Abstract
A series of composites based on the perfluorinated MF-4SK membrane and polyaniline was obtained under electrodiffusion of monomer and oxidizer. Aniline was used as monomer and potassium dichromate as oxidizer. Chronopotentiometry was proposed as the method of monitoring the formation of polyaniline in the near-surface layer of the membrane. The electronic absorption spectra of the composites and optical microphotographs of the surfaces were obtained right after membrane modification. A combined interpretation of results revealed the following stages: the accumulation of monomer in the reaction space accompanied by the onset of polymerization, the polymerization with a subsequent increase in rate, and the formation of modifier layer on the membrane surface. The polarization behavior of composite membranes was studied using voltammetry. It was shown that the presence of two limiting currents (pseudo-limiting and limiting) on the current-voltage curves when the composite was oriented with a polyaniline layer to the counterion flow but only for membranes obtained at synthesis times more than 35 min. Also for these composite membranes, no plateau of the limiting current on the current-voltage curve was observed in the case of reverse membrane orientation. But at the same time, the fluctuations of potential drop on the current-voltage curve indicated the overlimiting state of electromembrane system. A correlation between the shape of the chronopotentiogram recorded during membrane modification and the polarization behavior of the obtained composites was found.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A wide range of applications of perfluorinated cation-exchange membranes such as Nafion® and its Russian equivalent MF-4SK is tied with their unique properties. They have a cluster- channel structure and high ionic conductivity. In addition, such type of membranes is chemically and thermally stable. Modification of ion-exchange membranes (IEMs) with additives of organic and inorganic nature makes it possible to extend the functional properties of the membranes [1, 2]. Polyaniline (PANI) is one of the effective modifiers of IEMs today. Composite membranes with deposited PANI layer on the surface are successfully applied to the separation of poly- and singly charged ions [3,4,5,6,7]. Perfluorinated membranes modified by PANI are used in sensors and fuel cells [8,9,10,11,12].
Various methods for preparing composites based on IEMs and PANI are mentioned in the literature [5, 13, 14]. Methods are divided into two large groups. The first group includes introduction of the prepared PANI into the membrane at the stage of its formation. For example, at first, PANI is synthesized from monomer aniline with a further sulfonation of the vacuum-dried PANI granules. Then the blend of Nafion and sulfonated PANI solutions is casted on flat glass plates and kept in an oven for 24 h under a constant temperature [11]. The advantage of the method is that the prepared PANI has optimal conductive properties, which are defined primarily by synthesis conditions [15]. In addition, the exact amount of introduced modifier is known. The disadvantage of the method is the possible nonuniform distribution of PANI particles in the membrane due to its sedimentation during the solvent evaporation.
The second group includes template synthesis of PANI directly in the membrane matrix. In this case, the matrix of IEM serves as a template, which determines the structure of the resulting PANI phase and its spatial arrangement. For example, at first, a solution of aniline dissolved in acid is used to impregnate the cleaned membrane. Then the membrane is immersed in the solution of oxidizer for polymerization in static conditions [16]. Another way is chemical synthesis of PANI under electrodiffusion of a monomer and an oxidizer. In the latter case, it is possible to modify the IEMs directly in the electrodialyzer. At the first stage of synthesis, the membrane saturates with a monomer than the monomer polymerizes under the influence of the oxidizer. Membranes with different content of modifier are obtained by varying the time of contact between the membrane and the oxidizer or the duration of the electric current flow [17, 18]. The advantage of the methods is that it is quite easy to obtain membranes of any size and use industrially produced polymer materials with well-balanced physicochemical properties as basic materials. However, significant disadvantages are the uncertainty in the quantity of introduced PANI and impossibility of creating synthesis conditions that would ensure the maximum conductivity of PANI [15, 19].
PANI can be synthesized both in the bulk and in the surface layer of the IEM. The higher the PANI content in the membrane, the lower the values of electrical conductivity, diffusion, and electroosmotic permeability [13, 20, 21]. Such composite membranes are promising for use in the process of limiting electrodialysis concentration of electrolyte solutions, since the electroosmotic transfer of the solvent limits the maximum achievable degree of concentration [22].
The study of polarization behavior of anisotropic composites based on perfluorinated membranes and PANI has shown that there is an asymmetry of the current-voltage curve (CVC) of the composites [23]. The greater the amount of synthesized PANI, the more pronounced the asymmetry: the length of plateau and the slope of the ohmic region differ significantly at reverse current. The appearance of a pseudo-limiting current has been observed when the composite membranes are oriented with PANI layer toward the counterion flow. It is associated with the appearance of an internal bipolar boundary [18]. Such materials may be promising for the creation of membrane switches. However, the important problem is the control of the formation process of the modifier in the surface layer of IEM in order to obtain materials with desired properties.
The modification of Nafion® films deposited on the surface of an electrode or conductive glass as a result of electrochemical polymerization of aniline is closest to the systems under study [15, 24]. However, there are substantial differences. Firstly, the chemical polymerization of aniline occurs under the influence of an oxidizer, and the reagents are delivered to the reaction zone by the migration mechanism in the system under study. Secondly, the free-standing film is obtained. The process of PANI formation in the near-surface layer of a perfluorinated membrane under the conditions of electrodiffusion of a monomer and an oxidizer has not yet been studied despite the extensive literature in this field. Moreover, the use of electrochemical methods to control the formation of PANI is of particular interest. Therefore, the aim of the paper is to study the patterns of PANI formation on the surface of the perfluorinated membrane under the conditions of electrodiffusion of a monomer and an oxidizer. The objectives of the study include an analysis of chronopotentiograms (ChPs) recorded during the preparation of composites to establish a correlation between the character of ChPs and various stages of oxidative polymerization of aniline in the membrane, as well as studying the features of the CVC of the obtained composites.
Experimental
Membranes
The commercial cation-exchange MF-4SK membrane was used to polymerize aniline on the surface. The MF-4SK membrane is a homogeneous film (JSC “Plastpolymer”, Russia) obtained by extrusion from sulfo group containing perfluorinated polymer.
The commercial anion-exchange MA-40 membrane (LLC “Shchekinoazot”, Russia) was used as an auxiliary membrane at the stage of preparing MF-4SK/PANI samples and measuring CVCs. The heterogeneous MA-40 membrane is a composite made from ion-exchange resin, polyethylene, and reinforcing fabric (polycaproamide fiber). The fixed groups are secondary and tertiary amino groups and quaternary ammonium bases.
Method for obtaining MF-4SK/PANI composites
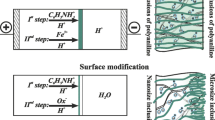
The set of MF-4SK/PANI membranes was obtained in a four-chamber electrodialysis cell under conditions of an external electric field (Fig. 1). The time required to the modification process varied from 5 to 60 min. In total, 14 samples were obtained. They are identified as “MF- 4SK/PANI_N,” where N is the time of PANI synthesis in min.
Electrode chambers were supplied with 0.05-M HCl solution at flowrate 14 mL/min (Fig. 1, №4). Desalination chamber was fed with a 0.01-M C6H5NH2 solution in 0.05-M HCl solution (Fig. 1, №3), concentrating chamber – with a 0.002-M K2Cr2O7 solution in 0.05-M HCl solution (Fig. 1, №2). The solutions in the desalination and concentrating chambers were stagnant. The temperature of these solutions was 25 °C. The membrane area was 7.1 cm2. The original MF-4SK membrane was placed between concentrating and desalination chambers. The studied system (MF- 4SK membrane and two adjacent solution layers) was isolated from products of electrode reactions by the auxiliary MA-40 and MF-4SK membranes. The direct current was supplied to the platinum electrodes (Fig. 1, №5). The current density was fixed, i = 200 A m−2. The anilinium cations (An+) formed as a result of aniline protonation migrate through the membrane under study. The dichromate anions migrate to the surface of the membrane under study. Thus, the oxidation of aniline to PANI takes place on the surface of MF-4SK membrane side facing the concentrating chamber. PANI layer forms in the near-surface layer of MF-4SK membrane looking toward the cathode.
ChPs were measured during the PANI synthesis. The potential drop (PD) across the membrane under study is registered by means of two Ag/AgCl electrodes (Fig. 1, №6). These electrodes are placed in the Haber-Luggin capillaries. The Haber-Luggin tips are installed at both sides of the membrane under study in its geometric center at a distance of about 0.5 mm from the membrane surface.
Voltammetry measurements
The CVCs of obtained composite membranes were measured using the same four-chamber electrodialysis cell as that used for the membrane modification (Fig. 1). The difference consists in that all chambers were fed with 0.05-M HCl solution at flowrate 14 mL/min. The current step was 10−4 A s−1. The slope of the ohmic region, the limiting current density (ilim), the PDs for the corresponding limiting and overlimiting currents, and the plateau length were determined from CVC by the tangent method [25, 26]. In addition, ilim was estimated as the maximum of derivative of CVC obtained by numerical differentiation of CVC.
Ultraviolet spectroscopy and optical microscopy
The electronic absorption spectra of the obtained composite membranes were measured within a wavelength range of 310–900 nm using a LEKI SS 2109 scanning spectrophotometer. The measurements were performed in relation to the original MF-4SK membrane.
The visualization of the surfaces of the swollen composite membranes was carried out using an optical microscope Altami BIO-2 equipped with a digital eyepiece USB camera UCMOSO5100KPA (4x, 10x, 40x magnification).
Results and discussion
Study of polyaniline formation on membrane surface using chronopotentiometry
The polarization behavior of a membrane is determined primarily by the state of the surface [27]. The appearance of a new material on the surface of a perfluorinated membrane should lead to a change in the behavior of the representative ChP. The representative ChP of homogeneous MF-4SK membrane in 0.05М HCl solution at overlimiting current density i = 200 A m−2 (i/ilim = 1.3) without solution flow is shown in Fig. 2. ChP is split into five separate segments. The height of I segment is equal to the value of the ohmic PD (ΔφOhm) due to the initial ohmic resistance of the system consisted of working solutions and membrane between the tips of the Haber-Luggin capillaries. Then there is very slow increase of PD in time (Fig. 2, II segment). This growth is due to a decrease in the concentration of counterions in the diffusion layer in the desalination chamber. In this case, the mass transfer mechanism is electrodiffusion in the absence of coupled effects of concentration polarization (water splitting and convection). After the inflection point τ, called the transition time, there is a sharp increase of PD (Fig. 2, III segment). The transition time corresponds to the drop in concentration at the membrane/solution interface toward zero; meanwhile PD becomes close to infinity in the mathematical model that does not take into account the coupled effects of concentration polarization. In real systems, there is a change in the delivery mechanism of ions from bulk solution to the membrane interface after the transition time. It means that coupled effects of concentration polarization start making a contribution in the mass transfer mechanism. The system switches to a quasi-steady state mode after III segment, when the PD slightly changes in time on representative ChP of homogeneous membrane obtained as the solution circulates in all chambers of an electrodialysis cell [28]. However, under conditions of our experiment, since the solutions on either side of the membrane under study are stagnant, the thickness of the boundary layer increases with time. Therefore, PD remains increasing with time and growth rate slows down (Fig. 2, IV segment). It can be assumed that the thickness of the boundary layer reaches the finite thickness after reaching Δφmax. In addition, coupled effects of concentration polarization affect the value of PD. Finally, the quasi-steady state is reached characterized by large fluctuations in PD (Fig. 2, V segment). The fluctuations of PD in time indicate the emergence of coupled effects of concentration polarization. The most likely cause of low-frequency oscillations is the hydrodynamic instability of electroconvective flows near the membrane interface or water splitting reaction at the membrane/solution interface [28,29,30]. The amplitude of these fluctuations clearly increases with increasing applied current density [28].
ChPs (Fig. 3) have been measured in the process of PANI synthesis in the near-surface layer of MF-4SK membranes. They are reproducible, and the shapes of the curves are similar. However, the nature of the curves obtained during the PANI synthesis (Fig. 3) differs significantly from ChP measured at the original MF-4SK membrane (Fig. 2). The ChP measured in the process of PANI synthesis during the longest time of modification (60 min) is shown in Fig. 3. Electronic absorption spectra of the composites (Fig. 4) have been obtained right after membrane modification. The analysis of the electronic absorption spectra of the composites shows that the spectra of all samples contain characteristic absorption peaks for the emeraldine form of PANI (not oligomers): about 350 nm, due to π – π * electron transitions in the benzene rings of PANI, and a wide band at 800 nm corresponding to radical cations (Fig. 4) [31, 32].
Let us first consider the ChP registered in the process of PANI synthesis in the near-surface layer of the MF-4SK membrane (Fig. 3). Initially, the ChP has a classical form for a membrane which is in an overlimiting current mode under conditions of natural convection (Fig. 2) during the first 3 min (Fig. 3, I segment). An increase of the PD on the membrane to 2.7 V is observed. Then, there is a sharp decrease of PD to 1.5 V till 7 min (Fig. 3, II segment). Apparently, it is due to the onset of the reaction of PANI formation. It is confirmed by the electronic absorption spectra of the composites. The absorption bands characteristic of PANI appear on the electronic absorption spectra of the composites after 5 min of synthesis (Fig. 4).
An increase in the color intensity of composites is observed (Fig. 5) with an increase in the synthesis time till 15 min, as well as a slight increase in the optical density at the absorption maxima. All of this points to an increase in the quantity of PANI in the composites. However, the rate of aniline polymerization remains low, due to the low monomer concentration in the reaction zone. Apparently, this happens due to the accumulation of aniline in the membrane at the initial stage, resulting from the transition of the membrane from H+- to An+-form. An analysis of the dependence of optical density at the absorption maximum to time shows that there is a sharp increase of optical density at the absorption maximum from about 20 min (Fig. 4). It indicates an increase in the rate of polymerization reaction of PANI (Fig. 6).
Along with this, the PD on the ChP does not reach quasi-steady state mode, but slowly increases with time within 25–30 min (Fig. 3, III segment). The similar pattern of ChP was observed for electromembrane systems in the process of precipitate formation on the surface of IEMs [33]. It is expected that PANI is synthesized around the outlets of the transport channels on the membrane surface at this time. The monotonous growth of Δφ is replaced by a noticeable decrease, which is associated with the formation of continuous layer of PANI from 35 min (Fig. 3, IV segment).
As is well-known, PANI has anion-exchange properties, and the MF-4SK membrane has cation- exchange properties. The appearance of a PANI layer on the membrane surface leads to the formation of a bipolar junction of the base membrane and the modifying layer. H+ cations are formed in the process of polymerization of aniline [34]. They must migrate toward the cathode through the PANI layer. Therefore, H+ cations can accumulate at the bipolar boundary, which may explain the decrease in PD observed at ChP in IV segment (Fig. 3). However, a further increase in PANI content leads to a decrease in the conductivity of the electromembrane system. This is reflected in a growth of PD at ChP from 45 min of synthesis. It must be noted that PANI has a lower ionic conductivity compared with the MF-4SK membrane. At the same time, the optical density in the maxima of the absorption spectra of the composites becomes higher than the working range of the spectrophotometer at about 40 min of synthesis.
The increase in the polymerization rate after 15 min of PANI synthesis allows us to conclude that the rate-limiting step is the electrodiffusion of the monomer through the membrane into the reaction space. One of the interesting questions is the site of polymerization reaction. On the one hand, it can be a receiving diffusion layer, and the formation of PANI occurs in solution, like systems with inert substrates [35]. On the other hand, the formation of PANI can be on the membrane surface. In this case, the oxidizer is in the solution and the monomer in the membrane.
Alternatively, the site of polymerization reaction can be the near-surface pore space of a homogeneous membrane. According to the model representation, a homogeneous membrane has a cluster-channel structure [36], wherein two phases can be conventionally distinguished. One phase has unipolar conductivity that is provided by counterions of sulfo groups. The composition of this phase will change as the H+ are replaced by An+. The second phase is a solution identical to the external bulk solution. In our system, the solutions on both sides of the membrane have different composition. In addition, the MF-4SK membrane is in the H+ form at the initial moment of time. Besides the direct migration of An+ through the membrane, An+ accumulates in the membrane phase as a result of the ion-exchange reaction:
PANI formation has not been observed in the solutions containing 0.002-M K2Cr2O7, 0.01 M C6H5NH2 and 0.05-M HCl for a long time comparable to the time of the described experiments. However, we observed the formation of PANI in the membrane. This may be attributed to an increase of the concentration of An+ in the membrane phase as a result of the ion-exchange reaction to H+ ions. A study of aniline sorption by the IEM under static conditions was conducted [37]. It was shown that An+ completely replaced hydrogen ions in the case when the amount of aniline in the solution exceeded the ion-exchange capacity of the membrane. In this regard, let us estimate the concentration of An+ in the membrane phase. The ion-exchange capacity of MF-4SK membrane of present instalment is 0.6 meq g-1 in swollen state, the density of the membrane swollen in water is 1.55 g cm-3. Accordingly, the concentration of An+ will be equal to 0.93 M. It was shown that the current efficiency of H+ is 4–5 times higher than that of An+ at their initial concentrations of 0.05 and 0.01 M, respectively, during electrodialysis process [38]. Therefore, the monomer did not reach measurable concentration in the diffusion layer after 5–10 min of experiment. At the same time, the concentration of the oxidizer is rather higher in the near-surface membrane layer than in the bulk solution from the outset of experiment due to the migration of Cr2O 2- anions from solution to the membrane surface. Thus, it can be assumed that the most likely site of the polymerization reaction is the near-surface pore space of the membrane. The formation of PANI primarily will be observed at the outlets of the mesopores on the membrane surface, because the electroneutral external solution containing Cr2O72- co-ions can be found in mesopores. The process is accompanied by subsequent germination of PANI into the membrane phase, which is confirmed by published data [39].
Current-voltage curves of MF-4SK/PANI composites
A representative CVC of homogeneous MF-4SK membrane in 0.05-М HCl solution is presented in Fig. 7a. CVC can be divided into 3 segments: the ohmic region (Fig. 7a, I segment), the plateau of the limiting current (Fig. 7a, II segment), and the overlimiting region (Fig. 7a, III segment). The slope of the ohmic region is determined by the electrical resistance of the membrane under study and the diffusion layers adjacent to the membrane. The concentration polarization increases linearly with an increase in applied direct current. A deviation from linearity takes place in II segment. The solution concentration at the membrane/solution interface is significantly reducing in comparison with the bulk solution concentration. It leads to a sharp increase in the PD on ChP. The electrolyte concentration at the membrane/solution interface approaches zero when the system reaches the limiting state and the plateau of the limiting current is observed. The emergence of III segment on the CVC is associated with the development of the coupled effects of concentration polarization: water splitting and convection. Appearance of oscillations of PD on the CVC is a typical sign of demonstration of coupled effects of concentration polarization.
The derivative of CVC as a function of current density is presented in Fig. 7b. The dV/di value increases slightly with increasing current at currents below the limiting (~150 A m−2) pointing to a slightly pronounced concentration polarization. The dV/di value increases sharply reaching a maximum at the peak at i from 150 to 170 A m−2. The maximum on the derived CVC corresponds to the inflection point of the CVC and is taken for the limiting current density. Strong fluctuation in the overlimiting current region is observed due to the electrical noise on the CVC.
The influence of polymerization time during the aniline synthesis on the CVCs of obtained composites has been studied. The results are presented in Fig. 8 in the case when non-modified surface of the composite (Fig. 8a, so called “1 side”) and PANI layer (Fig. 8b, so called “2 side”) are oriented toward the desalination chamber. Table 1 presents some characteristics of experimental CVCs of initial MF-4SK membrane and MF-4SK/PANI composites: the experimental limiting current density (ilim); the plateau length (Δ); the slopes of ohmic region (1/R1); and overlimiting region (1/R3), determined as shown in Fig. 7a.
Experimental data shows that the intercalation of PANI into the near-surface layer of the original MF-4SK membrane leads to a decrease in the limiting current density (Table 1). Furthermore, an increase in the synthesis time while obtaining the composite membranes results in an increase in the resistance of the electromembrane system, with attendant decrease in the slope of the ohmic region. The decrease in the limiting current and electrical conductivity of the composite membrane in comparison with the initial membrane is due to the barrier effect of the PANI layer on the membrane surface. PANI, which has anion exchange properties, blocks part of the cation-exchange membrane surface. The larger the amount of PANI is formed, the lower the limiting current density and the higher the membrane resistance is observed (Table 1). However, in general, the shape of the CVC of the composites obtained during 15 and 30 min of PANI synthesis is similar to the original MF-4SK membrane despite the observed changes in the conductivity and the limiting current density. The asymmetry of the CVCs of the obtained composite membranes has been discovered. Moreover, the longer the synthesis time, the more pronounced the effect of asymmetry. An increase in ilim, 1/R1, and 1/R3 and a decrease in the plateau length with increasing amount of introduced PANI are observed in the case when the composite membrane is oriented with the non-modified surface to the desalination chamber (Table 1, side 1) compared with the reverse orientation (Table 1, side 2).
A sharp change in all parameters and the shape of the CVC of the composites is observed with an increase in the synthesis time over 35 min. The CVCs and derived CVCs are deemed abnormal for composite membranes obtained for synthesis time of 45 and 60 min for both membrane orientations. The detailed study of the CVCs of composite membranes obtained at different current density showed the similar data [18]. However, in this case, all CVCs [18] were similar to the CVCs of the composites obtained within 45 and 60 min. There is no plateau of the limiting current in the case when the non-modified surface is oriented toward the counterion flux. However, the oscillations of the PD characteristic for the overlimiting state are observed at current densities above 9 A m−2. Two local maxima on the derived CVC are evidenced when the PANI layer is oriented toward the counterion flux (Fig. 9). The first maximum in the region of low PD is associated with the depletion of the internal bipolar boundary inside the composite membrane. The internal bipolar boundary forms at the junction of PANI having anion-exchange properties and the base cation-exchange membrane. Cations migrate from the bipolar boundary into the membrane bulk and anions through the PANI layer into the bulk solution under the action of an external electric field. Diffusion of the anions from bulk solution through a thin PANI layer allows one to reduce the deficit of ions at the bipolar boundary. However, this is not enough, and the ion concentration at the bipolar boundary becomes low at a certain current density. This limiting current density caused by the depletion of the bipolar boundary can be called a pseudo-limiting current density. Moreover, the longer the time of PANI synthesis, the more pronounced the first maximum on the derived CVC. Therefore, the internal bipolar boundary in the composite membrane plays a large role. The second maximum on the derived CVC corresponds to the depletion of the external boundary between the composite membrane and depleted solution, as in the case of a monopolar membrane. However, the limiting current is significantly lower than for the initial MF-4SK membrane. This confirms not only the role of the membrane surface but also the membrane phase in the formation of concentration polarization. The presence of two maxima on the CVC confirms the formation of PANI layer on the membrane surface.
Thus, the analysis of the CVCs has shown that the PANI synthesis on the membrane surface leads to a decrease in the limiting current density and an increase in the resistance of the electromembrane system. The asymmetry of the CVCs of obtained composites has been observed. The effect grows with an increase in modification time of the membrane. It is important to note that there is a correlation between ChPs obtained during the PANI synthesis in membranes and CVCs. The shape of the CVCs of composites obtained during modification time less than 35 min does not differ from the original membrane. A steady rise in PD is observed at the ChPs recorded during the modification process of such samples (Fig. 3, III segment). Conversely, the shape of the CVCs becomes abnormal and differs significantly from the original membrane for composites obtained within 45 and 60 min. Two limiting currents appear at the CVC when the composite membrane is oriented toward the counterion flow. This points to the fact that the formed internal bipolar boundary is starting to provide input to the ion transport. The sharp drop in PD replaced by its further growth is observed at the ChP recorded during modification time more than 35 min. The minimum region of the ChP between the IV and V segments corresponds to a change in the shape of the CVC. Thus, one can predict the polarization behavior of the desirable composites based on ChPs.
Conclusions
The method of polyaniline synthesis in the near-surface layer of a perfluorinated membrane under the conditions of electrodiffusion of the monomer and the oxidizer has been studied by chronopotentiometry. The analysis of chronopotentiograms during polyaniline synthesis, supplemented by measurements of the electronic absorption spectra of the composite membranes right after their obtainment and visualization of the membrane surfaces, makes it possible to use chronopotentiometry as a method of monitoring the formation of polyaniline layer on the membrane surface. A detailed study of the formation of polyaniline in the near-surface layer of MF-4SK membrane by chronopotentiometry has revealed the stages of polyaniline formation: the accumulation of monomer in the reaction space accompanied by the onset of polymerization, the polymerization with a subsequent increase in rate, and the formation of modifier layer on the membrane surface.
By means of voltammetry, it has been shown that all obtained composites have asymmetric current-voltage curves due to the presence of two layers in the composite membrane. The effect of asymmetry grows with increasing the modification time of the sample and, accordingly, the amount of polyaniline. A peculiarity of polarization behavior is the absence of the plateau of limiting current on the current-voltage curve when the membrane is oriented with the non-modified layer to the flow of counterions and the onset of both the limiting and quasi-limiting state in the electromembrane system in the case of reverse membrane orientation. For the first time, the correlation between the nature of chronopotentiograms recorded during the synthesis of polyaniline in the near-surface layer of the membrane and polarization behavior of the obtained composites is shown. The minimum region of the chronopotentiogram corresponds to a change in the shape of current-voltage curve pointing to a brightly formed bipolar boundary.
The established correlation between the stages of polyaniline formation in the membrane and the character of chronopotentiograms provides an opportunity not only to control the process of obtaining composites based on a perfluorinated membrane and polyaniline but also to predict polarization behavior of the obtained composites.
Abbreviations
- IEM:
-
ion-exchange membrane
- PANI:
-
polyaniline
- CVC:
-
current-voltage curve
- ChP:
-
chronopotentiogram
- An+ :
-
anilinium cations
- PD:
-
potential drop
References
Luo T, Abdu S, Wessling M (2018) Selectivity of ion exchange membranes: a review. J Membr Sci 555:429–454
Ran J, Wu L, He Y, Yang Z, Wang Y, Jiang C, Ge L, Bakangura E, Xu T (2017) Ion exchange membranes: new developments and applications. J Membr Sci 522:267–291
Sata T, Ishii Y, Kawamura K, Matsusaki K (1999) Composite membranes prepared from cation exchange membranes and polyaniline and their transport properties in electrodialysis. J Electrochem Soc 146:585–591
Sata T, Sata T, Yang W (2002) Studies on cation-exchange membranes having permselectivity between cations in electrodialysis. J Membr Sci 206:31–60
Nagarale RK, Gohil GS, Vinod SK, Trivedi GS, Rangarajan R (2004) Preparation and electrochemical characterization of cation- and anion-exchange/polyaniline composite membranes. J Colloid Interface Sci 277(1):162–171
Sivaraman P, Chavan JG, Thakur AP, Hande VR, Samui AB (2007) Electrochemical modification of cation exchange membrane with polyaniline for improvement in permselectivity. Electrochim Acta 52:5046–5052
Li J, Zhu J, Wang J, Yuan S, Lin J, Shen J, Van der Bruggen B (2018) Charge-assisted ultrafiltration membranes for monovalent ions separation in electrodialysis. J Appl Polym Sci. https://doi.org/10.1002/app.45692
Hosseini MG, Shahryari E (2017) Fabrication of novel solid-state supercapacitor using a Nafion polymer membrane with graphene oxide/multiwalled carbon nanotube/polyaniline. J Solid State Electrochem 21:2833–2848
Naveen MH, Gurudatt NG, Shim Y-B (2017) Applications of conducting polymer composites to electrochemical sensors: a review. Appl Mater Today 9:419–433
Zakil FA, Kamarudin SK, Basri S (2016) Modified Nafion membranes for direct alcohol fuel cells: an overview. Renew Sust Energ Rev 65:841–852
Dutta K, Das S, Kundu PP (2015) Partially sulfonated polyaniline induced high ion- exchange capacity and selectivity of Nafion membrane for application in direct methanol fuel cells. J Membr Sci 473:94–101
Xiangguo T, Jicui D, Jing S (2015) Effects of different kinds of surfactants on Nafion membranes for all vanadium redox flow battery. J Solid State Electrochem 19:1091–1101
Tan S, Belanger D (2005) Characterization and transport properties of Nafion/Polyaniline composite membranes. J Phys Chem B 109(49):23480–23490
Compan V, Riande E, Fernandez-Carretero FJ, Berezina NP, Sytcheva AA-R (2008) Influence of polyaniline intercolations on the conductivity and permselectivity of perfluorinated cation-exchange membranes. J Membr Sci 318:255–263
Stejskal J (2015) Polymers of phenylenediamines. Prog Polym Sci 41:1–31
Abdo N, Easton EB (2016) Nafion/Polyaniline composite membranes for hydrogen production in the Cu–Cl thermochemical cycle. Int J Hydrog Energy 41:7892–7903
Huang QM, Zhang QL, Huang HL, Li WS, Huang YJ, Luoc JL (2008) Methanol permeability and proton conductivity of Nafion membranes modified electrochemically with polyaniline. J Power Sources 184:338–343
Loza NV, Dolgopolov SV, Kononenko NA, Andreeva MA, Korshikova YS (2015) Effect of surface modification of perfluorinated membranes with polyaniline on their polarization behavior. Russ J Electrochem 51:538–545
Stejskal J, Gilbert RG (2002) Polyaniline. Preparation of a conducting polymer (IUPAC technical report). Pure Appl Chem 74:857–867
Kononenko NA, Loza NV, Shkirskaya SA, Falina IV, Khanukaeva DY (2015) Influence of conditions of polyaniline synthesis in perfluorinated membrane on electrotransport properties and surface morphology of composites. J Solid State Electrochem 19:2623–2631
Berezina NP, Kononenko NA, Sytcheva AA-R, Loza NV, Shkirskaya SA, Hegman N, Pungor A (2009) Perfluorinated nanocomposite membranes modified by polyaniline: electrotransport phenomena and morphology. Electrochim Acta 54:2342–2352
Protasov KV, Shkirskaya SA, Berezina NP, Zabolotskii VI (2010) Composite sulfonated cation-exchange membranes modified with polyaniline and applied to salt solution concentration by electrodialysis. Russ J Electrochem 46:1131–1140
Kononenko NA, Dolgopolov SV, Berezina NP, Loza NV, Lakeev SG (2012) Asymmetry of voltammetric characteristics of perfluorinated MF-4SK membranes with polyaniline-modified surface. Russ J Electrochem 48:857–861
Wu W, Pan D, Li Y, Zhao G, Jing L, Chen S (2015) Facile fabrication of polyaniline nanotubes using the self-assembly behavior based on the hydrogen bonding: a mechanistic study and application in high-performance electrochemical supercapacitor electrode. Electrochim Acta 152:126–134
Choi J-H, Park J-S, Moon S-H (2002) Direct measurements of concentration distribution within the boundary layer of an ion-exchange membrane. J Colloid Interface Sci 251(2):311–317
Loza NV, Kononenko NA, Shkirskaya SA, Berezina NP (2006) Effect of modification of ion-exchange membrane MF-4SK on its polarization characteristics. Russ J Electrochem 42:815–822
Volodina E, Pismenskaya N, Nikonenko V, Larchet C, Pourcelly G (2005) Ion transfer across ion-exchange membranes with homogeneous and heterogeneous surfaces. J Colloid Interface Sci 285(1):247–258
Krol JJ, Wessling M, Strathmann H (1999) Chronopotentiometry and overlimiting ion transport through monopolar ion exchange membranes. J Membr Sci 162:155–164
Rubinstein I, Staude E, Kedem O (1988) Role of the membrane surface in concentration polarization at ion-exchange membrane. Desalination 69:101–114
Taky M, Pourcelly G, Lebon F, Gavach C (1992) Polarization phenomena at the interfaces between an electrolyte solution and an ion exchange membrane. Part I: Ion transfer with a cation exchange membrane. J Electroanal Chem 336:171–194
Mažeikienė R, Niaura G, Malinauskas A (2019) A comparative multiwavelength Raman spectroelectrochemical study of polyaniline: a review. J Solid State Electrochem 23:1631–1640
Nekrasov AA, Ivanov VF, Vannikov AV (2001) A comparative voltabsorptometric study of polyaniline films prepared by different methods. Electrochim. Acta 46:3301–3307
Martí-Calatayud MC, Buzzi DC, García-Gabaldón M, Bernardes AM, Tenório JAS, Pérez-Herranz V (2014) Ion transport through homogeneous and heterogeneous ion-exchange membranes in single salt and multicomponent electrolyte solutions. J Membr Sci 466:45–47
Trchová M, Morávková Z, Bláha M, Stejskal J (2014) Raman spectroscopy of polyaniline and oligoaniline thin films. Electrochim Acta 122:28–38
Stejskal J, Sapurina I, Trchová M (2010) Polyaniline nanostructures and the role of aniline oligomers in their formation. Prog Polym Sci 35:1420–1481
Gierke TD, Munn GE, Wilson FC (1981) The morphology in Nafion perfluorinated membrane products, as determined by wide- and small-angle x-ray studies. J Polym Sci 19:1687–1704
Loza NV, Falina IV, Popova DS, Kononenko NA (2016) Influence of conditions of polyaniline template synthesis on its distribution in perfluorinated membrane. Sorbtsionnye Khromatograficheskie Protsessy 16:663–671
Loza NV, Loza SA, Romanyuc NA, Kononenko NA (2019) Experimental and theoretical investigation of electrodialysis process of model solutions containing aniline and sulfuric acid. Russ J Electrochem 55:1091–1098
Kononenko NA, Fomenko MA, Volfkovich YM (2015) Structure of perfluorinated membranes investigated by method of standard contact porosimetry. Adv Colloid Interf Sci 222:425–435
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 18-38- 20069 mol_a_ved).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Andreeva, M., Loza, N., Kutenko, N. et al. Polymerization of aniline in perfluorinated membranes under conditions of electrodiffusion of monomer and oxidizer. J Solid State Electrochem 24, 101–110 (2020). https://doi.org/10.1007/s10008-019-04463-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-019-04463-7