Abstract
The energetic and electronic structure of various water dimer isomers has been explored through DFT methodology. Six different possible water dimers come in two broad categories, planar and non-planar. In each of the categories, three distinct topologies (i) linear, (ii) ring and (iii) bifurcated, have been obtained. The linear dimer has the highest interaction energy, followed by the ring dimer and then comes the bifurcated dimer. For each of these type, a planar dimer having all six atoms organized in a single plane come very close to, but has a slightly higher energy than the corresponding non-planar counterpart. Bader’s atoms in molecules (AIM) theory, reduced density gradient (RDG) method and non-covalent interaction (NCI) analysis reveal that the electron density distribution among the interacting water molecules correlates exactly in the sequence of interaction energies of different isomers of water dimer.

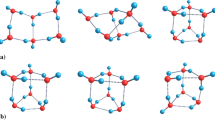
Various possible water dimer isomers
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is the most important as well as an enigmatic substance responsible for the sustenance of life on the earth. Several anomalous properties of water compared with other common solvents render water a special status [1]. A complete understanding of various peculiar characteristics of water is still lacking despite continuous efforts by scientists all around the world. These efforts have considerably advanced our knowledge towards understanding the nature of interfacial water [2] as well as bulk water, according to which, the bulk water can be considered as a statistical mixture of various small water clusters like dimer, trimer, tetramer, pentamer and hexamer leading to the advancement of continuum model of bulk water according to which various small clusters continuously transform among each other dynamically [3, 4]. The main hindrance behind the complete understanding of the properties of bulk water is the lack of understanding of the many-body effect not only between two water molecules but also among more than two water molecules when high nuclearity water clusters are considered. Theoretical modelling of these many-body effects is quite difficult. Among various small water clusters, water dimer is assumed to be the most important cluster [5] determining the lion’s share of the properties of bulk water. Water dimer is the smallest and basic water cluster which incorporates this many-body effect. Though water dimer is the most widely explored water cluster both experimentally [6] and theoretically [7], it is the subject of intense investigation even today. The simple system of two water molecules—the water dimer—is rich in diversity and complexity as this system has several local minima corresponding to different isomers. Different isomers of water dimer result from various possible ways two water molecules establish hydrogen bond with each other. There are at least six different isomers of water dimer (Fig. 1). Among these six isomers, linear non-planar (LNP) water dimer is the minimum energy structure, but a liner planar water dimer (LP) is a closely lying congener of it. In the linear planar water dimer, all four hydrogen atoms and two water oxygen atoms all lie in the same plane. Another water dimer isomer with ring topology (ring dimer) has been found where two water molecules act as a double donor and double acceptor resulting hydrogen-bonded closed ring. This again comes in non-planar (RNP) and planar form (RP). Among two other isomers of water dimer, one is bifurcated non-planar dimer (BNP) and the other is bifurcated planar dimer (BP), in each of which one water molecule acts as double acceptor and the other one acts as a double donor. In the bifurcated planar dimer (BP), all six atoms lie in the same plane but in the bifurcated non-planar dimer (BNP), the plane of water molecules are rotated 90° with respect to each other. In the present report, we have carried out an in-depth analysis regarding the energetic as well as the electronic structure of these six isomers with an aim to understand the interrelationship among these isomers. Besides ranking these isomers with respect to their interaction energies, we have carried out the atoms in molecules (AIM) [8] analysis for these six isomers which has provided a nice comparative view of the topological space of these isomers. Reduced density gradient (RDG), as well as non-covalent interaction (NCI) [9] study for these isomers, provides a bird’s eye view of the nature of intermolecular interaction among six water isomers which also nicely correlates with the ranking of these isomers.
Computational methods
To determine the optimized geometry, interaction energy and electronic structure of various possible water dimers, density functional theory (DFT) computational approach was adopted. To find out the optimized geometries and the energies corresponding to these clusters, geometry optimization has been carried out using the Gaussian 03 program package [10]. All calculations have been performed using DFT methodology with B3LYP hybrid density functional employing 6-311G++(d,p) basis set. Multiwfn [11] was used for carrying out AIM, RDG and NCI analysis.
Geometrical comparison of the structures
Computationally obtained optimized geometries of six different water dimer isomers have been depicted in Fig. 1. Three distinct topologies are present among these six water dimers. These are respectively (i) linear water dimer, (ii) cyclic or ring water dimer and (iii) bifurcated water dimer. Each of these has again a planar and a non-planar form. In the planar clusters, all six atoms (two O atoms and four H atoms) lie in a single plane. For linear water dimer, an oxygen atom of one of the water molecules acts as hydrogen donor whereas the oxygen atom of the other water molecule act as an acceptor for this hydrogen. In this case, a single hydrogen bond joins two water molecules.
On the other hand, in the case of ring dimer, each water molecule plays a dual role of donor and acceptor for each other and thus, a pair of hydrogen bonds glues two water molecules leading to closed hydrogen-bonded circuit. This hydrogen-bonded circuit has a parallelogram shape. The shorter arms of this parallelogram are formed by OH bonds of two participating water molecules. Two remaining OH bonds align outwards along one of the diagonals of the parallelogram.
In the case of bifurcated water dimer, one of the water molecules donates both hydrogen atoms and the other one acts as an acceptor for these hydrogen atoms. Again, a hydrogen bonding ring is formed but in this case, the geometry of the geometry of the hydrogen bonded ring resembles an elongated quadrilateral. Two adjacent arms of this quadrilateral are equal as two OH bonds of water molecules form the shorter pair of arms and two H∙∙∙O hydrogen bonds form the long pair of arms.
Linear non-planar
Computed vibrational frequency analysis for this dimer reveals that for this structure, there is no imaginary frequency. This means that LNP is a true minimum structure. The O∙∙∙O distance for this dimer is 2.901 Å. The O-H∙∙∙O angle is 175.55°, which implies that for this cluster, the alignment of donor, hydrogen and acceptor is nearly linear. Other relevant geometrical parameters for this isomer have been jotted down in Table 1. The interaction energy of this dimer is − 5.05 kcal/mol. Linear non-planar water dimer has the lowest energy among six different isomers of water dimer (Table 2).
Linear planar
Figure 1 b depicts a linear planar dimer. The energy value of this isomer is − 152.9252 au which is more than the linear non-planar dimer. The interaction energy of this cluster is − 4.54 kcal/mol which is 0.51 kcal/mol higher in energy than the non-planar linear water dimer. In this isomer, the O∙∙∙O distance is 2.912 Å. It can be noted that this O∙∙∙O distance is 0.011 Å larger than that of the linear non-planar dimer. The H∙∙∙O distance is 0.021 Å larger than the linear non-planar dimer. The corresponding O-H∙∙∙O angle is 169.45°, which is slightly larger than the corresponding angle of the linear non-planar dimer. The H-O-H angle is 106.32° for one of two water molecules and that for the other is 105.62°.
Ring non-planar
Figure 1 c shows the optimized configuration of the non-planar water ring dimer. The interaction energy of the non-planar ring dimer is − 4.16 kcal/mol. The interaction energy of the non-planar ring dimer is lower than that of both the linear planar and linear non-planar dimer (Table 2). It has 0.89 kcal/mol less interaction energy than the linear non-planar water dimer. In this isomer, two O-H∙∙∙O bonds lead to the formation of a ring topology between the two water molecules. The two O-H∙∙∙O bond lengths are equal having value 2.266 Å (H∙∙∙O distance)which is 0.333 Å larger than the length of linear non-planar water dimer. The O-H∙∙∙O angle is 113.73°. The H-O-H angle for both the water molecule is equal as well, having value 105.89°.
Ring planar
Figure 1 d shows the optimized geometry of the planar ring dimer. Here, both donor oxygen, a hydrogen atom and the acceptor oxygen atom from the two water molecules, lead to a ring in the same plane, unlike the non-planar ring dimer. The O-H∙∙∙O bond length is 2.279 Å (H∙∙∙O distance) where the calculated difference is 0.346 Å from the LNP. The corresponding O-H∙∙∙O angle is 109.31°. The H-O-H angle for both water molecules are 106.25°. The interaction energy of this dimer is − 3.75 kcal/mol which has 1.30 kcal/mol less interaction energy than that of the linear non-planar water dimer.
Bifurcated non-planar
Figure 1 e shows the optimized configuration of bifurcated non-planar water dimer. Two water molecules are aligned in such a way that the hydrogen atoms of one of the water molecules make bonds with the oxygen atom of another water molecule. Such an arrangement leads to the bifurcated non-planar topology. The two O-H∙∙∙O bond lengths are equal, 2.470 Å (H∙∙∙O distance), which is 0.537 Å larger than that of linear non-planar water dimer, and the O-H∙∙∙O angle is 111.44°. The two non-planar water molecules have angles of 105.63° and 101.88° respectively. The interaction energy of this dimer is − 3.29 kcal/mol which has 1.76 kcal/mol less interaction energy than the linear non-planar water dimer.
Bifurcated planar
Figure 1 f shows the optimized geometry of the bifurcated planar water dimer. It has the same topology as that of the bifurcated non-planar dimer. The only difference is that here, all atoms of both the water molecules lie in the same plane. This isomer of water dimer has the highest energy value among the six isomers of value − 152.9214 au. The lengths of both the bifurcated O-H∙∙∙O bond are same which is 2.643 Å (H∙∙∙O distance), 0.710 Å more than linear non-planar dimer, and the corresponding O-H∙∙∙O angle is 112.40°. The angle H-O-H of one of the two water molecules is 105.11° and that of another water molecule is 102.27°. The interaction energy of this dimer is − 2.39 kcal/mol. This dimer has 2.66 kcal/mol less interaction energy than the linear non-planar water dimer.
The interacting canonical molecular orbitals in different dimers
The nature of the canonical molecular orbitals for each of the water dimer isomer has been analysed to have an idea regarding the variation in the hydrogen bonding topologies of the water dimer isomers. From Fig. 2, it can be seen that there are two interacting molecular orbitals for the linear non-planar water dimer, but for the planar water dimer, there exists only one interacting molecular orbital. For linear non-planar water dimer, orbital no. 6 and orbital no. 8 shows substantial electron delocalization among the two water molecules which is along the intermolecular hydrogen bond direction. On the other hand, for the linear planar dimer, this delocalization is seen only for orbital no. 6.
In the case of non-planar ring dimer, there is only one interacting orbital (orbital no. 5) but for planar ring dimer, there exist two interacting orbitals orbital no. 5 and orbital no. 9. Though there are two interacting molecular orbitals in the case of planar ring dimer, the degree of electronic overlap is more in molecular orbital no.-5 for non-planar ring dimer in comparison with planar ring dimer (Fig. 2). Orbital no. 9 in the planar ring dimer has least electronic overlap.
For both non-planar and planar bifurcated water dimer, there exists only one interacting molecular orbital, which is -molecular orbital no. 7 for both. The overlapping orbital has axial symmetry. The symmetry axis is along the line joining the O atoms of two interacting water molecules (Fig. 2). It can be noticed that the degree of overlap is higher in the case of non-planar bifurcated dimer as compared with the planar bifurcated dimer.
The orbital interaction analysis hints that the higher interaction energy of the non-planar dimers as compared with the corresponding planar dimers can be assigned to the higher degree of orbital overlap in the interacting molecular orbital.
NCI, RDG and AIM analysis
To understand the nature of inter-atomic interaction between two water molecules in water dimer and to differentiate the set of water dimers from each other, the interaction energy was estimated according to Bader’s atoms in molecules (AIM) [12] theory. Through the analysis of several topological parameters like the electron density (ρ), Laplacian of electron density (∇2ρ), the potential energy density (V) and the kinetic energy density (G), the nature and strength of hydrogen bonds among various water dimers can be distinguished. We plotted figures of all critical points (CPs) [13] which depict the corresponding bond critical points (BCPs) along with bond paths. For all the dimers, critical points satisfy the Poincare-Hopf relationship. Among the total number of 11 CPs, there are 6 nuclear critical points (NCPs) and 5 bond critical points (BCPs). For a deeper view about these water dimers, we have computed AIM topological parameters at the intermediate BCPs between two water clusters for all the dimers. The values of these parameters have been listed in Table 3. In this table, the ratio of |V(r)|/G(r) as well as hyrogen bond enegy EHB (= V(rbcp)/2) have been provided. The |V(r)|/G(r) ratio provides information regarding the nature of interaction [14] and EHB provides information regarding the strength of the hydrogen bond [15]. EHB is related to the potential energy density at the bond critical point.
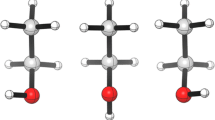
Along with the AIM critical points, non-covalent-interaction (NCI) analysis also has been performed for the visual understanding of the interaction type. It is based on electron density and its derivatives. In NCI, the reduced density gradient (RDG) function is plotted against the product of the second eigenvalue of the hessian and the electron density (sign(λ2)ρ). Characteristic spikes in this colour plot help one visualize and identify the strength of interaction of the corresponding non-covalently interacting molecules. In the graph, different colours correspond to different interactions, i.e. the van der Waals interaction (green), hydrogen bond (blue) and steric repulsion (red) from which one can easily perceive and compare the relative importance among a set of closely related systems having intermolecular interaction. The NCI iso-surface plot gives one the same idea, but now, one can visualize the interaction region in a three-dimension space between the interacting molecules in the form of iso-surface which is again colour-coded to distinguish between hydrogen bonding, van der Waals interaction and steric interaction.
If we compare both the planar and the non-planar linear dimer from Fig. 3, we can find a sharp visual difference in the NCI plot of them. Comparing the nature of the graph, one can see that the commonality among these is the presence of a single spike corresponding to the intermediate hydrogen bond. According to the adopted colour convention, this greenish-blue colour ellipsoidal iso-surface is located exactly at the position of the bond critical point (BCP) between H2A and O1 atoms for this cluster. The strength of this interaction is 5.77 kcal/mol, which is nearly the same as the hydrogen bond interaction energy of the non-planar linear dimer. For the planar linear dimer, the bond strength EHB is 5.0828 kcal/mol.
Figure 4 depicts the RDG and NCI iso-surface plot for planar as well as non-planar ring dimer. Both the ring dimers are characterized by a distinct green spike at a slightly negative value of sign(λ2)ρ. The green ellipsoidal NCI iso-surfaces depicted in the lower panel correspond to the van der Waals interaction. The interacting surface lies at the BCP between O1 and O2. The bond energy is 3.5141 kcal/mol for the non-planar one and 3.1689 kcal/mol for the planar case.
In Fig. 5, the results of both the planar and the non-planar bifurcated dimer have been given. Here, for both the dimers, we can see two sharp spikes in NCI plot; one corresponds to van der Wall range and another for the steric effect. There is a difference of value in the steric effect in NCI plot of them. There is also only one BCP in the dimer interaction region which is in between O2 and O1 of the cluster. This interaction has 2.3845 kcal/mol hydrogen bond energy for non-planar and 1.5688 kcal/mol EHB for the planar linear dimer Table 3.
Potential energy curve and barrier to rotation
The study of potential energy barriers has been carried out to better understand the relationship between the planar and the non-planar dimer for each of the three categories where the O∙∙∙O distance of respective dimers has been kept fixed at its optimized geometries and the second water molecule has been rotated with respect to the first one. Figure 6 a and b depict the nature of the potential energy curve for the non-planar water dimer. Figure 6 a depicts the potential energy profile for rotation of the second water molecule (O2) with respect to the first (O1) water molecule around the z-axis which has been set perpendicular to the xy-plane containing two water oxygen atoms (O1, O2) and the dangling hydrogen atom (H2B) of the second water molecule with origin at the oxygen atom O2. This rotation takes the bonded hydrogen atom (H1B) away from O1 and brings H2B closer to O1 in a complete rotation around the z-axis. At nearly 104° rotation, H2B forms the hydrogen bond with O1 and a potential minima is observed at this angle. One can observe a small potential barrier (4 kcal/mol) for this transition which is followed by a large potential barrier (9.5 kcal/mol) for the rest of the rotation. Figure 6 b depicts the potential energy profile for rotation of the second water molecule around the y-axis which takes away the bonded hydrogen atom (H2A) in the horizontal xy-plane. This potential energy variation is symmetric in nature. The barrier height to this transition is 8 kcal/mol and there is depth in the potential energy curve at 180° rotation of H2A making it farthest from O1 atom. In this configuration, H2B is closest to O1.
For the ring dimer, the xy-plane has been set through O1 and O2 and two hydrogen-bonded H atoms, x-axis running from O2 to O1 with origin at O2 and z-axis perpendicular to this plane. The rotation of second water molecule was carried out around z-axis . The corresponding potential energy variation has been depicted in Fig. 6c. The asymmetric potential energy curve has two unequal peaks separated by a shallow deep. The lower peak (8 kcal/mol) occurs at 166°, with shallow depth at 218°, and higher peak (15 kcal/mol) occurs at 287° degree.
For the planar bifurcated dimer, the xy-plane has been set in the plane of the dimer with origin at O1 and x-axis along O1 to O2. O1 water molecule has been rotated around the z-axis. The potential energy curve (Fig. 6d) is symmetric with two equal barriers (12 kcal/mol) with a small deep at 180° separating these two barriers. At 180°, two water molecules are in a face-to-face configuration where barrier height is 11 kcal/mol.
For the non-planar bifurcated dimer, the x-axis passes along O1 to O2 with origin at O1 and O1 water molecule has been rotated with respect to the x-axis. The potential curve (Fig. 6e) is symmetric with two equal barriers (1.4 kcal/mol) separated by a deep at 180°. At 90° rotation, the non-planar bifurcated dimer turns into a planar bifurcated dimer and the corresponding barrier is lowest among other dimers.
Fuzzy bond order analysis
Bond order, which was proposed by Linus Pauling can be used as a measurement of bond strength. Fuzzy bond order (FBO) was introduced by Mayer [16] which in comparison with the other bond order analysis is more stable with respect to the change in the basis set. FBO is essentially a measure of delocalization index calculated in fuzzy atomic space. The FBO values (Table 4) for O1∙∙∙H2A hydrogen bond nicely correlates with the relative strengths of the abovementioned water dimers. The highest bond order has been observed for the LNP dimer which is also the strongest bound system.
Discussion
A comparison of AIM, NCI and RDG results among three sets of non-planar and planar water dimers shows a nice correlation. In the AIM analysis, one can see that the electron density value at the bond critical points decreases continuously in the analogous sequence of decreasing bond energy as well the interaction energy among different water dimers. If one compares the location of the spikes corresponding to intrawater hydrogen bonding, the highest negative value (− 0.025 au) of sign(λ2)ρ occurs for the linear non-planar (LNP) water dimer which has the highest interaction energy. The sequence of sign(λ2)ρ for other dimers is − 0.022 au for linear planar (LP); − 0.014 au for non-planar ring dimer (RNP); − 0.012au for planar ring dimer (RP); − 0.011au for bifurcated non-planar dimer (BNP); and − 0.008au for bifurcated planar dimer (BP). So according to RDG analysis, the sequence of the strength of water dimers is LNP, LP, RNP, RP, BNP and BP. The sequence of colour of the 3D NCI iso-surface also correlates very well with this sequence. This is the sequence according to which the interaction energies of the water dimers also vary. One can note that the V(r)/G(r) ratio in each case is less than one, which means the interaction is closed-shell in nature. It is to be noted that hydrogen bonding is a σ-hole interaction [17, 18] and for different dimer isomers, the nature of the σ-hole may be studied further.
Conclusions
Water dimer is the most important cluster component of liquid water as well as water vapour. A detailed understanding of the energetic and electronic structural aspect of water dimer is quite crucial in gaining deeper insight into the dynamic nature of the hydrogen bonding network of water molecules. Various configurations of four hydrogen atoms among two water molecules can give rise to six isomers of water dimer. These six isomers are again divided into two broad classes, (i) non-planar and (ii) planar. The non-planar group has three distinct topologies: (i) linear topology, (ii) cyclic or ring topology and (ii) bifurcated topology. In the planar group, also same three topologies occur, but in this case, quite interestingly, all six atoms of two water molecules lie in the same plane. Highest interaction energy occurs for linear topology, followed by cyclic topology which is followed by the bifurcated topology. The planar and non-planar version for each topology comes quite close in interaction energy. The spread in interaction energy among different water isomers is 2.66 kcal/mol, which is quite small. Electron density at bond critical point correlates exactly with the interaction energy sequence of the six water isomers. RDG and NCI analysis also shows the same sequence. In summary, in the present paper, we have explored the energetic and electronic nature of six different water isomers which has provided deeper insight into the various possible ways the two water molecules can bind with each other. Though the non-planar linear water dimer has the lowest energy, the five other isomers have a very small energy difference with this one. This indicates that a slight temperature fluctuation or environmental perturbation can lead to reorganization of water molecules in a dimer and the dynamic nature of the water hydrogen-bonded network can be better understood in terms of interconversion of various water dimer isomers among each other. The exact pathway of this dynamic interconversion is currently being investigated and will be the subject of a future communication.
References
Eisenberg D, Kauzmann W (1969) The structure and properties of water. Oxford University Press, New York
Willard AP, Chandler D (2014) The molecular structure of the interface between water and hydrophobic substrate is liquid-vapor like. J Chem Phys 141:18C519
Angell CA, Rodgers V (1984) Near infrared spectra and the disrupted network model of normal and super cooled water. J Chem Phys 80:6245–6252
Ludwig R (2001) Water: from clusters to the bulk. Angew Chem Int Ed 40:1808–1827
Scheiner S (1994) Ab initio studies of hydrogen bonds: the water dimer paradigm, Annu Rev. Phys Chern 45:23–56
Mukhopadhyay A, Cole WTS, Saykally R (2015) The water dimer I. Experimental verification. J Chem Phys Lett 633:13–26
Mukhopadhyay A, Xantheas SS, Saykally R (2018) The water dimer II. Theoretical investigations. J Chem Phys Lett 700:163–175
Bader RFW (1985) Atoms in molecules. Acc Chem Res 18:9–15
Johnson ER, Keinan S, Sánchez PM, García JC, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JJA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacomb M, GillP MW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision C.02. Gaussian, Inc., Wallingford CT
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91:893–928
Kumar PSV, Raghabendra V, Subramanian V (2016) Bader’s theory of atoms in molecules (AIM) and its applications to chemical bonding. J Chem Sci 128(10)
Espinosa E, Alkorta I, Elguero J, Molins E (2002) From weak to strong interactions: a comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–HF–Y system. J Chem Phys 117:5529
Espinosa E, Molins E, Lecomte C Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem Phys Lett 285:170–173
Mayer I, Salvador P (2004) Overlap populations, bond orders and valences for ‘fuzzy’ atoms. Chem Phys Lett 383:368
Politzer P, Murray JS (2019) Electrostatics and polarization in σ- and π-hole noncovalent interactions: an overview. Chem Phys Chem. https://doi.org/10.1002/cphc.201900968
Politzer P, Murray JS (2019) An overview of strengths and directionalities of noncovalent interactions: σ-holes and π-holes. Crystals 9(1–15):165
Funding
Atish Dipankar Jana acknowledges the financial support from the department of Science, Technology and Biotechnology, Government of West Bengal, India, through the project no ST/P/S&T/16G-47/2017.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghosh, S.R., Debnath, B. & Jana, A.D. Water dimer isomers: interaction energies and electronic structure. J Mol Model 26, 20 (2020). https://doi.org/10.1007/s00894-019-4274-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4274-2