Abstract
Nano-Al2O3 was doped in poly(acrylonitrile-co-methyl methacrylate) (P(AN-co-MMA)), and polyethylene(PE)-supported P(AN-co-MMA)/nano-Al2O3 microporous composite polymer electrolyte (MCPE) was prepared. The performances of the prepared MCPE for lithium ion battery use, including ionic conductivity, electrochemical stability, interfacial compatibility, and cyclic stability, were studied by scanning electron spectroscopy, linear sweep voltammetry, and electrochemical impedance spectroscopy. It is found that the nano-Al2O3 significantly affects the MCPE performances. Compared to the MCPE without any nano-Al2O3, the MCPE with 10 wt.% nano-Al2O3 reaches its best performances. Its ionic conductivity is improved from 2.0 × 10−3 to 3.2 × 10−3 S cm−1, its decomposition potential is enhanced from 5.5 to 5.7 V (vs Li/Li+), and its interfacial resistance on lithium is reduced from 520 to 160 Ω cm2. Thus, the battery performance is improved.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microporous composite polymer electrolyte (MCPE) is attractive for lithium ion batteries due to its high safety, good cycle life, and easy processing compared with its liquid electrolyte counterpart. However, relative low ambient performances (e.g., low mechanical property and ionic conductivity) have limited its use. One of the ways to solve mechanical problem is to use a mechanical support such as polyethylene (PE) or non-woven fabrics to prepare supported MCPE [1–7]. On the other hand, the ionic conductivity of MCPE at room temperature can be enhanced by incorporation of nanoparticles, such as nano-SiO2 and nano-Al2O3, into the polymer matrix [8–16].
In our previous work [17], PE-supported poly(acrylonitrile-co-methyl methacrylate) (P(AN-co-MMA)) MCPE was prepared and its performances was understood. It was found that this supported MCPE has appropriate performances for lithium ion battery use, including appropriate ionic conductivity and good electrochemical compatibility with anode and cathode of lithium ion battery. The purpose of this paper was to further improve the performances of this PE-supported P(AN-co-MMA) MCPE using nano-Al2O3 as a filler. The effect of the content of the filler on the pore structure and electrolyte uptake of the MCPE matrix and the ionic conductivity and electrochemical stability of the MCPE are considered. It is found that nano-Al2O3 can improve the performances of the PE-supported P(AN-co-MMA) MCPE.
Experimental
Preparation
P(AN-co-MMA) copolymer, in which the mole ratio of AN to MMA is 4:1, was prepared by solution polymerization as previous report [17]. This ratio was used because it provided the copolymer with the best performance. A certain amount of nano-Al2O3 (Japan, average particle size of 10 nm) was dispersed ultrasonically in anhydrous N,N-dimethylformamide in which the P(AN-co-MMA) copolymer was dissolved under stirring to obtain nano-Al2O3-containing solution. The solution was cast on a microporous PE separator (Celgard 2400, USA; thickness, 16 μm) and dried in dry atmosphere and then under vacuum at 60 °C for 24 h to obtain PE-supported P(AN-co-MMA)/nano-Al2O3 membrane. The membrane was transferred into a glove box (Supper1220/750, Belgium) and soaked with 1 M LiPF6 in dimethyl carbonate (DMC)/diethylene carbonate (DEC)/ethylene carbonate (EC) (1:1:1, in volume) for 1 h to obtain PE-supported P(AN-co-MMA)/nano-Al2O3 MPCE. The membranes with 0, 5, 10, 15, 20, and 30 wt.% nano-Al2O3 were obtained.
Characterization
The morphology of the membrane was examined with scanning electron microscope (JEOL, JSM-6380LV, Japan). The electrolyte uptake (A) of the membrane was determined by immersing the membrane in 1 M LiPF6 (DMC/DEC/EC; 1:1:1, in volume) for 30 min and obtained by:
where W 1 and W 2 are the mass of the dry and wet membranes, respectively.
The ionic conductivity of the MCPE was determined by electrochemical impedance spectroscopy on electrochemical instrument (CHI650B, Shanghai) using alternative current signal with potential amplitude of 10 mV and frequencies from 100 KHz to 1 Hz. In the determination of the ionic conductivity, the MCPE was sandwiched between two parallel stainless steel (SS) discs (diameter Φ = 16 mm). The ionic conductivity was calculated from the bulk electrolyte resistance (R) according to:
where l is the thickness of the PE-supported MCPE and S is the contact area between MCPE and SS disc. The bulk electrolyte resistance (R) was obtained from the complex impedance diagram.
The interfacial stability of the MCPE was also determined by electrochemical impedance spectroscopy. In the determination of the interfacial stability, the MCPE was sandwiched between two lithium electrodes to form a symmetrical Li/MCPE/Li cell, with one lithium electrode as working electrode and the other as counter electrode and reference electrode. The lithium ion transference number was measured by the combination of chronoamperometry (CA) and electrochemical impedance spectroscopy (EIS) using the cell Li/MCPE/Li. The electrochemical stability of the MCPE was examined in the configuration of the cell Li/MCPE/SS by linear sweep voltammetry. To determine the battery performance, a cell Li/MCPE/LiCoO2 was set up and tested with charging/discharging instrument (PCBT-138-64D WUHAN LISUN).
Results and discussion
Morphology of membranes
Figure 1 shows the scanning electron microscopy (SEM) images of PE-supported P(AN-co-MMA)/nano-Al2O3 composite membranes. It can be seen from Fig. 1 that the pore distribution and structure of the membranes are affected by the content of nano-Al2O3 in the composites. Without nano-Al2O3, the membrane has large pores, with an average diameter of 8 μm, as shown in Fig. 1a. With the composite of nano-Al2O3 in P(AN-co-MMA), the pore diameter of the membrane becomes smaller; for example, the membrane with 5 wt.% nano-Al2O3 has the pores with an average diameter of 1 μm, as shown in Fig. 1b. When the content of nano-Al2O3 in P(AN-co-MMA) increases from 5 to 10 wt.%, the pore diameter of the membrane remains almost unchanged, but the pores disperse in the membrane more uniformly, as shown in Fig. 1c. As the content of nano-Al2O3 increases further, the pore number decreases, as shown in Fig. 1d. When the content of nano-Al2O3 is over 20 wt.%, the pore structure of the membrane almost disappears, as shown in Fig. 1e, f. This can be ascribed to the aggregating of nano-Al2O3 in P(AN-co-MMA). Therefore, the pore structure of P(AN-co-MMA) membrane can be improved by the composite of 5–15 wt.% nano-Al2O3 in P(AN-co-MMA). It can be expected that the better pore structure of the membrane will improve the ionic conductivity of the MCPE.
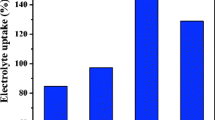
Electrolyte uptake
Figure 2 shows the dependence of electrolyte uptake of PE-supported P(AN-co-MMA)/nano-Al2O3 composite membranes on the content of nano-Al2O3. It can be seen from Fig. 2 that the electrolyte uptakes of the membranes are also affected by the composite of nano-Al2O3 in P(AN-co-MMA). It can be found by comparing Fig. 2 with Fig. 1 that the electrolyte uptakes of the membranes depend greatly on their pore structure. With 5–20 wt.% nano-Al2O3, the membrane has larger electrolyte uptake than that without Al2O3; especially, the membrane with 10 wt.% nano-Al2O3 has the largest electrolyte uptake, 280%, compared to 150% for the membrane without nano-Al2O3.
The electrolyte uptake of membrane is related to its pore structure [18–20]. The pore size of the copolymer without nano-Al2O3 is too large to retain the electrolyte, resulting in the smaller electrolyte uptake of only 150%. As the amount of nano-Al2O3 increases in the P(AN-co-MMA), the pores become smaller and their ability to retain electrolyte increases, resulting in the larger electrolyte uptake. It reaches the maximum level of 280% when the nano-Al2O3 content is 10 wt.%. When the content of nano-Al2O3 increases further, the pore sizes become too small to retain the electrolyte.
Ionic conductivity
To determine the ion conductivity of the PE-supported P(AN-co-MMA)/nano-Al2O3 MCPE, electrochemical impedance experiment was carried out on the cell SS/PE-supported MCPE/SS. Figure 3 presents the Nyquist plot of the electrochemical impedance for the PE-supported MCPE with 10 wt.% nano-Al2O3. It can be seen from Fig. 3 that the imaginary part of the impedance is linearly related to its real part and the imaginary part increases more quickly than the real part as the frequency becomes lower. This is characteristic of an equivalent of a resistor and a capacitor in series, which corresponds to the resistance of the gel electrolyte and the double capacitance of the cell in this case. Therefore, the resistance of the gel electrolyte can be determined by the intersection of linear relation between the imaginary and real parts of the impedance with the real part axis.
Figure 4 show the dependence of ionic conductivity of PE-supported P(AN-co-MMA)/nano-Al2O3 MCPE on the content of nano-Al2O3 at room temperature. The ionic conductivity of the MCPE shows the same dependence tendency on the content of nano-Al2O3 as the electrolyte uptake does. With 5–20 wt.% nano-Al2O3, the MCPE has higher ionic conductivity than that without nano-Al2O3; especially, the MCPE with 10 wt.% nano-Al2O3 has the largest ionic conductivity, 3.2 × 10−3 S cm−1, compared to 2.1 × 10−3 S cm−1 for the MCPE without nano-Al2O3. This ionic conductivity of PE-supported P(AN-co-MMA)/nano-Al2O3 MCPE is larger than those of similar MCPEs reported in literatures [13, 21–23]. For example, the ionic conductivity is 1.9 × 10−3 S cm−1 for P(AN-MMA)/SiO2 MCPE [13] and 1.25 S cm−1 for P(AN-MMA) MCPE [21]. When the content of nano-Al2O3 is over 20 wt.%, the ionic conductivity of the MCPE becomes less than that without nano-Al2O3. This can be ascribed to the lower electrolyte uptake of the MCPE with higher content of nano-Al2O3. It should be noted from Figs. 2 and 4 that the MCPE with 15 wt.% Al2O3 has lower electrolyte uptake but higher ionic conductivity than the MCPE with 5 wt.% Al2O3. This suggests that the nano-Al2O3 in the MCPE contributes to the ionic conductivity of the MCPE.
Transference number
The lithium ion transference numbers (t +) of PE-supported P(AN-co-MMA)/nano-Al2O3 MCPE were determined by the combination of CA and EIS using the cell Li/MCPE/Li, and the obtained results are shown in Table 1. The t + was calculated using the following equation [24, 25]:
where I 0, I S, and ΔV are the initial current, the steady-state current, and the potential applied to the cell in CA, respectively, and R 0 and R S are the initial interfacial resistance and the steady-state interfacial resistance obtained by EIS, respectively.
It can be seen from Table 1 that the t + of PE-supported P(AN-co-MMA) MCPE is enhanced by the doping of nano-Al2O3, especially for the MCPE with 10 wt.% nano-Al2O3 which has the largest transference number value of the order of 0.56. This value is higher than those reported in the literatures [26–29]. The MCPE with 15 wt.% Al2O3 has higher t + than the MCPE with 5 wt.% Al2O3, suggesting that the nano-Al2O3 in the MCPE contributes to the ionic conductivity of the MCPE by enhancing the lithium ion transfer number.
The effect of nano-Al2O3 results from Lewis acid–base reactions between the surface of the nanoparticle and the P(AN-co-MMA) segments [30, 31]. The Lewis acid of nano-Al2O3 competes with the Lewis acid of the lithium cations for the formation of complexes with the P(AN-co-MMA) chains. Thus, the nanoparticles act as cross-linking centers for the P(AN-co-MMA) segments, lowering the polymer chain reorganization tendency and promoting an overall structure stiffness. Such a structure modification provides lithium ion conducting pathways at the surface of the nanoparticles, resulting in the improvement in ionic transportation. On the other hand, the interactions of Lewis-acidic Al2O3 with PF6 − anions lead to the liberation of higher amounts of free Li+, which increases the Li+ transference number and thus the ionic conductivity of the MCPE.
Electrochemical stability
Figure 5 presents the linear voltammogram obtained for the cell Li/PE-supported MCPE/SS. It can be seen from Fig. 5 that the PE-supported MCPE without nano-Al2O3 decomposes at about 5.5 V (vs Li/Li+), but the PE-supported MCPE with 10 wt.%nano-Al2O3 does not decompose at the potential up to 5.7 V (vs Li/Li+). This indicates that the electrochemical stability of PE-supported MCPE can be further improved by the composite of nano-Al2O3 in it. Higher electrochemical stability is necessary to lithium ion battery, especially for the cathodic materials with high voltage.
Interfacial property with lithium electrode
The interfacial property between anode and electrolyte in a lithium ion battery is one of the important factors that determine the safety and cyclic stability of the battery [32, 33]. Interfacial stability of the PE-supported P(AN-co-MMA) MCPE with the lithium metal electrode was estimated by EIS. The time evolution of the impedance response was monitored with Li/PE-supported MCPE/Li cells at open circuit for 3 weeks. The obtained results are shown in Fig. 6. The semicircles at the high frequencies in the electrochemical impedance spectra of Fig. 6 are associated with the passivation layer and the charge transfer resistances on the lithium electrode, which are considered to constitute the interfacial resistance [34]. It can be seen from Fig. 6 that the PE-supported P(AN-co-MMA) MCPE with 10 wt.% nano-Al2O3 has lower interfacial resistance than that the MCPE without nano-Al2O3. The interfacial resistance is 160 and 520 Ω cm2 for the PE-supported P(AN-co-MMA) MCPE with and without nano-Al2O3, respectively. Obviously, the interfacial property between anode and PE-supported P(AN-co-MMA) MCPE can be improved by the composite of nano-Al2O3. This “interfacial stabilizing” effect has also been observed for the composite of ceramics in polymer [35–40].
Cyclic stability of lithium ion battery
In order to verify the electrochemical performance of the PE-supported P(AN-co-MMA) MCPE/nano-Al2O3 MCPE, coin cells Li/PE-supported MCPE/LiCoO2 were assembled for charging/discharging test at a constant current of 0.5 mA (0.2C) with cutoff voltages of 4.2 V at the upper limit and 3.0 V at the lower limit. Figure 7 presents the charging/discharging curve of the cell using the MCPE with 10 wt.% nano-Al2O3, and Fig. 8 presents the cyclic stability of the cell with a comparison of the cell with the MCPE without nano-Al2O3. It can be seen from Fig. 7 that the charging–discharging plateaus of the cell is less changed with increasing cycle number, indicating that the cell has good cyclic stability. The initial discharge capacity of the cell using PE-supported P(AN-co-MMA)/nano-Al2O3 MCPE is 2.54 mAh, very close to that of the cell using the MCPE without nano-Al2O3, which is 2.52 mAh. The discharge capacity of the cell using PE-supported P(AN-co-MMA)/nano-Al2O3 MCPE remains 91% of its initial capacity after 50 cycles, but the cell using the MCPE without nano-Al2O3 remains only 86.3% of its initial capacity after same cycles, as shown in Fig. 8. This indicates that the performance of lithium ion battery using PE-supported P(AN-co-MMA) as electrolyte can be improved by the composite of nano-Al2O3 in P(AN-co-MMA).
Conclusions
PE-supported P(AN-co-MMA))/nano-Al2O3 MCPE was prepared and its performance as the electrolyte for lithium ion battery use was studied. It is found that the composite of Nano-Al2O3 in P(AN-co-MMA) can improve the pore configuration of the PE-supported P(AN-co-MMA) membrane, the lithium ion transfer number, and the ionic conductivity of the MCPE at room temperature, and thus the performance of lithium ion battery. The PE-supported P(AN-co-MMA) MCPE with 10 wt.% nano-Al2O3 has best performance.
References
Kim DW, Oh B, Park JH, Sun YK (2000) Solid State Ion 138:41 doi:10.1016/S0167-2738(00)00763-3
Song MK, Kim YT, Cho JY, Cho BW, Popov BN, Rhee HW (2004) J Power Sources 125:10 doi:10.1016/S0378-7753(03)00826-7
Gao K, Hu XG, Yi TF, Dai CG (2006) Electrochim Acta 52:443 doi:10.1016/j.electacta.2006.05.049
Lee YM, Kim J-W, Choi N-S, Lee JA, Seol W-H, Park J-K (2005) J Power Sources 139:235 doi:10.1016/j.jpowsour.2004.06.055
Lee YM, Ko D-H, Lee JY, Park J-K (2006) Electrochim Acta 52:1582 doi:10.1016/j.electacta.2006.02.066
Yang MJ, Li WL, Wang GG, Zhang JQ (2005) Solid State Ion 176:2829 doi:10.1016/j.ssi.2005.07.011
Lee YM, Choi N-S, Lee JA, Seol W-H, Cho K-Y, Jung H-Y, Kim J-W, Park J-K (2005) J Power Sources 146:431 doi:10.1016/j.jpowsour.2005.03.047
Pitawala HMJC, Dissanayake MAKL, Seneviratne VA, Mellander B-E, Albinson I (2008) J Solid State Electrochem 12:783 doi:10.1007/s10008-008-0505-7
Subba Reddy CV, Wu GP, Zhao CX, Zhu QY, Chen W, Rajamohan RK (2007) J Non-Cryst Solids 353:440 doi:10.1016/j.jnoncrysol.2006.12.010
Park JW, Jeong ED, Won M-S, Shima Y-B (2006) J Power Sources 160:674 doi:10.1016/j.jpowsour.2006.01.029
Wu C-G, Lu M-I, Tsai C-C, Chuang H-J (2006) J Power Sources 159:295 doi:10.1016/j.jpowsour.2006.04.108
Ye H, Xu JJ (2007) J Power Sources 165:500 doi:10.1016/j.jpowsour.2006.10.042
Lee K-H, Lee Y-G, Park J-K, Seung D-Y (2000) Solid State Ion 133:257 doi:10.1016/S0167-2738(00)00708-6
Jiang Y-X, Chen Z-F, Zhuang Q-C, Xua J-M, Dong Q-F, Huang L, Sun S-G (2006) J Power Sources 160:1320 doi:10.1016/j.jpowsour.2006.02.029
Kalyana Sundaram NT, Subramania A (2007) Electrochim Acta 52:4987 doi:10.1016/j.electacta.2007.01.066
Park CH, Kim DW, Prakash J, Sun Y-K (2003) Solid State Ion 159:111 doi:10.1016/S0167-2738(03)00025-0
Rao MM, Liu JS, Li WS, Liang Y, Zhou DY (2008) J Membr Sci 322:314 doi:10.1016/j.memsci.2008.06.004
Kim D, Oh B, Choio Y (1999) Solid State Ion 123:243 doi:10.1016/S0167-2738(99)00099-5
Zhang HP, Zhang P, Li ZH, Sun M, Wu YP, Wu HO (2007) Electrochem Commun 9:1700 doi:10.1016/j.elecom.2007.03.021
Rao MM, Liu JS, Li WS, Liang Y, Liao YH, Zhao LZ (2009) Performance improvement of poly(acrylonitrile-vinyl acetate) by activation of poly(methyl methacrylate). J Power Sources. doi:10.1016/j. jpowsour.2008.08.049
Zhou DY, Wang GZ, Li WS, Li GL, Tan CL, Rao MM, Liao YH (2008) J Power Sources 184:477 doi:10.1016/j.jpowsour.2008.05.027
Jeong YB, Kim DW (2004) J Power Sources 128:256 doi:10.1016/j.jpowsour.2003.09.073
Vijayakumar G, Karthick SN, Sathiya Priya AR, Ramalingam S, Subramania A (2008) J Solid State Electrochem 12:1135 doi:10.1007/s10008-007-0460-8
Bruce PG, Vincent CA (1987) J Electroanal Chem 225:1 doi:10.1016/0022-0728(87)80001-3
Evans J, Vincent CA, Bruce PG (1987) Polymer (Guildf) 28:2324 doi:10.1016/0032-3861(87)90394-6
Xu W, Siow KS, Gao Z, Lee SY (1999) J Electrochem Soc 146:4410 doi:10.1149/1.1392652
Choe HS, Carroll BG, Pasquariello DM, Abraham KM (1997) Chem Mater 9:369 doi:10.1021/cm9604120
Liu Y, Lee JY, Hong L (2004) J Power Sources 129:303 doi:10.1016/j.jpowsour.2003.11.026
Liao YH, Zhou DY, Rao MM, Li WS, Cai ZP, Liang Y, Tan CL (2009) Self-supported poly(methyl methacrylate-acrylonitrile-vinyl acetate)-based gel electrolyte for lithium ion battery. J Power Sources. doi:10.1016/j.jpowsour.2008.10.027
Wieczorek W, Florjancyk Z, Stevens JR (1995) Electrochim Acta 40:2251 doi:10.1016/0013-4686(95)00172-B
Przyluski J, Siekierski M, Wieczorek W (1995) Electrochim Acta 40:2101 doi:10.1016/0013-4686(95)00147-7
Lee YG, Park JK, Moon SI (2000) Electrochim Acta 46:533 doi:10.1016/S0013-4686(00)00631-9
Choi NS, Lee YM, Park JH, Park JK (2003) J Power Sources 610:119–121 doi:10.1016/S0378-7753(03)00305-7
Nookala M, Kumar B, Rodrigues S (2002) J Power Sources 111:165 doi:10.1016/S0378-7753(02)00303-8
Appetecchi GB, Croce F, Persi L, Ronci F, Scrosati B (2000) Electrochim Acta 45:1481 doi:10.1016/S0013-4686(99)00363-1
Slane S, Salonmon MJ (1995) J Power Sources 55:7 doi:10.1016/0378-7753(94)02148-V
Li Q, Sun HY, Takeda Y, Imanishi N, Yang J, Yamamoto O (2001) J Power Sources 94:201 doi:10.1016/S0378-7753(00)00587-5
Kim KM, Ryu KS, Kang S-G, Chang SH, Chung IJ (2001) Macromol Chem Phys 202:866 doi:10.1002/1521-3935(20010301)202:6<866::AID-MACP866>3.0.CO;2-C
Kim KM, Park N-G, Ryu KS, Chang SH (2002) Polymer (Guildf) 43:3951 doi:10.1016/S0032-3861(02)00215-X
Subramania A, Kalyana Sundaram NT, Sathya Priya AR, Vijaya Kumar G (2007) J Membr Sci 294:8 doi:10.1016/j.memsci.2007.01.025
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rao, M.M., Liu, J.S., Li, W.S. et al. Polyethylene-supported poly(acrylonitrile-co-methyl methacrylate)/nano-Al2O3 microporous composite polymer electrolyte for lithium ion battery. J Solid State Electrochem 14, 255–261 (2010). https://doi.org/10.1007/s10008-009-0784-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-009-0784-7