Abstract
UB3LYP computation including dispersion and toluene solvation has been carried to elucidate the mechanisms of alkene hydrogenation catalyzed by bis(imino)pyridine iron dinitrogen complex (iPrPDI)Fe(N2)2, which has low stability towards N2 dissociation. The coordinatively unsaturated complexes, (iPrPDI)Fe(N2) and (iPrPDI)Fe(1-C4H8), favor open-shell singlet ground states. On the basis of our computations, we propose a new mechanism of 1-butene coordination and hydrogenation after N2 dissociation. The hydrogenation of 1-butene undergoes a concerted open-shell singlet transition state involving H2 dissociation, C-H bond formation and C=C bond elongation, as well as the subsequent C-H reductive elimination. In the whole alkene hydrogenation, the H-H bond cleavage is the rate-determining step.

The alkene hydrogenation catalyzed by redox-active pyridine(diimine)-chelate iron complex follows the open-shell singlet state path
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogenation of alkenes catalyzed by homogeneous transition metal compounds is one of the widely studied reactions in modern chemistry [1]. This powerful transformation has been widely applied in commercial processes for pharmaceutical, fine, and commodity chemical synthesis [2,3,4,5,6]. Since the discovery of the Wilkinson catalyst (Ph3P)3RhCl over half a century ago [7, 8], the most commonly employed protocols involve the use of catalysts based on precious metals like rhodium, iridium, and ruthenium [9,10,11,12,13]. Alternatively, earth-abundant base iron catalysts have been developed by several laboratories during the past few decades [14, 15].
One interesting type of hydrogenation catalysts is the aryl-substituted bis(imino)pyridine transition metal (PDI)M complexes [PDI = 2,6-(R1N=CR2)2C5H3N; R1 = alkyl, aryl, amino; R2 = H, Me]. Initial studies by Brookhart and Gibson revealed that Co(II) and Fe(II) complexes containing (imino)pyridine ligands with bulky aryl substituents at the imine position are highly active and long-lived for ethylene polymerization [16, 17]. Among different hydrogenation catalysts, cobalt and rhodium bis(imino)pyridine complexes have been utilized for the hydrogenation of mono- and disubstituted olefins [18].
The first bis(imino)pyridine iron bis(dinitrogen) complex (iPrPDI)Fe(N2)2 [iPrPDI = (2,6-(2,6-iPr2-C6H3N=CMe)2C5H3N)] (Scheme 1) was reported by Chirik et al. [19] They found that (iPrPDI)Fe(N2)2 is an effective pre-catalyst for olefin hydrogenation, like 1-hexene, under ambient temperature and one atmosphere H2 pressure with high turnover frequencies. Later, Chirik et al. [20], prepared the phenyl-substituted bis(imino)pyridine iron bis(dinitrogen) complex (iPrPhPDI)Fe(N2)2 [iPrPhPDI = 2,6-(2,6-iPr2-C6H3N=CPh)2C5H3N] and demonstrated that this phenyl-substituted catalyst (iPrPhPDI)Fe(N2)2 is more productive than (iPrPDI)Fe(N2)2 for 1-hexene hydrogenation, but inferior for the traditionally more hindered substrates like cyclohexene and (+)-(R)-limonene. In addition, complex (iPrPDI)Fe(N2)2 can be used for the hydrogenation of aryl azides to the corresponding anilines [21]. The catalytic performance of (iPrPDI)Fe(N2)2 on the hydrogenation of a range of substituted alkenes, such as amino- and oxygen-substituted alkenes, have been explored by Chirik et al. [22] The new dimeric, aryl-substituted bis(imino)pyridine iron dinitrogen complexes were synthesized and characterized [23, 24]. Compared with the original complex (iPrPDI)Fe(N2)2, complex [(MePDI)Fe(N2)]2(μ2-N2) offers dramatically improved activity for the hydrogenation of ethyl-3-methylbut-2-enoate [23, 24]. Despite the exploration of iron catalysts with the redox-active bis(imino)pyridine ligand or the weak-field ligand, the catalytic reaction pathways still remain unclear due to the short lifetime of catalyst and intermediates. Redox-active ligands may occur in several different formal oxidation states when bound to the first row transition metals [25]. The spin state crossing (“two-state reactivity”) is necessary when there are different spin states in catalysts and intermediates [26]. When spin-orbit coupling is sufficient to allow the molecule(s) to traverse between the potential energy surfaces (PES) within a reaction, spin state changes can occur [27]. A recent report shows that bis(imino)pyridine iron alkyl complexes have a high-spin iron(II) center, which is antiferromagnetically coupled to chelate radical anions [28].
Although previous work proposed a plausible mechanism (Scheme 2) [19], the detailed kinetic information and crucial intermediates were unclear. It is proposed that the initial step is the generation of the active catalyst via N2 dissociation, following by 1-butene coordination. In path I, direct H2 oxidative addition to the Fe center of (iPrPDI)Fe(CH2 = CHCH2CH3) leads to iron(II) dihydride complex (iPrPDI)Fe(H)2(CH2 = CHCH2CH3) and the next step is the stepwise transfer of hydride ligand to 1-butene and butyl ligands. In path II, the isomerization of 1-butene catalyzed by (iPrPDI)Fe takes place to form 2-butene-coordinated iron complex and the next step is the stepwise transfer of hydride ligand to 2-butene and 2-butyl ligands. To elucidate the alkene hydrogenation mechanism catalyzed by (iPrPDI)Fe(N2)2, we carried out detailed density functional theory calculations. Possible reaction paths including closed-shell singlet, open-shell singlet, and open-shell triplet states were investigated comprehensively, in order to identify different pathways of H2 oxidative addition and obtain the insights into the reaction mechanism of olefin hydrogenation. These insights should be helpful for the understanding into the catalytic activity of low-oxidation-state iron complexes.
Proposed mechanism of butene hydrogenation with [Fe](N2)2, [Fe] = (iPrPDI)Fe [19]
Computational details
Geometry optimizations were performed at the level of UB3LYP density functional theory [29,30,31], which was adopted in previous works on the detailed mechanisms of alkene polymerization and oligomerization process initiated by bis(imino)pyridyl-iron catalysts [32] and N-H insertion reactivity of iron porphyrin carbene [33]. We used the real (iPrPDI)Fe(N2)2 complex without any simplifications as pre-catalysts and 1-butene as substrate. The validity of our treatment has been established in the previous study of similar systems and it is not expected to affect the mechanistic results [34, 35]. The iron atom was performed with the effective core potential based LANL2DZ basis set [36] and the 6-31G(d) basis set was used for all other atoms [37, 38]. This basis set is denoted as BSI. The harmonic vibrational frequencies were calculated at the same level to characterize the nature of the stationary points as true minima without imaginary frequencies or authentic transition states with only one imaginary frequency. Especially, the validity of transition states was confirmed by intrinsic reaction coordinate (IRC) computation and the connectivity between stationary points was established [39, 40]. All complexes in the open-shell singlet were calculated by using the symmetry-broken method as used in previous studies [41,42,43]. Natural bond orbital (NBO) analysis has been explored to provide natural population analysis (NPA) charges [44].
To confirm the reliability of the chosen theory level, we made specific search on geometry optimization and energy computations (Fig. S1). The computed bond distances of (iPrPDI)Fe(N2)2 at B3LYP/BSI and B3LYP/BSII (BSII denotes the combination of LANL2DZ for Fe and 6–31 + G(d,p) for other atoms) differ very slightly (Table S1), and they are in good agreement (> 97%) with the available data from X-ray structure diffraction analysis (Table S2). To save computing costs, we used B3LYP/BSI to optimize the structures of all intermediates and transition states at first and then refined the energies with B3LYP/6–311++G(d,p)//B3LYP/BSI and B3LYP-D3/6–311++G(d,p)//B3LYP/BSI single-point energy calculations with solvent effects accounted by the conductor-like polarizable continuum model (CPCM) [45] and polarizable continuum model (PCM) [46, 47]. The dielectric constant (ε) of the polarizable medium toluene was set to 2.379, which was the solvent used in related experiments. The final B3LYP-D3 (with PCM)/6–311++G(d,p)//B3LYP/BSI electronic energies were added to the Gibbs free energy correction calculated at B3LYP/BSI level to obtain the final presenting Gibbs free energy in solution. The functional B3LYP-D3 shows good performance due to its inclusion of dispersion effects [48, 49]. In addition, we have compared M06 and B3LYP functional (Table S5) to reevaluate the relative energy and found that for 3a the single-point M06/6–311++G(d,p) or M06-2X/6–311++G(d,p) energies including dispersion and toluene solvation are close to that B3LYP-D3; and for the dissociation of N2 from (iPrPDI)Fe(N2)2, the B3LYP-D3 method is best (Table S4). Although the thermal and entropy contributions to the Gibbs free energy were incorporated from the gas phase frequency calculations at 1 atm pressure and 298 K, the entropy contribution was overestimated from the gas-phase calculations, especially for the cases where the numbers of reactant and product molecules are different, i.e., correction is added to the free energies according to the free volume theory. For one-to-one or two-to-two transformation, no correction was made. For two-to-one (or one-to-two) transformation, a correction of − 1.89 (or 1.89) kcal/mol was made at the temperature of 298.15 K [50].
In the reaction pathways, the species with OS denotes the open-shell singlet, such as OS2a, and the 3 denotes the triplet state, such as 32a. The “guess = alter” keyword was employed to obtain the open-shell singlet electronic structures. The wave function stability has been performed on all open-shell singlets by using a “stable = opt” calculation [51, 52]. The wave function of all open-shell singlets is stable and the <S2> values have been given in Table S6 to show the spin contaminations. Table S6 shows the energy changes upon Yamaguchi correction [53] for the open-shell singlet species. The correction stabilizes the open-shell singlet species, but the annihilation of the spin contamination is incomplete in these species. In order to consider all possible open-shell singlet solutions, a spin-unrestricted broken-symmetry (BS) model was investigated using the fragment guess feature. In the BS calculations, we defined three fragments for all species; Fe/Fe-H, PDI, and N2/C4H8. In BS(m,n) formulation, m is the number of electrons on the Fe center, and n is the number of electrons on the PDI fragment; and both types of electrons couple in an antiferromagnetic way. Two different BS approaches, BS(1,1) corresponding to the antiferromagnetic coupling between FeI (d7, SFe = 1/2) and the PDI doublet anion ligand (PDI−, SPDI = 1/2) as well as BS(2,2) corresponding to the antiferromagnetic coupling between FeII (d6, SFe = 1) and PDI triplet dianion ligand (PDI2−, SPDI = 1), were carried. It is found that both BS approaches converged to the one solution, which is the same as obtained using “guess = alter”. It is noted that most of the open-shell singlets have the same energy by using two methods except for OS5a and OS5b. The relative energies of OS5a and OS5b using “guess = fragment” are slightly lower than those using “guess = alter” (− 8.80 vs. − 12.11 kcal/mol; and − 10.00 vs. −13.97 kcal/mol before Yamaguchi correction, respectively). All calculations were performed with the Gaussian 09 software package [54].
Results and discussion
Catalyst activation as well as H2 and 1-butene coordination
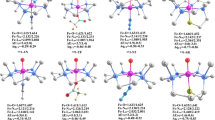
The optimized molecular structure of the real catalyst (iprPDI)Fe(N2)2 [1-(N2)2] in singlet state has a distorted square pyramid with one N2 ligand completing the fourth site of the basal plane, while the other N2 ligand occupies the apical position. This is in full agreement with the single crystal structure of 1-(N2)2 [19]. Similar to the previous suggested electron structure for 1-(N2)2, the BS(1,1) solution is 2.63 [11.66] kcal/mol more stable than the closed-shell singlet [55]. Attempts to optimize the triplet and quintet states of 1-(N2)2 led to the dissociation of one N2 ligand, indicating the instability of 1-(N2)2 in high spin states. The obvious difference between the susceptibility of the open-shell singlet and that of the closed-shell singlet is that a closed-shell singlet is usually diamagnetic, except when the temperature-independent paramagnetic interactions with relatively high energy excited states are strong enough, whereas the open-shell singlet is a temperature-independent paramagnet [56]. For the open-shell singlet, the spin is zero, but the orbital angular momentum is not. Therefore, the calculated open-shell singlet electronic structure for 1-(N2)2 is antiferromagnetic, which is consistent with the paramagnetic 1-(N2)2 in solid state examined by the SQUID data from 4 to 300 K [19]. Our calculations show that OS1-(N2)2 has spin density at Fe (ρ = 0.954) and the PDI fragment (ρ = − 0.858). The NMR spectroscopy of 1-(N2)2 shows the dynamic coordination and dissociation of N2 ligand from 1-(N2)2 [19]. We looked back to the SQUID data for 1-(N2)2 in the original paper of Chirik [19]. We analyzed the χT-T figure and endued that the paramagnetism in the high temperature region (above circa 30 K) is originated from 1-N2 and the antiferromagnetism in the low temperature region is originated from 1-(N2)2. The Weiss constant (θ) fitted out with Curie-Weiss law has a small negative value of − 0.64(2), which ruled out the diamagnetic behavior in the low-temperature region. Based on our calculations, 1-(N2)2 has an open-shell singlet ground state, which verified the antiferromagnetism in the low temperature. Therefore, the description of the diamagnetic ground state of 1-(N2)2 in the later paper of Chirik is probably not correct [55].
Prior to catalysis, the pre-catalyst needs to discard one or two N2 ligands to form the coordinatively unsaturated and active species; (iprPDI)Fe(N2) [1-N2] or (iprPDI)Fe [1]. Experimentally, it is found that (iPrPDI)Fe(N2)2 in toluene undergoes N2 dissociation and forms equilibrium between (iPrPDI)Fe(N2)2 and (iPrPDI)Fe(N2), in favor of (iPrPDI)Fe(N2). The dissociation of the first N2 ligand from 1-(N2)2 into the singlet state (1-N2) is endergonic by 14.04 kcal/mol, while exergonic into the triplet state (31-N2) by 2.08 kcal/mol and the open-shell singlet state (OS1-N2) by 3.71 [5.80] kcal/mol (Fig. 1), indicating the low thermodynamic stability of 1-(N2)2 as well as the preference of (iPrPDI)Fe(N2) as well as the possible equilibrium between (iPrPDI)Fe(N2)2 and (iPrPDI)Fe(N2).
On the potential energy surface, 31-N2 and OS1-N2 are much more stable than 1-N2 by 16.12 and 17.73 [19.82] kcal/mol at ambient temperature (298.15 K), close to 16.0 kcal/mol reported by the Chirik’s group [55]. Such energetic changes also have been found by using different methods (Tables S3 and S4). The scene of spin-forbidden ligand dissociation is very common in organometallics [57,58,59]. To afford 31-N2 the spin-change should take place. As shown in Fig. 2, 31-N2 involves spin density at Fe(I) (ρ = 1.238) and PDI− (ρ = 0.779); and OS1-N2 has spin density at Fe (ρ = 1.358) and the PDI fragment (ρ = − 1.182). This indicates the contribution of antiferromagnetic coupling between Fe(II) and a diradical dianion PDI2−, in agreement with the study on the multireference electronic structure of (PDI)FeN2 [60]. The calculated stability of OS1-N2 is in line with that of the (iPrEtPDI)FeN2 and (iPriPrPDI)FeN2 complexes [55]. The recent multireference study on the different spin states of the PDI-ligated Fe complexes show that the BS(4,2) septet, BS(3,1) quintet, and BS(3,1) triplet states with ρ(Fe) > 3 are not only higher in energy than the triplet and open-shell singlet BS(1,1) but also have the Fe–N bond distance significantly longer than the experimentally determined value [60]. Thus, we did not further consider the high spin states on the Fe center.
Without alkene or H2 coordination, the dissociation of the second N2 ligand from 31-N2/OS1-N2 into the triplet state 31 or the singlet 1 is endergonic by 18.94/20.57 [22.66] or 34.70/36.33 [38.42] kcal/mol, respectively, indicating the high thermodynamic stability of 31-N2 and OS1-N2. The open-shell singlet for 1 cannot be obtained. Furthermore, we computed the coordination of H2 and 1-butene (1-C4H8) to 31-N2 and OS1-N2. Our results show that 1-butene coordination to 31-N2/OS1-N2 to form 1-(N2)(1-C4H8) is endergonic by 16.15/17.78 [19.87] kcal/mol and H2 coordination to 31-N2/OS1-N2 to form 1-(N2)(H2) is endergonic by 15.35/16.98 [19.07] kcal/mol.
To show the participation of substrates in promoting the activation of 31-N2 and OS1-N2, we computed the substitution of N2 in 31-N2/OS1-N2 by H2 and 1-butene (1-C4H8). The substitution of N2 in 31-N2/OS1-N2 by 1-butene to form the singlet state 1-(1-C4H8), triplet state 31-(1-C4H8) and open-shell singlet state OS1-(1-C4H8) is endergonic by 21.52/23.15 [25.24], 21.84/23.47 [25.56], and 5.59/7.22 [−5.43/−1.71] kcal/mol, respectively, indicating that 1-butene prefers N2 substitution to form the open-shell singlet state OS1-(1-C4H8) rather than the coordination to form the singlet 1-(N2)(1-C4H8). The substitution of N2 in 31-N2/OS1-N2 by H2 to form the singlet state 1-H2, triplet state 31-H2 and the open-shell singlet state OS1-H2 is endergonic by 28.61/30.24 [32.33], 9.74/11.37 [13.46], and 12.62/14.25 [2.83/6.55] kcal/mol, respectively, indicating that H2 prefers N2 substitution to form the triplet state 31-H2 (the open shell singlet OS1-H2 after Yamaguchi correction) rather than coordination to form the singlet 1-(N2)(H2). It also shows that N2 substitution by 1-butene to form the open-shell singlet state OS1-(1-C4H8) is more favored than N2 substitution by H2 to form the triplet state 31-H2 by 4.15 [15.17] kcal/mol and to form OS1-H2 by 7.03 [8.26] kcal/mol.
In the geometry of 31-H2, the H2 ligand coordinates to the Fe center in η2 fashion vertically with respect to N-Fe-N plane and forms a distorted square planar coordination sphere, where the H2 ligand has molecular coordination as indicated by the H-H distance of 0.825 Å, which is slightly elongated as compared with free H2 molecule (0.743 Å). In 1-H2, the H2 ligand lies on the equatorial plane of N-Fe-N. Thermodynamically, 31-H2 is more stable than 1-H2 and OS1-H2 by 18.87 and 2.88 kcal/mol (OS1-H2 is more stable than 1-H2 and 31-H2 by 25.78 and 6.91 kcal/mol after Yamaguchi correction), respectively. In addition, the dihydride complexes 31-(H)2 and 1-(H)2 from H2 oxidative addition are less stable than 31-H2 by 6.56 and 15.79 kcal/mol (less stable than OS1-H2 by 13.47 and 22.70 kcal/mol after Yamaguchi correction), respectively. All these indicate that the stable intermediates 31-N2 and OS1-N2 are afforded at the initial stage of reaction and the next step should be 1-butene substitution with the formation of open-shell singlet state OS1-(1-C4H8) in the environment of 1-butene and H2. This supports the reaction intermediate (iPrPDI)Fe(CH2 = CHCH2CH3) suggested by Chirik and coworkers [19].
1-Butene hydrogenation
Considering that the spin state pre-equilibrium is established between OS1-N2 and 31-N2 due to the small energy difference (1.63 [3.72] kcal/mol), we computed the open- and closed-shell singlet states as well as triplet states for all intermediates and transition states. As shown in Fig. 3 and Fig. S2, the open-shell singlet OS1-(1-C4H8) exhibits anti-ferromagnetic coupling between the unpaired d-electron of Fe (ρ = 1.596) and the PDI fragment (ρ = − 1.417), with a charge distribution as Fe(+I)-(PDI)1−. The triplet 31-(1-C4H8) involves a high-spin Fe(I) (ρ = 1.260) and PDI− (ρ = 0.849).
Firstly, H2 coordination to OS1-(1-C4H8) forms the open-shell singlet complex [Fe](H2)(1-C4H8) (OS2) in distorted square pyramid coordination sphere. There are two possible conformers, one with the C2 carbon close to H2 ligand (OS2a); and one with C1 carbon close to H2 (OS2b). As expected, H2 coordination to OS1-(1-C4H8) to form OS2a and OS2b is endergonic by 11.50 [17.03] and 13.46 [18.84] kcal/mol. The closed-shell singlet and triplet states are higher in energy than the open-shell singlet state (Fig. 4 and Fig. S3). It is noted that only molecular coordinated H2 complexes are found in OS2a/2a and OS2b/2b; and it is not possible to find any dissociatively coordinated dihydride complexes from the directly oxidative addition, different from the proposed iron(II) dihydride complex (CH2 = CHCH2CH3)[Fe](H)2 by Chirik et al. [19]. Previous experiments showed that the pyridine bis(phosphine) iron(II) dihydride complexes were prepared from hydride addition [61, 62]. The electron-withdrawing redox-active bis(imino)pyridine disfavors H2 direct oxidative addition H2 to iron(0), while the electron-donating pyridine bis(carbene) ligand enables H2 oxidative addition to iron(0) [63].
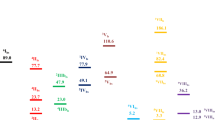
From complex OS2a, we computed 1-butene hydrogenation (Fig. 4). At first, we located the open-shell singlet transition state [OSTS(2a/3a)] for 1-butyl formation with hydrogen attacking the C2 carbon, i.e.; a transition state for H-H dissociation (1.120 Å) and C–H bond formation (1.543 Å) as well as C=C double bond elongation (1.434 Å). Since H2 coordination and H-H bond cleavage take place concurrently, OSTS(2a/3a) is a multi-bond concerted transition state [64]. For comparison, we further optimized 2a, TS(2a/3a), and 3a in toluene and found that structural parameters of these species in gas phase and toluene are almost the same.
The hydrogenation of 1-butene affords 1-butyl complex 3a, in which the 1-butyl ligand agostically interacts with the Fe center. In 3a, the Fe∙∙∙H–C agostic bond is 1.810 Å, and the C=C double bond is elongated to 1.498 Å as well as the dissociated H–H distance is 1.925 Å. We searched for the open-shell singlet state for 3a, but it collapsed to the closed-shell singlet. Starting from complex OS2a, 1-butene hydrogenation affording 1-butyl complex 3a is exergonic by 2.92 kcal/mol (however, it is endergonic by 3.57 kcal/mol after Yamaguchi correction), and the associated barrier is only 0.03 [3.37] kcal/mol, indicating that the σ-bond metathesis between the bound H2 and 1-butene takes place easily. This is similar to the fact that the σ-bond metathesis between the bound H2 and Ir-ethyl moiety overcomes relatively low energetic span in the iridium-catalyzed alkene hydrogenation [65], and iron dialkyl complexes have been reported [66, 67]. Noticeably, the closed-shell singlet [TS(2a/3a)] and triplet state [3TS(2a/3a)] transition states are 1.47 [3.62] and 16.74 [18.89] kcal/mol above the open-shell singlet transition state OSTS(2a/3a), respectively. The triplet 1-butyl complex 33a is less stable than 3a by 17.24 kcal/mol. On the basis of our calculations, the C=C bond activation and hydrogenation occurs through the open-shell singlet state path, and the overall barrier is 15.04 [12.89] kcal/mol.
After 3a, the C–H reductive elimination was considered. Since H1 and C1 atoms in 3a are on different sides of the N-Fe-N plane, the H1 atom cannot attack the C1 atom directly; and therefore 1-butyl rotation is indispensable. However, attempts to locate a transition state for 1-butyl clockwise rotation around the Fe–Npyridine axis failed. On the basis of the rotated 1-butyl ligand, we located species 4a, which is less stable than 3a by 5.38 kcal/mol. It is worth noting that a potential energy surface scan from 3a is uphill, indicating that no transition state exists. Interestingly, the open-shell singlet OS4a and the triplet state 34a are more stable than its singlet 4a by 7.09 [9.12] and 12.99 kcal/mol. We also located the authentic triplet three-center transition state 3TS(4a/5a) between 34a and 35a. Compared with the close-shell singlet transition state TS(4a/5a) and the triplet transition state 3TS(4a/5a), the open-shell singlet transition state OSTS(4a/5a) has the lowest energy, indicating that the C-H reductive elimination occurs via the open-shell singlet state path with low barrier of 0.65 kcal/mol. From 3a, the open-shell singlet state is below the triplet and closed-shell singlet states. Here, the 1-butyl hydrogenation from 4a proceeds via the open-shell singlet pathway, which is different in the styryl ligand hydrogenation catalyzed by triplet (TPB)Fe(μ-H)(H) [68]. Based on the singlet and the triplet potential energy surfaces (PES), from 3a, the weaker 1-butyl ligand dissociation or rotation features no spin acceleration [69]. Finally, one molecular C4H8 enters the coordinate site of iron and the 1-butane releases. The geometries of the closed-shell singlet intermediates and transition states are displayed in Fig. S4.
We investigated another alkene hydrogenation but it needs overcoming relatively higher barrier (Fig. S5 and S6). Starting from the less stable OS2b, we computed the transition state TS(2b/3b) for 2-butyl formation with hydrogen attacking the C1 carbon, and the barrier is 18.23 [29.25] kcal/mol, higher than that of 1-butyl formation (11.53 [20.40] kcal/mol). In addition, 3a is more stable than 3b by 8.83 kcal/mol. This indicates that 3a formation is more favored kinetically and thermodynamically than 3b formation. Nevertheless, we computed the subsequent reaction for 3b. The difference is that three states for 3b almost have the same high energy and the same geometric feature. From 3b, the triplet states lie below the closed- and open-shell singlet states (the open-shell singlet states OSTS(4b/5b) and OS5b lie below the corresponding closed- and triplet states after Yamaguchi correction). The C-H reductive elimination takes place via 3TS(4b/5b) with an moderate barrier of 10.60 kcal/mol (via OSTS(4b/5b) with an moderate barrier of 6.66 [1.73] kcal/mol after Yamaguchi correction), which is higher than that (0.65 kcal/mol) along the path initiated by species OS4a. Thus, the path along with 2a is more favorable than the path along with 2b. All computational details are listed in the Supplementary material for comparison.
Previous studies on the Pt- and Rh-catalyzed hydrosilylation of alkenes show that ethylene coordination with the Pt(II) or Rh(II) center can lower the barrier of Si–C or C–H reductive elimination [70,71,72]. Thus, we explored H2 or N2-promoted C–H reductive elimination (Fig. S7). It was found that the coordination of one molecular N2 or H2 to the Fe center of 3a by breaking the agostic interaction is endergonic by 8.97 or 13.25 kcal/mol, respectively; and the subsequently promoted transition state for C–H reductive elimination is higher than that of OSTS(4a/5a) by 9.70 [12.71] and 15.38 [18.39] kcal/mol, respectively. Such energy increase rules out the promotion effect for additional N2 and H2 coordination, different from the fact that the oxidative cleavage of the H2 to form Ir–H bond occurs before the C–H reductive elimination [73].
1-Butene isomerization and H2 addition
Since N2 substitution by 1-butene to form the open-shell singlet state OS1-(1-C4H8) is more favored than N2 substitution by H2 to form the triplet state 31-H2 by 4.15 kcal/mol (to form the open-shell singlet state OS1-H2 by 8.26 kcal/mol after Yamaguchi correction), we computed the potential energy surface according to the proposed isomerization and hydrogenation path for comparison (Fig. 5 and Fig. S8). Due to the planar geometry of 1-N2/OS1-N2, the incoming 1-butene may attack the axial site to form 1-(N2)(1-C4H8) or directly replace the N2 ligand to form 1-(1-C4H8); and the former is found endergonic by 17.78 [19.87] kcal/mol, while the latter is found endergonic by 7.22 kcal/mol (exoergic by 1.71 kcal/mol after Yamaguchi correction) to form OS1-(1-C4H8) and endergonic by 23.15 [25.24] kcal/mol to form 1-(1-C4H8). Obviously, the reaction undergoes the replacement of N2 in OS1-N2 by 1-butene to form OS1-(1-C4H8). The Fe∙∙∙H–C agostic interaction in 1-(1-C4H8) (Fe∙∙∙H-C distance is 1.880 Å) enables the C-H bond activation and the subsequent isomerization, as reported in the bifunctional ruthenium-catalyzed alkene isomerization via similar agostic Ru∙∙∙H–C intermediate [74]. Moreover, we found that the geometry of OS1-(1-C4H8) is also in favor of 1-butene isomerization. Complex OS1-(1-C4H8) dominates the reaction due to its relatively high stability over 1-(1-C4H8).
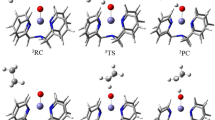
From 1-(1-C4H8), the shift of the agostic H1 to the Fe center, leading to the η3-allyl hydride species (η3-C4H7)[Fe]H (6a), takes place via the authentic transition state TS(1-(1-C4H8)/6a). This scene appeared in the olefin isomerization reaction [74]. One can see that the Fe–H1 bond in TS(1-(1-C4H8)/6a) is obviously shortened (1.556 Å) with respect to the length of Fe∙∙∙HC agostic value (1.880 Å). The imaginary frequency of TS(1-(1-C4H8)/6a) displays the desired displacement orientation. Similar η3-allyl intermediates have also been found in other iron carbonyl [75, 76] and palladium-catalyzed [77, 78] alkene isomerization reactions. This transformation process (1-(1-C4H8) → 6a) has a barrier of 3.31 kcal/mol, lower than that (8.7 kcal/mol) catalyzed by Fe(CO)3 fragment [79]. The conversion of 1-(1-C4H8) to 6a is predicted to be endergonic by 0.55 kcal/mol. For the isomerization reaction, the η3-allyl ligand has to rotate in such a way where the C1 carbon and the hydride should be close at the same side. We tried to locate the transition state for the η3-allyl ligand rotation, but failed. Only the conformer 6b was obtained. From 6a, the shift of H1 to C1 atom occurs easily through the transition state TS(6b/1-(2 t-C4H8)) with very low barrier of 2.49 kcal/mol, and leads to the trans-2-butene-coordinated complex 1-(2 t-C4H8). In 1-(2 t-C4H8), the trans-2-butene is stabilized by the Fe∙∙∙H–C agostic interaction in the vacant axis site and the Fe∙∙∙HC distance is 1.857 Å. The trans-2-butene coordinated complex 1-(2 t-C4H8) is less stable than OS1-(1-C4H8) by 16.28 [27.30] kcal/mol. We found that the release of trans-C4H8 from 1-(2 t-C4H8) is only exoergic by 3.14 kcal/mol and the whole 1-butene isomerization is an endergonic process (Fig. 5).
Fortunately, we obtained all the stationary points along the open-shell singlet path (Fig. 5). From OS1-(1-C4H8), the H-shift transition state OSTS(1-(1-C4H8)/6a) is slightly more stable by 1.03 [5.07] kcal/mol than TS(1-(1-C4H8)/6a). The first H-shift barrier is 18.21 [29.23] kcal/mol, higher than that (8.7 kcal/mol) catalyzed by Fe(CO)3 fragment [79]. The hydrogen transfer from Fe to the terminal carbon takes place via the open-shell singlet transition state OSTS(6b/1-(2 t-C4H8)) with barrier of 1.72 kcal/mol. Especially, the open-shell singlet adduct OS1-(2 t-C4H8) is more stable than the closed-shell singlet 1-(2 t-C4H8) by 15.41 [19.58] kcal/mol. The isomerization of 1-(1-C4H8) to 1-(2 t-C4H8) should be accomplished with C4H8 entering. One C4H8 molecule coordinates to Fe and trans-C4H8 releases with an exergonic energy of 19.25 kcal/mol. The open-shell singlet state path is more favorable thermodynamically and the closed-shell singlet mechanism can be ruled out. This is different from the fact that the closed singlet alkene-coordinated iron complex Fe(CO)3(η2–1-hexene) favors the alkene isomerization [79].
For the triplet state pathway, unfortunately, the crucial H-shift transition state could not be located by our much effort. This is not surprising because the C=C double in 31-(1-C4H8) (Fig. 4) is nearly in the distorted square planar plane and the methylene -CH2- in C4H8 is very far from the Fe center and not available for C-H bond activation and subsequent isomerization. The following triplet stationary points have been located and they are much less stable than the singlets (Fig. S8). Thus, the triplet mechanism for alkene isomerization can be ruled out.
Having OS1-(2 t-C4H8) in hand, we computed the following hydrogenation steps. Starting from OS1-(2 t-C4H8), one H2 coordination in η2 fashion affords the dihydrogen complex (C4H8)[Fe](η2-H2) OS7 (Fig. 6). The formation of OS7 is endergonic by 14.99 [26.01] kcal/mol; indicating that H2 coordination is not favorable thermodynamically. As in case of OSTS(2a/3a), we found a concerted open-shell singlet transition state for H2 breaking and C-H formation via the transition state OSTS(7/8) for the formation of hydride and 2-butyl complex 8. Along the triplet state surface (Fig. S10), the transition state 3TS(7/8) for the hydrogen shift in the H-H cleavage process and intermediate 38 are much higher in energy than the singlets. The final reductive elimination with the formation of butane from 8 has almost no barrier and is exergonic. It is worth noting that the energy of the open-shell singlet transition state OSTS(9/10) is lower than that of TS(9/10) and 3TS(9/10) by 6.06 and 1.92 kcal/mol, respectively. As shown in Fig. S10, the triplet intermediates involved in the C-H reductive elimination lie below the closed- and open-shell singlet states. This indicates that the spin crossing might take place from 8 to 39 due to the spin-orbit coupling and the triplet mechanism is in competition with the open-shell singlet mechanism in the C-H reductive elimination. On the potential energy surface, the first step of H2 coordination and insertion is unfavorable due to the higher barrier of 18.21 [25.19] kcal/mol (11.53 [20.40] kcal/mol in the direct hydrogenation of alkene) relative to OS1-(1-C4H8). Therefore, the reaction path of 1-butene coordination and hydrogenation is more favorable kinetically.
On the basis of our computations, we wish to propose a simplified reaction mechanism of alkene hydrogenation by using bis(imino)pyridine iron dinitrogen complex (iPrPDI)Fe(N2)2 under one atmosphere of H2 (Scheme 3). Due to the low thermodynamic stability of the parent complex, the initial step is the N2 dissociation, which generates intermediate (iPrPDI)Fe(N2). In the catalytic cycle, the first step should be the replacement of N2 by alkene to form the active species (iPrPDI)Fe(1-C4H8). The next step is H2 coordination to form (MePDI)Fe(H2)(1-C4H8). Subsequently, alkene hydrogenation undergoes a concerted open-shell singlet transition of H2 dissociation and C-H bond formation as well as C=C bond elongation, which results in the formation of the alkyl complex. The last step is the reductive elimination of the formed alkane. In the whole alkene hydrogenation, the open-shell singlet state reaction path is viable, and the H-H bond cleavage is the rate-determining step with barrier of 11.53 [20.40] kcal/mol. Our proposal differs from the mechanism of Chirik et al., where H2 coordination is oxidative with the formation of dihydride alkene complex. Our results also show that the proposed alkene coordination and isomerization followed by H2 oxidative addition is not favorable due to the high effective barrier.
Conclusions
We performed UB3LYP density functional theory computation to elucidate the mechanism for alkene hydrogenation catalyzed by bis(imino)pyridine iron dinitrogen complex (iPrPDI)Fe(N2)2, where the solvation effect of toluene and dispersion effect were included. We found several very interesting points regarding the catalysis steps; different spin states are shown to take place and crossover of several paths is not possible. The redox-active pyridine(diimine)-chelate iron complex shows the characteristic feature of the cooperative electron flow with the ligand and the iron metal in alkene hydrogenation.
-
(a)
The bis(imino)pyridine iron dinitrogen complex, (iPrPDI)Fe(N2)2, is unstable toward N2 dissociation and the unsaturated complex, (iPrPDI)Fe(N2), favors open-shell singlet ground state, which is in close energy with the triplet state, while the corresponding closed singlet state is unstable and high in energy.
-
(b)
The dihydrogen complex (iPrPDI)Fe(H2) favors triplet state ground state (however, the open-shell singlet is the ground state after Yamaguchi correction); and the dihydride complex from H2 oxidative addition is unstable and high in energy.
-
(c)
The formation of the dihydrogen (iPrPDI)Fe(H2) complex is more endergonic than that of 1-butene complex (iPrPDI)Fe(1-C4H8) in the open-shell singlet state. This is in agreement with the proposal in literature.
-
(d)
Starting from (iPrPDI)Fe(H2)(1-C4H8), 1-butene hydrogenation takes place via a concerted open-shell singlet transition state involving H-H dissociation, C-H bond formation and C=C double bond elongation. This reaction path is kinetically much more favorable than the alternative reaction path following 1-butene isomerization and H2 coordination as well as hydrogenation. From 1-(1-C4H8) the open-shell singlet path is generally low in free energy to become a more viable reaction channel. In the whole alkene hydrogenation, the H-H bond cleavage is the rate-determining step with barrier of 11.53 [20.40] kcal/mol.
References
Blaser H-U, Spindler F, Thommen M (2008) The handbook of homogeneous hydrogenation. de Vries JG, Elsevier CJ (eds) Wiley-VCH: Weinheim
Cipot J, McDonald R, Stradiotto M (2006) Organometallics 25:29–31
Hesp KD, Wechsler D, Cipot J, Myers A, McDonald R, Ferguson MJ, Schatte G, Stradiotto M (2007) Organometallics 26:5430–5437
Alvarez A, Maclas R, Bould J, Fabra MJ, Lahoz FJ, Oro LA (2008) J Am Chem Soc 130:11455–11466
Chirik PJ (2015) Acc Chem Res 48:1687–1695
Li L, Zhao H, Wang R (2015) ACS Catal 5:948–955
Osborn JA, Wilkinson G, Young JF (1965) Chem Commun 17
Osborn JA, Jardine FH, Young JF, Wilkinson G (1966) J Chem Soc A 1711–1732
Schrock RR, Osborn JA (1971) J Am Chem Soc 93:3091–3092
Crabtree RH (1979) Acc Chem Res 12:331–337
Knowles WS (2002) Angew Chem Int Ed 41:1998–2007
Noyori R (2002) Angew Chem Int Ed 41:2008–2022
Sperger T, Sanhueza IA, Kalvet I, Schoenebeck F (2015) Chem Rev 115:9532–9586
Bolm C, Legros J, Le Paih J, Zani L (2004) Chem Rev 104:6217–6254
Bauer I, Knölker H-J (2015) Chem Rev 115:3170–3387
Small BL, Brookhart M, Bennett AMA (1998) J Am Chem Soc 120:4049–4050
Britovsek GJP, Bruce M, Gibson VC, Kimberley BS, Maddox PJ, Mastroianni S, McTavish SJ, Redshaw C, Solan GA, Strömberg S, White AJP, Williams DJ (1999) J Am Chem Soc 121:8728–8740
Knijnenburg Q, Horton AD, van der Heijden H, Kooistra TM, Hetterscheid DGH, Smits JMM, de Bruin B, Budzelaar PHM, Gal AW (2005) J Mol Catal A Chem 232:151–159
Bart SC, Lobkovsky E, Chirik PJ (2004) J Am Chem Soc 126:13794–13807
Archer AM, Bouwkamp MW, Cortez MP, Lopkovsky E, Chirik PJ (2006) Organometallics 25:4269–4278
Bart SC, Lobkovsky E, Bill E, Chirik PJ (2006) J Am Chem Soc 128:5302–5303
Trovitch RJ, Lopkovsky E, Bill E, Chirik PJ (2008) Organometallics 27:1470–1478
Russell SK, Darmon JM, Lopkovsky E, Chirik PJ (2010) Inorg Chem 49:2782–2792
Tondreau AM, Atienza CCH, Weller KJ, Nye SA, Lewis KM, Delis JGP, Chirik PJ (2012) Science 335:567–570
Ward MD, McCleverty JA (2002) J Chem Soc Dalton Trans 275
Schröder D, Shaik S, Schwarz H (2000) Acc Chem Res 33:139–145
Bellows SM, Cundari TR, Holland PL (2013) Organometallics 32:4741–4751
Trovitch RJ, Lobkovsky E, Chirik PJ (2008) J Am Chem Soc 130:11631–11640
Lee CT, Yang WT, Parr RG (1988) Phys Rev B 37:785–789
Becke AD (1993) J Chem Phys 98:5648–5652
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) J Phys Chem 98:11623–11627
Khoroshun DV, Musaev DG, Vreven T, Morokuma K (2001) Organometallics 20:2007–2026
Sharon DA, Mallick D, Wang B, Shaik S (2016) J Am Chem Soc 138:9597–9610
Long GT, Weitz E (2000) J Am Chem Soc 122:1431–1442
Glascoe EA, Sawyer KR, Shanoski JE, Harris CB (2007) J Phys Chem C 111:8789–8795
Hay PJ, Wadt WR (1985) J Chem Phys 82:299–310
Hehre WJ, Ditchfield R, Pople JA (1972) J Chem Phys 56:2257–2261
Hariharan PC, Pople JA (1973) Theor Chim Acta 28:213–222
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154–2161
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523–5527
Bachler V, Olbrich G, Neese F, Wieghardt K (2002) Inorg Chem 41:4179–4193
Cheng L, Wang J, Wang M, Wu Z (2009) Dalton Trans 3286–3297
Peng D, Zhang Y, Du X, Zhang L, Leng X, Walter MD, Huang Z (2013) J Am Chem Soc 135:19154–19166
Reed AE, Weinstock RB, Weinhold F (1985) J Chem Phys 83:735–746
Cossi M, Rega N, Scalmani G, Barone V (2003) J Comput Chem 24:669–681
Miertus S, Scrocco E, Tomasi J (1981) J Chem Phys 55:117–129
Miertus S, Tomasi J (1982) J Chem Phys 65:239–245
Grimme S, Antony J, Ehrlich S, Krieg H (2010) J Chem Phys 132:154104
Grimme S, Ehrlich S, Goerigk L (2011) J Comput Chem 32:1456–1465
Winget P, Cramer CJ, Truhlar DG (2004) Theor Chem Accounts 112:217–227
Seeger R, Pople JA (1977) J Chem Phys 66:3045–3050
Bauernschmitt R, Ahlrichs R (1996) J Chem Phys 104:9047–9052
Yamaguchi K, Jensen F, Dorigo A, Houk KN (1988) Chem Phys Lett 149:537–542
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Camii R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ӧ, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09, Revision E.01. Gaussian, Inc., Wallingford
Stieber SCE, Milsmann C, Hoyt JM, Turner ZR, Finkelstein KD, Wieghardt K, DeBeer S, Chirik PJ (2012) Inorg Chem 51:3770–3785
Booth CH, Walter MD, Kazhdan D, Hu Y-J, Lukens WW, Bauer ED, Maron L, Eisenstein O, Andersen RA (2009) J Am Chem Soc 131:6480–6491
Smith KM, Poli R, Harvey JN (2000) New J Chem 24:77–80
Harvey JN (2004) Faraday Discuss 127:165–177
Ganguly G, Malakar T, Paul A (2015) ACS Catal 5:2754–2769
Ortuño MA, Cramer CJ (2017) J Phys Chem A 121:5932–5939
Trovitch RJ, Lobkovsky E, Chirik RJ (2006) Inorg Chem 45:7252–7260
Gorgas N, Alves LG, Stöger B, Martins AM, Veiros LF, Kirchner K (2017) J Am Chem Soc 139:8130–8133
Yu RP, Darmon JM, Semproni SP, Turner ZR, Chirik PJ (2017) Organometallics 36:4341–4343
Dub PA, Gordon JC (2017) ACS Catal 7:6635–6655
Polo V, Al-Saadi AA, Oro LA (2014) Organometallics 33:5156–5163
Hoyt JM, Sylvester KT, Semproni SP, Chirkik PJ (2013) J Am Chem Soc 135:4862–4877
Tondreau AM, Atienza CCH, Darmon JM, Milsmann C, Hoyt HM, Weller KJ, Nye SA, Lewis KM, Boyer J, Delis JGP, Lobkovsky E, Chirik PJ (2012) Organometallics 31:4886–4893
Li L, Lei M, Sakaki S (2017) Organometallics 36:3530–3538
Poli R, Harvey JN (2003) Chem Soc Rev 32:1–8
Ozawa F, Hikida T, Hayashi T (1994) J Am Chem Soc 116:2844–2849
Sakaki S, Mizoe N, Sugimoto M, Musashi Y (1999) Coord Chem Rev 190:933–960
Sakaki S, Sumimoto M, Fukuhara M, Sugimoto M, Fujimoto H, Matsuzaki S (2002) Organometallics 21:3788–3802
Cheng C, Kim BG, Guironnet D, Brookhart M, Guan CJ, Wang DY, Krogh-Jespersen K, Goldman AS (2014) J Am Chem Soc 136:6672–6683
Tao JC, Sun FS, Fang T (2012) J Org Chem 698:1–6
Harrod JF, Chalk AJ (1964) J Am Chem Soc 86:1776–1779
Harrod JF, Chalk AJ (1966) J Am Chem Soc 88:3491–3497
Davies NR (1964) Nature 201:490–491
Davies NR (1964) Aust J Chem 17:212–218
Sawyer KR, Glascoe EA, Cahoon JF, Schlegel JP, Harris CB (2008) Organometallics 27:4370–4379
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 21203115 and 21373131) and the Research Project Supported by Shanxi Scholarship Council of China (Grant No. 2012-057). We thank Dr. Ling Guo and Dr. Bingqiang Wang for helpful discussions. This work was also supported in part by Computer resources at the National Supercomputing Center in Shenzhen (Shenzhen Cloud Computing Center).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Dedicated to Prof. Dr. Tim Clark on the occasion of his 70th birthday
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to the Topical Collection Tim Clark 70th Birthday Festschrift
Electronic supplementary material
ESM 1
(DOC 2041 kb)
Rights and permissions
About this article
Cite this article
Guo, CH., Yang, D., Liu, X. et al. Exploring the mechanism of alkene hydrogenation catalyzed by defined iron complex from DFT computation. J Mol Model 25, 61 (2019). https://doi.org/10.1007/s00894-019-3942-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-3942-6