Abstract
LiNi0.8Co0.2O2 and Ca-doped LiNi0.8Co0.2O2 cathode materials have been synthesized via a rheological phase reaction method. X-ray diffraction studies show that the Ca-doped material, and also the discharged electrode, maintains a hexagonal structure even when cycled in the range of 3.0–4.35 V (vs Li+/Li) after 100 cycles. Electrochemical tests show that Ca doping significantly improves the reversible capacity and cyclability. The improvement is attributed to the formation of defects caused by the partial occupancy of Ca2+ ions in lithium lattice sites, which reduce the resistance and thus improve the electrochemical properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lithium ion batteries are considered to be the best available power source for most portable electronic devices, owing to their higher energy density, larger power capacity, and higher working voltage, compared with other secondary batteries [1]. The cycle life and capacity of lithium ion batteries critically depend on the structural and electrochemical properties of the cathode materials. Traditionally, LiCoO2 is used as commercial cathode material for lithium rechargeable batteries because of its high reversibility, reasonable rate capacity, and easy preparation. However, the toxicity of cobalt coupled with its high cost and low practical capacity limit further applications. Currently, LiNiO2 is being developed to replace LiCoO2, due to the comparatively low cost, high energy density, and environmental advantages of the former. Nevertheless, this material is known to be difficult to synthesize and suffers from instabilities at high states of charge [2, 3]. Therefore, much research has been undertaken to search for new cathode materials to overcome these shortcomings [4–6].
LiNi1−x Co x O2 (0<x<1) compounds, which are isostructural as LiCoO2 and LiNiO2 with hexagonal R-3m α-NaFeO2-layered structures and have the advantages of both LiCoO2 and LiNiO2, are considered as promising cathode materials for lithium secondary batteries [7–10]. Elements such as Fe, Al, Y, and Ga have been used for the partial substitution of Ni or Co to further enhance the electrochemical performance of the cathode materials [11–14]. The doped materials showed better structural stability and cycling behavior than the undoped ones. However, the electrochemically inactive dopant generally reduced the reversible cycling capacity.
In the present study, we report an improved reversible capacity and cycling performance of Ca-doped LiNi0.8Co0.2O2 cathode material. The structural and electrochemical properties were investigated to find the reasons for the improvement in electrochemical properties.
Experimental
LiNi0.8Co0.2O2 and Ca-doped LiNi0.8Co0.2O2 cathode materials were synthesized by a rheological phase reaction method [15]. LiOH H2O, Ni(OH)2, Co2O3, and Ca(OH)2 were fully mixed by grinding in a molar ratio of 1:0.8:0.1:0.02. An appropriate amount of deionized water was added to get a rheological body, and the mixture was heated at 80 °C for 2 h in a closed container. After being dried at 120 °C, the mixture was sintered at 730 °C for 10 h in an oxygen atmosphere, and then cooled to room temperature to yield a black product.
The identification of phases and structures was carried out on a Shimadzu 6000 X-ray diffractometer in the 2θ range of 15 to 120°, at a scanning rate of 2°/min and a step of 0.02°, using Cu Kα1 radiation (λ=1.54056 Å).
The charge/discharge tests were carried out using the coin-type cell (with a size of 2,016), which consisted of an active-material working electrode and a lithium foil counter electrode separated by a Celgard 2400 microporous membrane. The working electrode was prepared by mixing active material with 15 wt.% acetylene black and 5 wt.% polytetrafluoroethylene binder, compressing the mixture onto an aluminum mesh current collector. A 1 mol/L solution of LiPF6 dissolved in ethylene carbonate/dimethyl carbonate (DMC) (1:1 volume ratio) was used as the electrolyte. The cells were assembled in an Argon-filled glove box (Mikrouna, Super 1220/750, China). The cells were charged and discharged between 3.0 and 4.35 V (vs Li+/Li) at different current densities.
Results and discussion
The X-ray diffraction (XRD) patterns of LiNi0.8Co0.2O2 and Ca-doped LiNi0.8Co0.2O2 are shown in Fig. 1. Both compounds show sharp and narrow peaks, indicating a high degree of crystallinity. The patterns can be indexed based on the hexagonal α-NaFeO2 structure with the R-3m space group, except a trace amount of CaO peaks as impurity in the spectra of Ca-doped material (Fig. 1b). The spectrum shows a clear split of (006)/(102) and (108)/(110) peaks. According to previous studies [16, 17], the degree of ordering layered structure and the amount of transition metal in the interslab space is indicated from the XRD spectra with the intensity rations of I(003)/I(104), the c/a rations of lattice parameters, and the degree of either (108)/(110) or (006)/(102) peak splitting. Crystal lattice parameters of the compounds, which are calculated by means of the least-squares method in terms of hexagonal structure, are given in Table 1. The values of lattice parameters agree well with those reported in literature [18]. Lattice parameters of Ca2+-doped LiNi0.8Co0.2O2 are smaller than those of the undoped material. The rations of I(003)/I(104) and c/a of Ca2+-doped LiNi0.8Co0.2O2 are larger than those of the undoped material, indicating a better ordering layered structure, as well as a lower degree of cation mixing. This implies that the calcium doping has affected the crystal structure. This may be attributed to the formation of the defects, which occurs from the partial occupancy of Ca2+ ions in the lithium lattice sites. A Ca2+ ion occupies the Li+ ion site to form a CaLi ˙ defect and a lithium vacancy (VLi’). The formations of the defects shrink the unit cell, suppress the mixing of transition metal in the interslab space, and stabilize the hexagonal LiNi0.8Co0.2O2 structure. The defects in the material would increase the conductivity and diffusion of lithium ions, and thus improve the electrochemical properties [19] (see the discussion of Figs. 3 and 4).
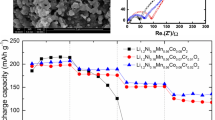
Figure 2 presents the initial charge and discharge curves of LiNi0.8Co0.2O2 and Ca-doped LiNi0.8Co0.2O2 electrodes between 3.0 and 4.35 V at a constant current density of 100 mA/g. Both profiles are similar, indicating similar electrochemical behaviors of the samples. From the figure, we can also observe that Ca-doped LiNi0.8Co0.2O2 has a lower charge voltage than the undoped material at the same charge capacity, while at the same discharge capacity, the former has higher discharge voltage than the latter. Figure 3 shows the cycle performances of the samples. The cathode material of Ca-doped LiNi0.8Co0.2O2 has an initial charge capacity of 212 mAh/g, followed by a discharge capacity of 166 mAh/g. The coulombic efficiency is 78.3%. Even after 100 cycles, the discharge capacity still keeps 163 mAh/g. Only less than 2% of the initial discharge capacity is lost, with an average capacity fade of 0.02% per cycle. In comparison, the LiNi0.8Co0.2O2 material delivers initial charge and discharge capacities of 200 and 153 mAh/g, respectively, with a coulombic efficiency of 76.5%, which is in agreement with the results reported in the literature [10]. The discharge capacity loses 19% in 70 cycles, with an average fade of 0.27% per cycle.
Figure 4 shows the cycle performances of LiNi0.8Co0.2O2 and Ca-doped LiNi0.8Co0.2O2 electrode at a current density of 25 mA/g between 3.0 and 4.35 V. Ca-doped LiNi0.8Co0.2O2 material maintains its initial discharge capacity (174 mAh/g) for up to 30 cycles. By contrast, the undoped material delivers an initial discharge capacity of 169 mAh/g, and the capacity decreases gradually with cycle number. This demonstrates that Ca-doped LiNi0.8Co0.2O2 cathode material has a good rate capacity.
XRD patterns of Ca-doped LiNi0.8Co0.2O2 electrodes discharged at 3.0 V in a voltage range of 3.0–4.35 V after 1 cycle and after 100 cycles are shown in Fig. 5. The discharged electrodes were taken out from the cells and the cathode materials were removed from the aluminum mesh using a doctor blade, washed by DMC and dried under vacuum at 80 °C, and then used for XRD tests. Comparing the XRD patterns, we note that the characteristic peak position of Ca-doped LiNi0.8Co0.2O2 phase maintains unchanged. The ration of I(003)/I(104) remains larger than 1.20, indicating that the material still maintains a good ordering layered structure even after 100 cycles. The sample completely recovers to its initial hexagonal structure after lithium reintercalation.
To understand the different cycling characteristics, AC impedance measurements of the two materials were carried out. Figure 6 presents the Nyquist plots obtained at charged state (4.35 V) in the first and fifth cycles. Each plot consists of a depressed semicircle in high and low frequency ranges. The high-frequency semicircle is attributed to the presence of a passivating film on the oxide surface and the low-frequency semicircle to the charge-transfer resistance of electrochemical reaction [20, 21]. The spectra clearly shows that the charge-transfer resistance of Ca-doped LiNi0.8Co0.2O2 electrode is much lower than that of the undoped material, which indicates that the material becomes more conductive with Ca doping. The charge-transfer resistance of the Ca-doped LiNi0.8Co0.2O2 electrode decreased after 5 cycles. By contrast, that of the undoped material increased. The lower resistance is attributed to the effect of Ca which causes the formation of defects (CaLi ˙ and VLi’). The defects in the material would increase the conductivity, decrease the activation energy for the hopping of lithium ions from one site to another, and improve the diffusion of lithium ions [19]. From these results, it can be considered that the improved electrochemical properties of Ca-doped LiNi0.8Co0.2O2 material were due to the low charge–discharge resistance.
Conclusions
In sum, the effects of Ca doping on the structure and electrochemcial properties of LiNi0.8Co0.2O2 powder were investigated. Ca-doped LiNi0.8Co0.2O2 material has smaller lattice parameters and its hexagonal structure maintains even after 100 cycles. It delivers higher reversible capacity and exhibits better cyclability than the undoped material. The superior electrochemical performance of Ca-doped LiNi0.8Co0.2O2 may be ascribed to the reduced resistance.
References
Tarascon JM, Mckinnon WR, Coowar F, Bowmer TN, Amatucci G, Guyomard D (1994) J Electrochem Soc 141:1421
Yamada S, Fujiwara M, Kanda M (1995) J Power Sources 54:209
Ebner W, Fouchard D, Xie L (1994) Solid State Ion 68:238
Koksbang R, Barker J, Shi H, Saidi MY (1996) Solid State Ion 84:1
Whittingham MS (2004) Chem Rev 104:4271
Scrosati B (2000) Electrochim Acta 45:2461
Liu HS, Li J, Zhang ZR, Gong ZL, Yang Y (2003) J Solid State Electrochem 7:456
Lu CH, Wang HC (2003) J Mater Chem 13:428
Wu MQ, Chen A, Xu RQ, Li Y (2003) Mater Sci Eng B, Solid-State Mater Adv Technol 99:336
Ying JR, Wan CR, Jiang CY (2001) J Power Sources 102:162
Prado G, Fournes L, Delmas C (2001) J Solid State Chem 159:103
Han CJ, Yoon JH, Cho WI, Jang H (2004) J Power Sources 136:132
Subba Rao GV, Chowdari BVR, Lindner HJ (2001) J Power Sources 97–98:313
Balasubramanian M, McBreen J, Pandya K, Amine K (2002) J Electrochem Soc 149:A1246
Sun JT, Xie W, Yuan LJ, Zhang KL, Wang QY (1999) Mater Sci Eng B, Solid-State Mater Adv Technol 64:157
Morales J, Vicente CP, Tirado JL (1990) Mater Res Bull 25:623
Ohuzuku T, Ueda A, Nagayama M (1993) J Electrochem Soc 140:1862
Gong ZL, Liu HS, Guo XJ, Zhang ZR, Yang Y (2004) J Power Sources 136:139
Ven AVD, Ceder G (2000) Electrochem Solid State Lett 3:301
Rodrigues Shalini, Munichandraiah N, Shukla AK (1999) J Solid State Electrochem 3:397
Nobili F, Croce F, Scrosati B, Marassi R (2001) Chem Mater 13:1642
Acknowledgement
This work was supported by the National Natural Science Foundation of China (No. 20471044).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Ma, X., Cheng, J. et al. Synthesis and electrochemical properties of Ca-doped LiNi0.8Co0.2O2 cathode material for lithium ion battery. J Solid State Electrochem 11, 361–364 (2007). https://doi.org/10.1007/s10008-006-0150-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-006-0150-y