Abstract
Degradation-related issues are among the main limitations to make solid oxide electrolysis cells (SOEC) meet performance targets economically viable for long-term operation. In this study, the considered cell presents a premature degradation during electrolysis operation observed after sealing the cell holder and the support pieces providing and releasing H2 electrode gas. This premature degradation is characterized by unusual polarization curve slopes, and appearance of a new impedance contribution at the lowest frequencies of the impedance diagrams recorded. To the best of our knowledge, this new contribution has never been reported for SOECs. Post-mortem analysis of the cell by scanning electron microscopy (SEM)/energy dispersive X-ray (EDX) shows the presence of Si, Al, Na, K, and Ca at the H2 electrode interface (surface and first dozens microns in the volume) and at the Ni-yttria-stabilized zirconia (YSZ)/YSZ interface, contrary to similar cells tested before sealing the pieces. This degradation is related to Si deposition, notably at the Ni/YSZ/H2O triple phase boundaries. Concomitantly, the new contribution observed, leading to a beneficial effect on the cell functioning, is assimilated to a “reactivation” contribution. This reactivation contribution is associated with an H2O adsorption phenomenon and characterized by a relaxation frequency of [1–10 mHz] and a capacitance of ~ 100 F cm−2. The evolution with time of the resistive and capacitive contributions is consistent with the interpretation of the premature cell degradation. A mechanism explaining the cell behavior after this premature degradation is proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Energy production through renewable sources is a critical challenge for the twenty-first century. The main route for producing energy is still nowadays based on fossil fuels, which are expected to significantly decrease in a few decades. It is hence crucial to rethink our approach by (i) diversifying energy sources; (ii) optimizing natural energy sources such as wind, sun light, or biomass; and (iii) avoiding as much as possible pollutant and greenhouse gas generation to prevent severe environmental issues, among others, global warming. Thus, the concept of a multi-source energy production, clean and renewable, is being considered. Hydrogen production through water high temperature electrolysis (HTE) meets the conditions to be part of this concept [1,2,3]. Solid oxide electrolysis cells (SOEC) can be used to perform HTE. Even though significant studies appeared during the 1970s [4,5,6,7], interest on SOEC really rose during the 1980s through projects and investigations such as the Westinghouse project [8, 9], the work of Barbi and Mari [10,11,12,13,14], or the HOTELLY project reported by Dönitz et al. [15,16,17,18]. However, cell degradation occurring during electrolysis still represents nowadays a critical issue to make SOEC meet performance targets economically viable for possible applications, especially the ones with long-term operations. Hence, a deeper understanding of SOEC functioning and degradation is needed. In addition to post-test characterization methods such as scanning electron microscopy (SEM), X-ray diffraction (XRD), or X-ray photoelectron spectroscopy (XPS), electrochemical impedance spectroscopy (EIS) is an in situ method that is very helpful for understanding cell degradation mechanisms.
Cell degradation often occurs consequently to apparition of impurities originating from glass sealing. Chen et al. observed a severe degradation effect of volatile boron species from borosilicate glass sealant on the electrochemical activity of LSM oxygen electrode [19]. Indeed, applying 0.2 A cm−2 for 2 h at T = 800 °C led to a significant increase in both polarization and ohmic resistances. This was furthermore characterized by Sr segregation as well as boron deposition at the LSM/YSZ interface, leading to formation of lanthanum borates and manganese oxide. Consequently, disintegration and delamination of LSM electrode was accelerated by the boron deposition. Hauch et al. tested several locally produced SOFCs in HTE mode for up to 766 h [20]. The cells mainly degraded during the first 100 h of electrolysis and could be partly reactivated. In terms of EIS, degradation and activation of the cell were respectively characterized by an increase and a decrease in the polarization resistance (Rp), while the ohmic resistance (Rs) remained constant during the entire test. Based on the observation of silica containing impurities and on the work done by Jensen et al. [21], Hauch et al. suggested that (i) degradation of the cell might be due to a build-up of glassy phase impurities at the triple phase boundary (TPB) of the H2 electrode leading to an increase in the diffusion path length at the TPB, and (ii) its partial activation could be explained by a break-up of the glass leading to a decrease in the diffusion path length at the TPB. Another study by Hauch et al. strengthened these suggestions [22]. Indeed, SOE cells tested for 1000 h were found to degrade because of silica impurities segregation at the Ni-yttria-stabilized zirconia (YSZ)/YSZ interface characterized by Rs and/or Rp increase. Silica impurities observed by SEM are supposed to segregate from glass sealing. In an additional study [23], Hauch et al. verified, by changing the type of sealing, that silica impurities segregate from the applied glass sealing to the H2 electrode/electrolyte interface. Besides, they showed that degradation of the cell was characterized by an increase in the resistance at higher frequency part of the impedance diagram assigned here to charge transfer at the H2 electrode [24, 25]. Ebbesen et al. examined a cell degradation for more than 1300 h in both H2O electrolysis and H2O/CO2 co-electrolysis modes [26]. In this case, impurities originated from inlet gases. Indeed, when inlet gases were used as received, degradation of the cell occurred at both short and long term. This degradation was characterized by an increase in Rp. The use of analysis of the difference in impedance spectra (ADIS) method allowed identifying two contributions to this degradation: one at the Ni-YSZ electrode characterized by a relaxation frequency in the 100–200 Hz range and one occurring either at the Ni-YSZ or Sr-doped LaMnO3 (LSM)-YSZ electrodes characterized by a relaxation frequency in the 1000–3000 Hz range. Cleaning the inlet gases leads to the disappearance of the former contribution, meaning that this contribution is surely caused by impurities adsorption at the Ni-YSZ/YSZ interface, similar to what mentioned above [21,22,23]. Besides cell poisoning, electrolysis operation under high currents applied (above − 1.0 A cm−2), delamination issues at the O2 electrode, or carbon formation at Ni-based electrodes represent other sources of detrimental degradation for SOECs [3, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42].
The present study deals with an early degradation phenomenon appearing after less than 200 h of test under electrolysis conditions and is attributed to cell contamination by Si impurities. We herein discuss the appearance and the evolution with time of this premature degradation based on the analysis of the EIS measurements, the post-test characterizations, and literature data. We strongly believe that this “electrochemical” approach is compulsory for understanding new kind of degradation phenomena in SOECs and optimizing the experimental conditions, which are a key step in all these high temperature devices operating with varied materials and gases.
Experimental
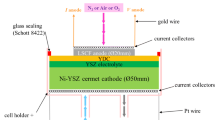
In this study, a LSCF//YSZ//Ni-YSZ commercial anode-supported SOFC used in HTE mode (HC Starck) was considered in a two-electrode experimental set-up. An intermediate YDC (yttria-doped Ceria) layer is present between LSCF (La0.6Sr0.4Co0.2Fe0.8O3-δ) and YSZ. Several H2 leakage problems were encountered in the cathode compartment during the test bench validation process. In order to avoid such issues, the cell holder and the support pieces (alumina) used to provide and release H2 electrode gas were sealed using Schott 8422 glass (composed of 69.8% SiO2, 12.0% Na2O, 7.8% B2O3, 4.1% K2O, 0.4% CaO, and 0.2% BaO) typically used for alumina sealing, as shown in Fig. 1. The sealing was realized by increasing the temperature to 900 °C at 0.5 °C/min in flowing N2. This temperature was maintained for 90 min. The system was further stabilized to the operating temperature of 850 °C at 0.5 °C/min. At the end of the test, the system was cooled down to room temperature at 0.5 °C/min. Additional details of the cell and the experimental set-up are given and justified elsewhere [43]. A scanning electron microscopy (SEM) cross section image of the different layers of the cell before the test is shown in Fig. 2. The cell was tested at 850 °C with PH2O/PH2 = 9 at the cathode side, air at the anode side, and a gas flow rate of 2.26 NL/h at each side. The circular cell has an active electrode area of 3.14 cm2, referring to the LSCF diameter (20 mm). A succession of impedance diagrams were measured, preceded and followed by current density-potential (i-U) curves. The overall 8-h procedure was repeated with time. Thus, to study the appearance and the evolution with time of the premature degradation, the cell is analyzed initially (0 h), after 144 h and after 168 h of working time. The i-U curves were recorded in potentiostatic mode with a scan rate of 1 mV/s. The electrochemical impedance diagrams were recorded applying a dc current with a 30 mA amplitude perturbation in the frequency range from 10 to 0.01 Hz with 10 points per decade. A Biologic SP-150 frequency analyzer with a 20 A booster was used for both chronopotentiometry and EIS measurements. Post-mortem analyses of the tested cell were performed using a Hitachi SU-70 SEM-FEG equipped with an Oxford X-Max 50 mm2 X-ray microanalysis system for the energy dispersive X-ray (EDX) spectroscopy (detection limit, 1.0 wt%). In our case, analyses were carried out with an acceleration voltage of 15 kV. No additional coating was required to clearly observe by SEM the surfaces and the considered layers.
Results and discussions on electrochemical measurements
Premature degradation appearance
In a similar study recently reported [43], the i-U curve measured showed three functioning regions (curve at 0 h in Fig. 3): region I where Rp decreases with increasing current densities corresponding to a decrease in the activation resistance, region II where Rp reaches a minimum and remains constant while current density increases showing an optimal use of the cell, and region III where Rp increases with current density resulting mainly from an increase in concentration overpotentials. According to Fig. 3, the i-U curve measured in this study (at t = 144 h) shows a particular trend: five functioning regions can be distinguished instead of three, which leads to a “double basin-like” evolution of the total resistance (Rt) with current density, as shown in Fig. 4a. Indeed, after a decrease in Rt during cell activation (region I), the “optimal cell utilization” region appears at − 0.2 A cm−2 (region II). From − 0.3 to − 0.6 A cm−2Rt increases again, indicating that the cell is already working under gas transport limitation (region III) before decreasing again to reach a minimum value of 0.41 Ω cm2 at − 0.8 A cm−2 (region IV). Note that Rt at − 0.8 A cm−2 is smaller than the 0.54 Ω cm2 obtained at − 0.2 A cm−2. Finally, Rt increases to reach 1.41 Ω cm2 at − 1.05 A cm−2 (region V). The degradation thus leads to a perturbation in cell operation from the very first current densities applied.
Polarization curve (i-U curve) of the cell after (□) 0 h and (◊) 144 h of test. The i-U curve measured after 144 h of test shows five functioning regions: region I corresponding to a decrease in the activation resistance, region II to an optimal use of the cell, region III resulting mainly from an increase of concentration overpotentials, region IV corresponding to the cell reactivation until limitation by H2O transport becomes the predominant phenomenon governing the cell functioning (region V). T = 850 °C, PH2O/PH2 = 9 (H2 electrode), air (O2 electrode), standard flow rate of 2.26 NL h−1 on both sides
Total and polarization resistances Rt and Rp (a), ohmic resistance Rs of the cell after 144 h of test (b). T = 850 °C, PH2O/PH2 = 9 (H2 electrode), air (O2 electrode), standard flow rate of 2.26 NL h−1 on both sides. The unfilled points correspond to the appearance of region IV. Rp values were measured as explained in Appendix A
According to the orders of magnitude for Rp and Rs as well as their variations with current density (Fig. 4a, b, respectively), the “double basin-like” evolution of Rt with current density is imposed by Rp variations. Thus, the cell behavior mainly depends on the reaction processes included in the polarization resistance. Nevertheless, the ohmic resistance tends to increase until − 0.4 A cm−2 (13% increase) to further decrease and even reaches a value inferior to the one at open-circuit voltage (OCV) (Rs = 0.141 Ω cm2 at i = 0 A cm−2 whereas Rs = 0.135 Ω cm2 at i = − 1.05 A cm−2). This transition happens for a cell voltage superior to 1.1 V, which is consistent with an endo/exothermic transition. Details on the measurement method of Rp values are given in Appendix A.
The impedance diagrams recorded in the five regions and shown in Fig. 5 lead to the following qualitative analysis:
-
Region I: Rp decreases during the activation of the cell at the first current densities applied. This is characterized by a slight decrease in the high frequency (HF) part of the impedance diagram, and a more important decrease in the middle frequency (MF) and low frequency (LF) parts (Fig. 5a).
-
Region II: Figure 5b shows that the optimal utilization region is severely shortened compared to our previous study in which the cell holder and support pieces were not sealed [43]. Indeed, a slight increase of Rp is observed between − 0.2 and − 0.4 A cm−2. This is characterized by (i) an important increase in the HF part associated with charge transfer at the Ni-YSZ/electrolyte interface [43,44,45,46,47]; (ii) a slight decrease in the MF part that maybe related to H2O transport phenomena at the Ni-YSZ electrode [43, 45, 48,49,50]; and (iii) a slight decrease in the LF part attributed to H2O diffusion at the Ni-YSZ electrode [44, 51,52,53,54]. This indicates that the cell starts being disturbed by a phenomenon affecting mainly H2 electrode interfacial charge transfer at this stage.
-
Region III: Rp still increases between − 0.4 and − 0.6 A cm−2 (Fig. 5c) with an even more important increase of the HF part, but also this time an important increase in the MF and LF parts of the diagram. This indicates that, in addition to interfacial charge transfer, this phenomenon now affects H2O transport at the H2 electrode, explaining the early appearance of gas transport limitation on the i-U curve (Figs. 3 and 4a).
-
Region IV: Above − 0.6 A cm−2 (Fig. 5c), the appearance of region IV corresponds to a decrease in Rp characterized by two contradictory trends. On the one hand, the overall impedance diagram increases until a certain frequency (0.1 Hz for i = −0.8 A cm−2) and, on the other hand, a positive contribution, which is akin to a “reactivation” of the cell, appears and leads to a decrease in the impedance diagram at the lowest frequencies, which explains the decrease in Rp. It should be noticed that, according to the Rt (and so Rp) value, this reactivation contribution is significantly more important than what is observed from the impedance diagram only recorded until 0.01 Hz.
-
Region V: Located above − 0.8 A cm−2, region V is characterized by the same trends as region IV, the transition happening for lower frequencies as the current density applied increases (Fig. 5d). Hence, considering that region III corresponds to gas transport limitation, the appearance of the reactivation contribution counterbalances transport limitation (region IV) up to a certain current density value above which transport limitation prevails over the other reaction phenomena, including the one related to the reactivation contribution (region V).
Table 1 contains relaxation frequency fo and capacitance C values obtained by deconvolution of the impedance diagrams shown in Fig. 5. Up to i = − 0.6 A cm−2, the equivalent electrical circuit shown in Fig. 6a was used; it is composed of an ohmic resistance Rs (corresponding to RΩ) in series with an inductance L and four combinations of a resistance R in parallel with a constant phase element CPE (R//CPE). For i = − 0.8 and − 1.0 A cm−2, a fifth R//CPE was required (Fig. 6b). This fifth element allows taking into account the reactivation contribution, firstly assigned to an inductive contribution, but finally modeled with a differential negative resistance in parallel with a differential negative capacitance (pseudo-capacitive negative behavior). Having a negative resistance is relevant with the beneficial effect of this reactivation contribution on the cell functioning. Further discussions involving these two negative elements will be based on the absolute values obtained from the fitting.
Electrical equivalent circuits used to fit the impedance diagrams from Fig. 5. a Up to i = − 0.6 A cm−2, an equivalent electrical circuit composed of a ohmic resistance Rs (corresponding to RΩ) in series with an inductance L and four combinations of a resistance R in parallel with a constant phase element CPE (R//CPE) was used. b For i = − 0.8 and − 1.0 A cm−2, a fifth R//CPE was required. RHF and CPEHF are associated with charge transfer at the Ni-YSZ/YSZ interface, RMF1 and CPEMF1 with H2O diffusion at the Ni-YSZ electrode, RMF2 and CPEMF2 with H2O gas conversion at the Ni-YSZ electrode, while RLF and CPELF are also associated with H2O diffusion at the Ni-YSZ electrode. RVLF and CPEVLF are related to the new reactivation contribution
Table 1 clearly shows that, depending on the current density considered, four to five main phenomena can be distinguished, each phenomenon characterized by a specific relaxation frequency and capacitance. Thus, there are four to five main phenomena governing the cell operation in this study.
The four to five frequency ranges identified after deconvolution of the impedance diagrams are designated as HF, MF1, MF2, LF, and VLF (for very low frequency) arcs, from high to low frequencies. Except for the VLF arc, the four other orders of magnitude correspond to those obtained in a previous study [43]: {[100 –1000 Hz], 10 mF cm−2}, {10 Hz, 0.1 F cm−2}, {1 Hz, 1 F cm−2}, and {0.1 Hz, 10 F cm−2}, respectively. The main difference in the present study is the sealing of the two alumina pieces that provide and release H2 electrode gas. Consequently, any change in the identified phenomena would mean that these are associated with the cathode side of the electrolyzer.
The first analysis of the impedance diagrams shows that, depending on the current density considered, all or part of the diagram is influenced by the appearance of the reactivation contribution. This allows us pointing out the following statements:
(i) This confirms that HF, MF2, and LF arcs are respectively associated with charge transfer at the Ni-YSZ/YSZ interface, H2O gas conversion at the Ni-YSZ electrode, and H2O diffusion at the Ni-YSZ electrode.
(ii) It also proves that the MF1 arc is also related to a cathode phenomenon. The influence of PH2O/PH2 ratio and cathode gas flow rate on the MF1 arc was highlighted elsewhere [43, 45, 48]. This allows to relate MF1 arc to a phenomenon associated with H2O transport at the Ni-YSZ electrode. Besides, the influence of H2O/H2 dilution with N2 on MF1 arc suggests its association with H2O diffusion on the cathode side [43]. This association is now confirmed as well; the MF1 arc is related to H2O diffusion at the Ni-YSZ electrode.
It is also important to underline that the new reactivation contribution is clearly distinguishable from the other contributions with orders of magnitude of [1–10 mHz] for the relaxation frequency and |100 F cm−2| for the capacitance.
The reactivation contribution is characterized by the appearance of the negative differential resistance at the lowest frequencies RVLF, which absolute value increases with current density (Table B1, Appendix B). Thus, as shown in Table 2, the appearance of this reactivation contribution improves the cell operation (|RVLF|/RMF1 = 2.9 and |RVLF|/RLF = 4.0), before H2O transport phenomena becomes dominant as shown by the decrease in the ratios |RVLF|/RMF1 (from 2.9 to 2.3), |RVLF|/RMF2 (from 1.7 to 1.0), and |RVLF|/RLF (from 4.0 to 3.4). Details of the resistance values obtained by fitting are shown in Table B1.
Premature degradation evolution with time
To understand the evolution of Rp with time, a qualitative analysis of the impedance diagrams recorded at the most relevant current densities applied was realized, leading to the following observations:
-
At OCV (Fig. 7a), an increase with time in the whole impedance diagram is observed, even if the lower frequency part becomes more difficult to observe with time because of the instability of the cell.
-
When i = − 0.2 A cm−2 is applied (Fig. 7b), the cell already reaches the “pseudo” optimal utilization region at 144 h and 168 h, while the system is still activating at 0 h. However, from 0 to 144 h, the whole impedance diagram increases as well, whereas from 144 to 168 h, it is the HF part of the diagram which mainly increases. This means that from the first current densities applied, all the main phenomena dominating the cell functioning, especially charge transfer at the Ni-YSZ/YSZ interface and the different phenomena related to H2O transport at the Ni-YSZ electrode, are disrupted, up to a certain time above which interfacial charge transfer is mainly disrupted.
-
When i = − 0.4 A cm−2 (Fig. 7c) is applied, the cell reaches the optimal utilization region at 0 h while it is already in the H2O transport limitation region at 144 h and 168 h. This is once again characterized by an increase in the whole impedance diagram from 0 to 144 h, but also from 144 to 168 h. Thus, at this functioning state of the cell, all the dominating phenomena are affected, whatever the time considered.
-
From i = − 0.6 A cm−2 (Fig. 7d) to i = − 0.8 A cm−2 (Fig. 7e) applied, the cell is in the region IV of reactivation for 144 h and 168 h while it only starts reaching the H2O transport limitation region at 0 h. In this case, the whole impedance diagram still increases with time but the reactivation contribution appears at the lowest frequencies for 144 h and 168 h, and this from − 0.6 A cm−2 for 168 h. Thus, even if the main phenomena dominating the cell are still disrupted, appearance of the reactivation contribution allows the renewal of the cell performance, and this for a lower value of current density applied with time. H2O transport limitation is, at this stage, counterbalanced by the reactivation contribution.
-
Above − 0.8 A cm−2 applied, the current demand of the cell is so large that the system is reaching a region where H2O transport limitation predominates the functioning of the cell, whatever the time considered. This is characterized notably at − 1.0 A cm−2 (Fig. 7f) by an increase in the whole impedance diagram.
It is important to notice that the Rp value evolves differently with time depending on the current density applied. Indeed, until − 0.5 A cm−2, Rp increases with time. However, above − 0.5 A cm−2 applied, this trend is reversing progressively until − 0.8 A cm−2, value from which Rp decreases with time. This trend is in accordance with the interpretation made previously regarding the appearance of the premature degradation.
According to Table C1 (cf. Appendix C), f0 and C values obtained from the fittings using the electrical equivalent circuits shown Fig. C1 (cf. Appendix C) allow distinguishing the same four to five main phenomena for the functioning of the cell as the ones obtained earlier. However, more or less important shifts of f0 and C can be noticed with time and current density change, and make this distinction less obvious. Nevertheless, this distinction remains clear when one duration is considered.
Table 3 shows the ratios R144 h/R0 h and R168 h/R144 h for the frequency ranges HF, MF1, MF2, LF, and VLF at the different current densities of the impedance diagrams presented in Fig. 7. Most of these ratios are above 1.0, which means an increase with time in the resistance for all the contributions. It should be however noted that for the LF part, the ratio R168 h/R144 h varies between 0.72 and 1.0, whereas R144 h/R0 h is mostly above 1.0. Thus, contrary to the other phenomena, the H2O diffusion phenomenon at the Ni-YSZ electrode associated with the LF part tends to be less resistive with time. This is related to the appearance of the reactivation contribution. Indeed, at 144 h, when this new contribution appears, the LF part is still present while at 168 h, it is not anymore. It is moreover interesting to notice that when the ratios R144 h/R0 h and R168 h/R144 h are considered, the values are particularly high for the HF and MF1 parts. Indeed, according to Table 3, for the HF part, R144 h/R0 h and R168 h/R144 h ratios reach up to 8.5 and 2.5, respectively, and, for the MF1 part, 3.9 and 2.3, respectively. On the contrary, except the values at 0 A/cm2, these ratios are mostly inferior to 2 for the MF2 and LF parts. Thereby, the phenomena related to the HF and MF1 parts are the most affected by the degradation with time, the one related to the HF part being especially affected initially according to the ratio R144 h/R0 h.
Table 4 shows the ratios C144 h/C0 h and C168 h/C144 h for the HF, MF1, MF2, LF, and VLF parts at different current densities. The ratio C144 h/C0 h is systematically inferior to 1.0, which means that all the capacitances related to the main phenomena decrease with time. This decrease is particularly important for the HF and MF1 parts which ratios are mostly between 0.1 and 0.2, meaning a capacitance variation with a factor from 5 to 10 between 0 and 144 h.
Thus, appearance of the premature degradation is characterized with time by a decrease in all capacitances, which is surprising since this trend is rather the sign of a favorable evolution of the cell functioning with time, while a degradation of the cell is observed. On the other hand, this trend is predominantly reversed when the ratio C168 h/C144 h is considered since most of the capacitances increase with time. In this case, the MF1, MF2, and LF parts are the most characteristic of this increase, meaning that the capacitances related to the H2O transport phenomena are mostly increasing between 144 and 168 h. This is in accordance with a more difficult H2O transport processing as a consequence of the premature degradation. A possible explanation might be that H2O transport in the cell is disturbed, leading locally to the accumulation of H2O in the porous path along the way from the surface to the Ni-YSZ/YSZ interface. With values between 0.63 and 1.1, the HF part ratios are mostly inferior to 1, meaning that the HF capacitances associated to charge transfer at the Ni-YSZ/YSZ interface are still decreasing between 144 and 168 h. This decrease is however much less important than between 0 and 144 h. This clearly shows that, with time, the premature degradation has more influence on the capacitive behavior of all the main electrochemical processes governing cell functioning, including charge transfer at the Ni-YSZ/YSZ interface. Consequently, the evolution of the degradation with time seems to be mainly shown above 144 h by an increase in most of the capacitances related to the phenomena dominating the cell functioning, except charge transfer which continues to decrease.
Discussion on the premature degradation origins
It is first worth reminding that the set-up was modified for this test by sealing the cell holder and the support pieces (see Fig. 1). The premature degradation was not observed for the tests realized before sealing the pieces, including the one discussed elsewhere [43]. Consequently, this degradation is related to the sealing done using Schott 8422 glass composed of SiO2 (69.8%), B2O3 (7.8%), Na2O (12.0%), K2O (4.1%), CaO (0.4%), and BaO (0.2%). We know that the two pieces provide and release H2 electrode gas composed of 90% H20 and 10% H2. Besides, according to Hauch et al. [22], in the experimental conditions of this test, SiO2 and H2O can generate gaseous Si(OH)4 as follows:
Once deposited, Si can segregate in the volume of Ni-YSZ electrode up to the Ni-YSZ/YSZ interface, this segregation being facilitated by the presence of Na2O and Al2O3 [55,56,57,58,59,60,61].
Figure 8 shows the post-mortem SEM images of the tested cell, with a transversal cut of the H2 electrode volume (Fig. 8a), a transversal cut of the complete cell (Fig. 8b), the H2 electrode surface (Fig. 8c), and a transversal cut of the first dozens of microns of the H2 electrode (Fig. 8d).
Post-mortem SEM images of the tested cell, with a transversal cut of the H2 electrode volume (a), a transversal cut of the complete cell (b), including LSCF, YDC, and YSZ layers, and the first dozens of microns of Ni-YSZ cathode (from the Ni-YSZ/YSZ interface), the H2 electrode surface (c), and a transversal cut of the first dozens of microns for the H2 electrode (d) with, from the surface, a Ni layer, a dense 8YSZ layer, and the Ni-YSZ cermet. It should be noticed that the Ni layer is for current collection at the H2 electrode surface while the dense 8YSZ layer aims at maintaining mechanically the cell [62]
Table 5 contains the amounts of Si, Al, Na, Ka, and Ca elements obtained by SEM/EDX analyses. All the results are presented in atomic percentage.
The MEB/EDX analyses do not show any presence of Si in the H2 electrode volume, but rather at the Ni-YSZ/YSZ interface. Besides, a regular presence of Si, Al, Na, K, and Ca is noticed at the H2 electrode surface and at the first dozens of microns in the volume from the surface. These elements are also detected in the LSCF and YDC volumes, as for tests anterior to the sealing of the two alumina pieces [63], meaning that this is not linked to the premature degradation of the cell. On the contrary, no trace of these elements was observed at the surface or the volume of the H2 electrode, or at the Ni-YSZ/YSZ interface for the tests anterior to the sealing [63]. Thus, this degradation is related to the presence of Si, at the H2 electrode surface and at low depth mainly, as well as at the Ni-YSZ/YSZ interface. There might have been formation of Si(OH)4 from SiO2 and H2O as Hauch et al. suggested [22] leading in our case to Si deposit on the H2 electrode surface and Si segregation in the first dozens of microns in the volume from the surface, and also at the Ni-YSZ/YSZ interface. Hence, in our electrolysis conditions, the presence of Si has an important influence on cell degradation, contrary to a study made by Virkar in SOFC mode [64].
We interpret the behavior of the cell and its premature degradation as follows. The presence of Si at the H2 electrode surface and low depth disturbs charge transfer at the cathode side from the lowest current densities applied, because of a direct contact with the current collectors, as well as H2O transport necessary for the reduction reaction at the Ni-YSZ/YSZ interface. Disruption of H2O transport, which is only partial first according to the earlier discussion, becomes increasingly important with the current density applied, to be finally generalized to all the processes related to H2O transport at the H2 electrode, while charge transfer is still disrupted.
We know that the appearance of the new phenomenon is characterized by the emergence of the positive contribution, assigned to a reactivation contribution, at the lowest frequencies. Moreover, a study conducted by Van Hassel et al. in SOFC mode showed that this kind of contribution can be associated with O2− adsorption phenomenon at the O2 electrode/electrolyte interface [65, 66]. In this work, the cell is functioning in electrolysis mode, and the new phenomenon is related to the Ni-YSZ cathode. Therefore, this phenomenon might be associated with H2O adsorption.
Mechanism of the premature degradation
As illustrated in Fig. 9, the premature degradation results from deposit of impurities containing Si at the H2 electrode interface (surface and first dozens microns in the volume) and at the Ni-YSZ/YSZ interface. As a direct consequence, current collection is disrupted at the Ni-YSZ/current collector interface, and so charge transfer, but also H2O transport. Thus, based on the analysis of the polarization curves and the impedance diagrams recorded in situ at different cell working times, and the SEM/EDX post-mortem characterization, a mechanism illustrated in Fig. 10 is proposed as follows to explain the cell behavior while in use further to the premature degradation:
Illustration of the mechanism explaining cell behavior while in use after impurity deposits containing Si at the root of the observed premature degradation. Initially (t = 0 h), optimal functioning after cell activation (a), with no H2O transport limitation for | i | ≤ 0.5 A cm−2; partial limitation for 0.5 < | i | < 0.8 A cm−2 (b); important limitation by H2O transport for | i | ≥ 0.8 A cm−2 (c). After premature degradation (t = 144 h), partial limitation by H2O transport from | i | < 0.4 A cm−2 (d); presence of Si impurities at some TPBs prevents H2O reduction reaction (e); Si impurities disturb or even prevent H2O crossing and so its transport toward TPBs mainly located at the Ni-YSZ/YSZ interface (f). For | i | ≥ 0.6 A cm−2, the applied current is sufficiently high to lead to a cell reactivation by H2O adsorption (g) at TPBs polluted by Si impurities and/or H2O shifting by surface adsorption/desorption (h) at Ni sites to “bypass” the pathways blocked by Si impurities. However, in the meantime, from | i | = 0.4 A cm−2 (i), high limitation by H2O transport which finally becomes predominant for cell functioning
- At t = 0 h, the cell has a typical functioning, with H2O supplied in excess for the lowest current densities applied (Fig. 10a), with less excess thereafter (Fig. 10b), before becoming limiting when the cell is used at the highest current densities applied (Fig. 10c);
- At t = 144 h, because H2O transport is (severely) limited by impurity deposits containing Si, the cell is functioning with a H2O supply already diminished from the very lowest current densities applied (Fig. 10d). Si deposits affect H2O transport in two ways: (i) H2O cannot reach some TPBs contaminated by these deposits (Fig. 10e), which prevents its reduction and so H2 formation and (ii) H2O crossing is disturbed or even prevented because of Si deposits (Fig. 10f), which leads to a H2O depletion at the Ni-YSZ/YSZ interface from the lowest current densities.
This trend continues until a certain i value from which the applied current leads to the appearance of an H2O adsorption phenomenon allowing H2O reduction at the contaminated reaction sites (Fig. 10g) and H2O transport in the blocked pathways (Fig. 10h). There is a reactivation of the cell resulting from this H2O adsorption phenomenon. This trend continues until a certain i value above which transport limitation is significant (Fig. 10i), H2O transport at the Ni-YSZ electrode becoming the predominant limiting phenomenon for the cell functioning. The cell behavior is then more the consequence of the cell under a too significant current demand than an effect of Si deposits. This mechanism, in fair agreement with the experimental data, can justify such an unexpected cell behavior not reported so far under electrolysis mode, to the best of our knowledge. Yet alternative phenomena such as adsorption of H2O on YSZ surface remain also plausible.
Conclusions
To avoid a critical issue that represents H2 leakage, glass sealing was used between the electrolysis cell holder and the support pieces. This beneficial effect was counterbalanced by the appearance of a premature degradation. This degradation was characterized by a deformation of the i-U curve, several frequency shifts in the impedance diagrams as well as the appearance of a new impedance reactivation contribution at the lowest frequencies. This new impedance contribution has not been reported to date, to the best of our knowledge, in the case of a solid oxide cell working in electrolysis mode. The post-mortem cell analysis by SEM/EDX revealed the presence of Si, Al, Na, K, and Ca at the H2 electrode interface (surface and first dozens microns in the volume) and at the Ni-YSZ/YSZ interface, by opposition to similar cells tested before without sealing of the pieces. Thus, this degradation was associated with Si deposition, including at the Ni/YSZ/H2O TPBs, and the reactivation contribution characterized by ([1–10 mHz], |100 F cm−2|) to an H2O adsorption phenomenon, including at these same TPBs. This contribution, which appears for the highest current densities applied, allows a renewal of the cell performance until limitation by H2O transport becomes the predominant phenomenon governing the cell functioning. The evolution with time of the different resistive and capacitive contributions is consistent with the interpretation of the premature cell degradation. Analysis of the impedance diagrams, including by use of electrical equivalent circuits, allowed to clearly distinguish four to five contributions related to the functioning of the electrolysis cell as follows and illustrated in Fig. 11:
-
HF part (f0 = [100 –1000 Hz]; C = 10 m F cm−2) is associated with charge transfer at the Ni-YSZ/YSZ interface;
-
MF1 part (f0 = 10 Hz; C = 100 m F cm−2) is related to an H2O diffusion phenomenon at the H2 electrode;
-
MF2 part (f0 = 1 Hz; C = 1 F cm−2) is associated with H2O gas conversion at the H2 electrode;
-
LF part (f0 = 0.1 Hz; C = 10 F cm−2) is also related to an H2O diffusion phenomenon at the H2 electrode; and
-
VLF part (f0 = [1–10 mHz]; C = |100 F cm−2|) is associated with an H2O adsorption phenomenon at the H2 electrode.
Hence, this deeper analysis highlights that (i) all the dominant phenomena identified as driving cell operation in electrolysis mode are associated with the H2 electrode and (ii) except charge transfer at the Ni-YSZ/YSZ interface, these phenomena are all related to H2O transport at the H2 electrode. Consequently, in order to enhance the performances of the system considered, further work should mainly focus on the H2 electrode, and more particularly on the ways to optimize H2O transport through the electrode. The observation of the premature degradation highlights the fact that the sealing process is a critical step that has to be reconsidered in future studies. Furthermore, this thorough electrochemical investigation based on chronopotentiometry and EIS sheds light onto how impurity deposit can dramatically affect the H2 electrode and consequently the cell functioning.
References
National Academy of Sciences, National Research Council (February 2004) The hydrogen economy: opportunities, costs, barriers. In: and R&D needs
Manage MN, Hodgson D, Milligan N, Simons SJR, Brett DJL (2011) Int J Hydrog Energy 36(10):5782–5796
Nechache A, Cassir M, Ringuedé A (2014) J Power Sources 258:164–181
Fischer GW, Gels HB, Gross F, Liemert K, Rohr FJ (1978) J Power Sources 3(4):331–345
Elektrochemische Prozesse (1975) DECHEMA-Studie
Isenberg AO, Brecher LE (1970) Water vapor electrolysis at high temperatures, Westinghouse Electric Corp., Final Report, Project Fuel Cell, Rep. No. 57, Proc. First Int. Energy Agency Water Electrolysis Workshop. Brookhaven National Laboratory, Sept. 1975
Rohr FJ High temperature solid oxide fuel cells, Proc. Int. Energy Agency Workshop on Solid Electrolyte Fuel Cells. Brookhaven National Laboratory, May 1977
Isenberg AO (1981) Solid state Ionics 3/4:431–437
Maskalick NJ (1986) Int J Hydrog Energy 11(9):563–570
Barbi GB, Mari CM (1981) Mater Chem 6(1):35–54
Barbi GB, Mari CM (1982) Solid State Ionics 6(4):341–351
Barbi GB, Mari CM Paper presented at the 4th Int. Conf. on Solid State Ionics. Grenoble (4–8 July 1983)
Barbi GB, Mari CM (1988) Solid State Ionics 26(3):243–250
Barbi GB, Mari CM (1984) Int J Hydrog Energy 9(11):895–899
Dönitz W, Schmidberger R, Steinheil E (1980) Int J Hydrog Energy 5(1):55–63
Dönitz W, Schmidberger R (1982) Int J Hydrog Energy 7(4):321–330
Dönitz W, Erdle E (1985) Int J Hydrog Energy 10(5):291–295
Dönitz W, Dietrich G, Erdle E, Streicher R (1988) Int J Hydrog Energy 13(5):283–287
Chen K, Hyodo J, Ai N, Ishihara T, Jiang SP (2016) Int J Hydrog Energy 41(3):1419–1431
Hauch A, Jensen SH, Ramousse S, Mogensen M (2006) J Electrochem Soc 153(9):A1741–A1747
Jensen SH, Hauch A, Hendriksen PV, Mogensen M, Bonanos N, Jacobsen T (2007) J Electrochem Soc 154(12):B1325–B1330
Hauch A, Jensen SH, Mogensen M, Bilde-Sørensen JB (2007) J Electrochem Soc 154(7):A619–A626
Hauch A, Ebbesen SD, Jensen SH, Mogensen M (2008) J Electrochem Soc 155(11):B1184–B1193
Barfod R, Mogensen M, Klemenso T, Hagen A, Liu YL, Hendriksen PV (2007) J Electrochem Soc 154(4):B371–B378
Barfod R, Mogensen M, Klemenso T, Hagen A, Liu YL, Hendriksen PV (2005) In: Singhal SC, Mizusaki J (eds) Solid Oxide Fuel Cells (SOFC IX), PV 2005–07, p 524. The Electrochemical Society Proceedings Series, Pennington, NJ
Ebbesen SD, Graves C, Hauch A, Jensen SH, Mogensen M (2010) J Electrochem Soc 157(10):B1419–B1429
Laguna-Bercero MA (2012) J Power Sources 203:4–16
Moçoteguy P, Brisse A (2013) Int J Hydrog Energy 38(36):15887–15902
Keane M, Fan H, Han M, Singh P (2014) Int J Hydrog Energy 39(33):18718–18726
Chen K, Ai N, Jiang SP (2014) Int J Hydrog Energy 39(20):10349–10358
Hjalmarsson P, Sun X, Liu YL, Chen M (2014) J Power Sources 262:316–322
Chen T, Liu M, Yuan C, Zhou Y, Ye X, Zhan Z, Xia C, Wang S (2015) J Power Sources 276:1–6
Knibbe R, Traulsen ML, Hauch A, Ebbesen SD, Mogensen M (2010) J Electrochem Soc 157(8):B1209–B1217
Pan Z, Liu Q, Lyu R, Li P, Chan SH (2018) J Power Sources 378:571–578
Duboviks V, Maher RC, Kishimoto M, Cohen LF, Brandon NP, Offer GJ (2014) Phys Chem Chem Phys 16(26):13063–13068
Tao Y, Ebbesen SD, Mogensen MB (2014) J Electrochem Soc 161(3):F337–F343
Tao Y, Ebbesen SD, Zhang W, Mogensen MB (2014) ChemCatChem 6:1220–1224
Skafte TL, Blennow P, Hjelm J, Graves C (2018) J Power Sources 373:54–60
Duboviks V, Lomberg M, Maher RC, Cohen LF, Brandon NP, Offer GJ (2015) J Power Sources 293:912–921
Zheng Y, Li Q, Chen T, Wu W, Xu C, Wang WG (2015) Int J Hydrog Energy 40(6):2460–2472
Kim SJ, Kim KJ, Choi GM (2015) J Power Sources 284:617–622
Hjalmarsson P, Sun X, Liu YL, Chen M (2013) J Power Sources 223:349–357
Nechache A, Mansuy A, Petitjean M, Mougin J, Mauvy F, Boukamp BA, Cassir M, Ringuedé A (2016) Electrochim Acta 210:596–605
Brisse A, Schefold J, Zahid M (2008) Int J Hydrog Energy 33(20):5375–5382
Leonide A, Sonn V, Weber A, Ivers-Tiffée E (2008) J Electrochem Soc 155(1):B36–B41
Dasari HP, Park SY, Kim J, Lee JH, Kim BK, Je HJ, Lee HW, Yoon KJ (2013) J Power Sources 240:721–728
Fan H, Keane M, Singh P, Han M (2014) J Power Sources 268:634–639
Schefold J, Brisse A, Zahid M (2009) J Electrochem Soc 156(8):B897–B904
Wang W, Huang Y, Jung S, Vohs JM, Gorte RJ (2006) J Electrochem Soc 153(11):A2066–A2070
Hanifi AR, Laguna-Bercero MA, Etsell TH, Sarkar P (2014) Int J Hydrog Energy 39(15):8002–8008
Laguna-Bercero MA, Campana R, Larrea A, Kilner JA, Orera VM (2011) J Power Sources 196(21):8942–8947
Jiang SP, Badwal SPS (1999) Solid State Ionics 123(1-4):209–224
Laguna-Bercero MA, Kilner JA, Skinner SJ (2010) Chem Mater 22(3):1134–1141
Chen S, Xie K, Dong D, Li H, Qin Q, Zhang Y, Wu Y (2015) J Power Sources 274:718–729
Anderson JC, Leaver KD, Rawlings RD, Alexander JM (1990) Materials science. Chapmann & Hall, New York
Doremus RH (1973) Glass science. John Wiley & Sons, New York
Holland L (1964) The properties of glass surface. Chapman and Hall, London
Butler EP, Drennan J (1982) J Am Ceram Soc 65(10):474–478
Jewell JM, Spess MS, Shelby JE (1990) J Am Ceram Soc 73(1):132–135
Jewell JM, Shelby JE (1990) J Cryst Growth 73:1446
Shelby JE, Mcvay GL (1976) J Non-Cryst Solids 20(3):439–449
Mansuy A (2012) PhD Thesis, Université Bordeaux 1
Nechache A (2014) PhD thesis, Université Pierre et Marie Curie, Paris
Virkar AV (2007) J Power Sources 172(2):713–724
van Hassel BA, Boukamp BA, Burggraaf AJ (1991) Solid State Ionics 48:139–154
van Hassel BA, Boukamp BA, Burggraaf AJ (1991) Solid State Ionics 48:155–171
Funding
This work is supported by the French Research National Agency (ANR) through Hydrogène et piles à combustible program (project FIDELHYO n°ANR-09-HPAC-005).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Nechache, A., Boukamp, B.A., Cassir, M. et al. Premature degradation study of a cathode-supported solid oxide electrolysis cell. J Solid State Electrochem 23, 109–123 (2019). https://doi.org/10.1007/s10008-018-4116-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-018-4116-7