 76:398–405. https://doi.org/10.1016/j.msec.2017.03.031
Donini CA, da Silva MKL, Simões RP, Cesarino I (2018) Reduced graphene oxide modified with silver nanoparticles for the electrochemical detection of estriol. J Electroanal Chem 809:67–73. https://doi.org/10.1016/j.jelechem.2017.12.054
Da Silva MKL, Plana Simões R, Cesarino I (2018) Evaluation of reduced Graphene oxide modified with antimony and copper nanoparticles for levofloxacin oxidation. Electroanalysis. https://doi.org/10.1002/elan.201800265
Gong Y, Li D, Fu Q, Pan C (2015) Influence of graphene microstructures on electrochemical performance for supercapacitors. Prog Nat Sci 25:379–385. https://doi.org/10.1016/j.pnsc.2015.10.004
Casero E, Parra-Alfambra AM, Petit-Domínguez MD, Pariente F, Lorenzo E, Alonso C (2012) Differentiation between graphene oxide and reduced graphene by electrochemical impedance spectroscopy (EIS). Electrochemical Commun 20:63–66. https://doi.org/10.1016/j.elecom.2012.04.002
Wang D, Liang Y, Su Y, Shang Q, Zhang C (2019) Sensitivity enhancement of cloth-based closed bipolar electrochemiluminescence glucose sensor via electrode decoration with chitosan/multi-walled carbon nanotubes/graphene quantum dots-gold nanoparticles. Biosens Bioelectron 130:55–64. https://doi.org/10.1016/j.bios.2019.01.027
Funding
FAPESP (grants 2017/03925-6 and 2017/24274-3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Donini, C.A., Silva, M.K.L., Bronzato, G.R. et al. Evaluation of a biosensor based on reduced graphene oxide and glucose oxidase enzyme on the monitoring of second-generation ethanol production. J Solid State Electrochem 24, 2011–2018 (2020). https://doi.org/10.1007/s10008-019-04471-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-019-04471-7






 ), rGO (
), rGO ( ) and rGO-GOx (
) and rGO-GOx ( )
)

