Abstract
A solid state polyvinyl chloride (PVC) membrane Li+-selective electrode was prepared and used as a detector in a low-dead volume flow through cell for the determination of Li+ in pharmaceutical formulations and human serum samples. The potentiometric performance characteristics of the electrode were calculated under the optimized flow conditions. The electrode had near-Nernstian behavior in the concentration range of 0.1–100 mM (R 2 = 0.9981) with a slope of 61.34 mV decade−1 and detection limit of 0.080 mM. The relative standard deviation of the electrode response for eight replicate measurements of 100, 10, and 1 mM Li+ was 0.43%, 0.45%, and 0.99%, respectively. The designed flow-through cell detector system revealed sampling rates of approximately 70 injections per hour. Flow injection potentiometry (FIP) results obtained for the pharmaceutical formulations were in good harmony with the atomic emission spectrophotometry results. However, the electrode could not be used successfully for the direct analysis of real serum samples in FIP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Utilization of ion selective electrodes (ISEs) as detectors in flow-through automatic analyzers and continuous monitoring systems for the analysis of pharmaceutical formulations and clinical samples are very common because of their ease of use, low-cost, high sampling-rate, and practicability [1–3]. More importantly, the qualified experts and the sophisticated constituents are not needed for the use of these kinds of systems.
Up to now, various kinds of lithium-selective electrodes such as polyvinyl chloride membrane, solid contact membrane, and ceramic membrane electrodes were fabricated and their potentiometric performance characteristics were described in some of the reviews and papers [4–17]. The number of flow injection potentiometric applications of the Li+-selective electrodes among them resulted in failure [18–25]. In most of the flow injection potentiometric studies available in the literature, Li+-selective electrodes were generally tested in synthetic aqueous solutions. The measurements taken in real samples were frequently avoided. Li-selective electrodes were widely used in monitoring of serum lithium levels of the patients with bipolar disorders. Bipolar disorder is a psychiatric diagnosis that describes a category of mood disorders defined by the presence of one or more episodes of abnormally elevated mood. It can result in damaged relationship, poor job or school performance, and even suicide [26]. In the treatment of bipolar disorders, a number of chemical salts of lithium are used as a mood-stabilizing drug [27, 28]. The therapeutic range of Li+ is very narrow (0.6–1.5 mmol L−1) and close to toxic level (>2 mmol L−1). If the Li+ level is below the curable dose, the treatment resulted in failure. On the other hand, overdose of the Li+ may cause fatal and toxic effects. Therefore, it is important to monitor the lithium level continuously in the blood of this kind of patient for making the treatment adequate and safe [29, 30].
In our earlier paper [31], an all-solid state Li+-selective electrode was fabricated and its potentiometric performance characteristics were fully investigated in batch conditions. The prepared electrode was also successfully utilized to determine lithium levels of human serum samples and pharmaceuticals which are used in the treatment of manic depressive patients.
The goal of the present work was to develop and optimize a flow injection system for rapid and reliable determination of Li+ in pharmaceutical formulations and serum samples by potentiometry with Li+-selective electrode mentioned above.
In the study, we combined superior properties of ISEs and flow injection analysis method by constructing a low-dead volume flow-through cell. The solid-state Li+-selective electrode was used as a detector in this cell. The potentiometric performance characteristics of the electrode were investigated under dynamic conditions by using this detector cell. The practical applicability of the electrode for the sample analysis under dynamic conditions was studied and lithium contents of some pharmaceuticals and human serum samples were determined. The use of low-dead volume flow-through cell offers a simple procedure for continuous monitoring of lithium contents of some pharmaceuticals with high accuracy, selectivity, sensitivity, and good analytical frequency.
Experimental
Reagents and solutions
Tetrahydrofuran (THF), high molecular weight poly(vinyl chloride; PVC), o-nitrophenyl octyl ether (NPOE), potassium tetrakis (para-chlorophenyl) borate (KTpClPB), graphite and ionophore, 6,6-dibenzyl-1,4,8-11-tetraoxosiclotetradecane (Lithium Ionophore VI), used in the construction of the ISE, were purchased from Fluka (Bucks, Switzerland). Epoxy (Macroplast Su 2227) and hardener (Desmodur RFE), used in the preparation of conductive solid contact, were purchased from Henkel (Istanbul, Turkey) and Bayer AG (Darmstadt, Germany), respectively. Human blood serum samples were provided from one of the local hospitals. Two drug tablets (Lithuril) containing various amount of Li2CO3 (300 and 400 mg) analysed in the present study was purchased from Ilac and Kimya San. A. S. (Istanbul, Turkey). Distilled deionised water was used throughout for the preparation of the aqueous solutions. All solutions prepared were of the analytical grade nitrate or chloride salts of the relevant cations.
Fabrication of the flow-through all-solid state Li+-selective electrode
For the preparation of the flow-through all-solid state electrode, the similar steps described in our previous works [31, 32] were followed. The electrode was prepared at two steps. At the first step, a mixture of conductive material consisting of 50% (w/w) graphite, 35% (w/w) epoxy, and 15% (w/w) hardener was prepared by mixing in sufficient THF. This conductive material were filled into a 5-mm copper pipe with a diameter of 4 mm and kept at 30 °C overnight. Then, for the electrical connection, an electric cable was soldered to the copper pipe and a hole of 0.1 mm radius was opened in the centre of the conductive material. At the second step, a membrane cocktail consisting of 1% (w/w) Lithium Ionophore VI, 29% (w/w) PVC, 69.5% (w/w) NPOE, and 0.5% (w/w) KTpClPB was prepared in 3 ml THF; and the hole opened in the centre of the conductive material at the first step was covered with this membrane cocktail. The coated surface was left to be dried at laboratory conditions overnight. The flow-through electrode was conditioned by soaking into 100 mM LiNO3 solution for at least 6 h before use. Then, the electrode combined with a miniaturized salt bridge was directly attached to the end of the flow-line consisting PVC tubing (30 cm length and 0.1 mm i.d.) to minimize possible dilution of the sample in the carrier solution. When not in use, sensors were stored in the laboratory conditions.
Apparatus and FIA system
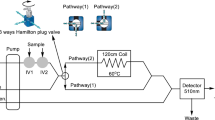
Figure 1 indicates the schematic diagram of the used potentiometric flow injection analysis (FIA) system. The carrier solution was aspirated through the tubing and electrodes by the P680 HPLC pump (Dionex). Samples and standards were injected into the carrier solution using a two-position injection unit (Vici). Potentiometric measurements were recorded by using a laboratory-made computer-controlled high-input impedance multi-channel potentiometric measurement system which has a home-made software program. The flow-cell was equipped with a saturated calomel electrode (Gamry). The pH of the buffer solutions was monitored by using a glass pH electrode (Schott) with a Jenway 3040 model Ion Analyser. All measurements were carried out at room temperature (20 ± 2 °C).
Atomic emission spectrophotometry (AES) measurements were taken by Unicam-929 Atomic Absorption Spectrophotometer.
Solutions at required concentrations were homogenized using Ultrasonic LC30 (Germany) stirrer.
Results and discussion
FIP optimisation
After the electrode was inserted on the flow line, firstly, we desired to determine potentiometric performance characteristics of the prepared electrode in FIA system at dynamic conditions. Therefore, factors affecting signal shape and intensity such as suitable tubing length from injection valve to measurement cell, concentration, and composition of the carrier solution, injection volume and flow rate were optimized. The effects of sample injection volume (20–100 μL), carrier flow rate (0.1–1.1 mL min−1), and tubing length (10–100 cm) were examined.
Due to dispersion, the peak width of the potentiometric signal increases as the length of tubing from injection valve to the detection cell increases, so this tubing length is kept as short as possible to minimize dispersion. On the other hand, it is necessary to have an adequate mixture of the injected sample and the carrier solution before getting into the flow detection cell [33]. In the present FIP system, the optimum tubing length for minimum dispersion and well mixture of sample and carrier solution is found to be 30 cm (0.1 mm i.d.).
It is well-known that, in FIP, carrier solution composition affects the response behaviour of ion selective electrode in terms of baseline stability [34, 35]. We have examined various electrolyte solutions at different concentrations (100, 50, and 10 mM) such as MgSO4, MgCl2 and Na2SO4 as carrier solutions (flow rate: 1 mL min−1, injection volume 20 μL). Of the TRIS-HCl buffer, 10 mM pH = 8.3 was used as background for all the carrier solutions; 10 mM pH = 8.3 TRIS-HCl buffer was also merely used as carrier solution. Among the tested solutions, 10 mM MgCl2 in 10 mM pH = 8.3 TRIS-HCl buffer was found to provide best analytical characteristics with respect to baseline stability, response time, reproducibility, linear range, and detection limit.
To determine optimum flow-rate and injection volume, 10 mM Li+ standard was injected into the carrier solution, 10 mM MgCl2 in 10 mM TRIS-HCl at pH = 8.3. A slight decrease in peak height was observed when the flow rate was increased from 0.1 to 1.1 mL min−1. But the peak width (residence time) was significantly decreased at flow rate of approximately 1.0 mL min−1. At flow-rates above the 1.0 mL min−1, no significant decreases were seen in peak widths, thus peak shapes and reproducibilities were deteriorated beyond this flow rate. Therefore, flow rate of 1.0 mL min−1 was preferred as optimum and used during the rest of the study. Of the 10 mM standard lithium solutions 20, 50, and 100 μL were injected into the carrier solution to investigate the effect of the sample volume. In general, the higher are the injection volumes, the higher are the peak heights and longer residence time at the electrode surface. It requires more time to reach steady state and greater consumption of carrier solution [36]. In the present system, because the increasing injection volume caused very long residence time, utilization of higher injection volumes were avoided. Therefore, injection volume of 20 μL was chosen for the sake of shorter analysis time and less solution consumption.
Potentiometric performance characteristics of the electrode in flow conditions
Standard lithium solutions were injected into the carrier solution for the determination of the electrode performance under the preliminary determined optimum conditions (carrier: 10 mM MgCl2, flow rate: 1.0 mL min−1, injection volume: 20 μL), the obtained peaks and relevant calibration graphic are given in Fig. 2. These conditions provide about 96–98% of the maximum peak height and compromise between sensitivity, sample rate, and linear operational range.
Under the above optimum conditions, the response time (baseline-to-baseline time) of the electrode in the dynamic mode is 20–80 s depending on the concentration of Li+ in solutions. These data reveal that the proposed flow through detector cell provides a low dead volume, fast response time, and sampling rates approximately 70 injections per hour. Further calculations showed that the prepared electrode had a linear response over the concentration range of 100–0.1 mM. The electrode showed nearly Nernstian behaviour with a slope of 61.34 mV in the range of linear response. Limit of detection and limit of quantification of the electrode were calculated as 0.080 mM and 0.18 mM, respectively. The equation that describes the analytical performance of the electrode is given by:
The correlation coefficient (r) was 0.9981 for n = 8 (n, the number of repetition for the same electrode).
Potentiometric responses of the electrode to Li+ and some of interferents were indicated in Fig. 3. We preferred separate solution method [37] for the calculation of the selectivity coefficients of the electrode in the dynamic condition because other calculation methods are very time consuming and laborious. The selectivity coefficients obtained in the batch and FIP mode are listed in Table 1. When the calculated selectivity coefficients in FIP and batch mode are compared, it is seen that there are some differences. Previous works on the solid state membrane electrodes shows that the apparent selectivity coefficient calculated under dynamic conditions may differ significantly from the one calculated under batch conditions [38, 39]. This situation can be explained by the difference in time of interactions of interferents with the surface of the electrode membrane. In addition, selectivity coefficients are dependent on the diffusion rate and exchange reaction of the interferents [40].
While the electrode shows high sensitivity and selectivity towards Li+ ions in solution, the drift of the potential is of concern for continuous monitoring. Hence, the study of response stability of the electrode was also carried out. The potential stability of the electrode was tested with the eight successive measurements taken in the 100, 10, and 1.0 mM standard Li+ solutions as indicated in Fig. 4. Average potential values and standard deviations calculated for 100, 10, and 1.0 mM Li+ solutions at 95% confidence level were 202.1 ± 0.9, 140.1 ± 0.6, and 80.3 ± 0.8 mV, respectively. The drift of the baseline potential 1.5 mV h−1 and the noise level was about 0.1 mV.
Analytical applications
Analysis of pharmaceutical formulations
In this part of the study, two commercially available pharmaceutical formulations were analyzed. Sample solutions of the pharmaceutical formulations containing 300 mg and 400 mg Li2CO3 were prepared as follow. One tablet of the each pharmaceutical formulations was dissolved in 1 L TRIS-HCl buffer (10 mM and pH = 8.3) by using ultrasonic stirrer. The final pH of the solutions were adjusted to 8.3 adding a few drops of 100 mM HCl. Standards and samples were analyzed under the optimized conditions (injection volume: 20 μL, flow rate: 1.0 mL min−1 carrier solution: 10 mM MgCl2 in 10 mM pH = 8.3 TRIS-HCl). The obtained triplet peaks for the standards and samples and calibration graph were indicated in Fig. 5. For the validation of the FIP measurements, AES measurements were used as standard method. Results obtained in the FIP and AES for the determination of Li+ in each tablet were given in Table 2. It is seen that, the obtained results for each pharmaceutical formulations in FIP were in a good agreement with the AES results at % 95 confidence level. The obtained F and t values show that the present FIP method is of comparable precision to AES method and there is no significant difference between the mean values obtained by the two methods.
Analysis of serum samples
In this application, we attempted to determine Li+ levels of the spiked real serum samples by direct injection. Throughout the serum analysis study, artificial serum solution was used as a carrier solution to minimize any contributions of the significant interferents to the analytical signal of the electrode. Artificial serum solution was consisted of 140 mM NaCl, 4.5 mM KCl, 2.5 mM CaCl2, 0.8 mM MgCl2, 2.5 mM urea, and 4.7 mM glucose. 10 mM TRIS-HCl buffer was used as the background of the artificial serum solution. The final pH of the solution was fixed to 7.3 with the addition a few drops of concentrated HCl. Standard Li+ solutions were prepared from LiNO3 salt using the artificial serum electrolyte as a background solution. To investigate the interference effect of the Na+ ions in the serum analysis, three Li+-standard series were prepared using the artificial serum electrolyte in the similar composition but different in the amount of Na+ (120, 140 and 160 mM) as background. These Li+ standard series were injected into the carrier solution under the optimized conditions that injection volume and flow rate were 20 μL and 1.0 mL min−1, respectively. To observe the differences appearing in the potentiometric behaviour of the electrode with the injection of both artificial serum standards and real serum samples, lithium spiked real serum samples were also injected into the carrier solution as standard Li+ solutions. The obtained peaks and calibration graphic were indicated in Figs. 6 and 7, respectively.
Calibration graphics related to the peaks given in Fig. 6
Figure 7 shows that the sensor presented non-Nernstian behaviour in the measured concentration range of 0.8 and 7 mM Li+. No considerable potential differences were observed between the real serum samples of 0.8 and 1.0 mM Li+. Nevertheless, electrode still behaved linearly in the concentration range of 1.0–7.0 mM Li+. Using the calibration graphic obtained from the aqueous Li+ standard solutions with 140 mM Na+ as background, Li+ contents of both aqueous artificial serum samples (containing 120, 140, and 160 mM Na+) and real serum samples were determined and related relative errors were calculated. The calculated and expected concentration values, and related relative errors for each solution were summarized in Table 3. As could be seen from Table 3, Na+ amounts in the solutions provided significant contribution over the electrode responses. When the serum samples with 120 mM Na+ were injected, the peak heights became lower than expected and the errors were ranged between −59% and 1.4% depending on the Li+ concentration levels. More satisfactory results were obtained for the artificial Li+ serum samples containing 140 mM Na+. In this case, the errors ranged between −2.0% and 3.6% because the only source of the electrode responses is the Li+ ion in the solutions. When the artificial Li+ samples containing 160 mM Na+ were injected into the carrier stream, the size of the errors were ranged between 0.3 and 61.3% depending on the concentration levels. The errors were at positive directions as expected due to the excess of the Na+ compared with the carrier stream contained. As a result, the response of the Li+-selective electrode was highly dependent on the Na+ concentration in the measurement media. The levels of the errors were relatively high in the aqueous solutions which contain 120 or 160 mM Na+. It is known that the sodium concentration of the real serum sample is between 135 and 145 mM. Therefore, in the measurements of the real serum samples, the errors caused by Na+ ions should be lower. However, the potentiometric behaviour of the sensor in real serum samples is important as the selectivity over sodium. Actually, in the real serum sample measurement, the electrode is subject to serious interferences from serum components such as proteins, lipids, organic materials, and the other serum components. The measured potential difference in real serum samples was lower than the potential differences measured in aqueous solution as seen Fig. 7d. The obtained response pattern could relate to many effects such as pH, anions, proteins, lipo-proteins, and the varying electrolyte concentrations of the serum samples. When the aqueous artificial Li+ standards were used as calibration standards, the size of the errors calculated for the real serum samples were in the range of −18.2% and −40.4%. Therefore, the use of calibrations, obtained by using Li+ standards in artificial serum electrolyte, was not a convenient approach for the determination of Li+ in real serum samples.
Conclusions
In the study, a solid-state Li+-selective electrode was prepared and combined with a low-dead volume flow-through cell to the use as a detector in FIA. Potentiometric characteristics of the prepared electrode were investigated in FIA mode. Electrode had a linear response in the concentration range of 0.1–100 mM (R 2 = 0.9981) with a slope of 61.34 mV decade−1 and detection limit of 0.080 mM. Moreover, the electrode displays the best performance with great reproducibility of potentiometric signals, short response time, and good analytical frequency in comparison to the other electrodes [8, 23]. Some pharmaceuticals and human serum samples were analysed with the described system. The described system can be successfully used for the direct Li determination in the pharmaceuticals samples. Also, FIP system provides simple, sensitive, and selective method for the determination of Li+ in pharmaceutical formulations. The system is reasonably cost effective providing a good sample frequency of 70 injections per hour and should be useful for routine lithium analysis and quality control of pharmaceutical samples.
The results obtained from real serum samples demonstrated that the electrode was not adequate for the direct analysis of Li+ ions in dynamic conditions. Because of the interference effects of the Na+ ions and the complex proteinic structures, lipids, and organic materials present in the human serum samples, the aqueous solutions can not be used as a calibrant. In order to obtain more satisfactory results for the direct analysis of the serum samples, either pre-treatment processes for the eliminations of the serum components or a more selective Li+ electrode is necessary.
References
Badawy SS, Youssef AF, Mutair AA (2004) Anal Chim Acta 511:207–214
Issa YM, Abdel-Ghani MT, Shoukry AF, Ahmed HM (2005) Anal Sci 21:1037–1042
Garcia CAB, Junior LR, Neto GO (2003) J Pharm Biomed 31:11–18
Bühlmann P, Pretsch E, Bakker E (1998) Chem Rev 98:1593–1687
Bakker E, Buhlmann P, Pretsch E (1997) Chem Rev 97:3083–3132
Faridbod F, Norouzi P, Dinarvand R, Ganjali MR (2008) Sensors 8:2331–2412
Cretin M, Fabry P (1997) Anal Chim Acta 354:291–299
Teixeira MFS, Fatibello-Filho O, Ferracin LC, Rocha-Filho RC, Bocchi N (2000) Sens Actuators B 67:96–100
Kimura K, Oishi H, Miura T, Shono T (1987) Anal Chem 59:2331–2334
Zhukov AF, Erne D, Amman D, Guggi M, Pretsch E, Simon W (1981) Anal Chim Acta 131:117–122
Grekovich AL, Markuzina NN, Mikhelson KN, Bochenska M, Lewenstam A (2002) Electroanalysis 14:551–555
Lindfors T, Sjoberg P, Bobacka J, Lewenstam A, Ivaska A (1999) Anal Chim Acta 385:163–173
Gupta VK, Chandra S, Agarwal S, Lang H (2005) Sens Actuators B 107:762–767
Sugihara H, Okada T, Hiratani K (1993) Anal Sci 9:593–597
Okada T, Sugihara H, Hiratani K (1995) Analyst 120:2381–2386
Ermolenko Yu, Yoshinobu T, Mourzina Yu, Furuichi K, Levichev S, Vlasov Yu, Schöning MJ, Iwasaki H (2002) Anal Chim Acta 459:1–9
Sun L-X, Okada T, Collin J-P, Sugihara H (1996) Anal Chim Acta 329:57–64
Xie RY, Christian GD (1986) Anal Chem 58:1806–1810
Gadzekpo VPY, Moody GJ, Thomas JDR (1986) Analyst 111:567–570
Gadzekpo VPY, Moody GJ, Thomas JDR (1985) Analyst 110:1381–1385
Cretin M, Alerm LI, Bartroli J, Fabry P (1997) Anal Chim Acta 350:7–14
Bertholf RL, Savory MG, Winborne KH, Hundley JC, Plummer GM, Savory J (1988) Clin Chem 34:1500–1502
Teixeira MFS, Cavalheiro ETG, Bergamini MF, Moraes FC, Bocchia N (2004) Electroanalysis 16:633–639
Xie RY, Gadzekpo VPY, Kadry AM, Ibrahim YA, Ruzicka J, Christian GD (1986) Anal Chim Acta 184:259–269
Farrell JR, IIes PJ, Dimitrakopoulos T (1996) Anal Chim Acta 335:111–116
Spearing M (2002) Bipolar Disorder, Publication No: 02-3679, National Institue of Mental Healthy (USA)
Thase ME, Sachs GS (2000) Biol Psychiatry 48:558–572
Prien RF, Himmelhoch JM, Kupfer DJ (1988) J Affect Disord 15:9–15
Zeevi A, Margalit R (1991) J Membr Biol 121:133–140
Christian GD (2002) Sensors 2:432–435
Coldur F, Andac M, Isildak I, Saka T (2009) J Electroanal Chem 626:30–35
Isildak I, Yolcu M, Isildak O, Demirel N, Topal G, Hosgoren H (2004) Microchim Acta 144:177–181
Sánchez-Moreno RA, Gismera MJ, Sevilla MT, Procopio JR (2009) Anal Chim Acta 634:68–74
Shamsipur M, Mizani F, Mousavi MF, Alizadeh N, Alizadeh K, Eshghi H, Karami H (2007) Anal Chim Acta 589:22–32
Beheshti SS, Amini MK (2007) Int J Electrochem Sci 2:778–787
Yang X, Hibbert DB, Alexander PW (1998) Anal Chim Acta 372:387–398
Umezawa Y, Bühlmann P, Umezawa K, Tohda K, Amemiya S (2000) Pure Appl Chem 72:1851–2082
Davey DE, Mulcahy DE, O’Connel GR, Smart RS (1995) Electroanalysis 7:461–470
Shpigun LK, Basanova OV, Zolotov YA (1992) Sensor Actuators B 10:15–20
Hulanicki A, Lewenstam A (1981) Anal Chem 53:1401–1405
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coldur, F., Andac, M. & Isildak, I. Flow-injection potentiometric applications of solid state Li+ selective electrode in biological and pharmaceutical samples. J Solid State Electrochem 14, 2241–2249 (2010). https://doi.org/10.1007/s10008-010-1070-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-010-1070-4