Abstract
The surface structures, O2 adsorption, and CO oxidation reaction properties of Ce6O12/Cu(111) have been investigated using density functional theory including on-site Coulomb corrections (DFT + U). Results show that the supported ceria nanoparticles would gain electrons from the Cu(111) surface, and the Ce4+ are reduced to Ce3+. In addition, the oxygens at the interface have been largely activated, resulting in much low formation energy of O vacancies. For the CO oxidation reaction, two possible pathways are investigated, CO reacts with the O2 molecule adsorbed on Ce3+ and the lattice O at the interface, respectively. It has been found that CO reacting with the lattice O atom gives a lower reaction barrier than that of adsorbed O2 on Ce3+. These results are important for further understanding of the role of different active sites on the inverse CeOx/Cu(111) surface structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inverse catalysts with oxide nanoparticles (NPs) supported on metal surfaces have attracted much attention for their high catalytic activity, even at relatively low temperatures, in many areas, such as water-gas shift (WGS) [1,2,3], CO oxidation [4, 5], and methanol synthesis from carbon dioxide [6, 7]. Interestingly, compared with the regular metal/oxide catalysts, the inverse ones sometimes show better activity [8, 9]. For example, compared with Cu/CeO2, CeOx/Cu(111) has higher catalytic activity for the WGS reaction [10, 11]. Furthermore, Au(111) and CeO2 contribute no activity to the WGS reaction individually, but together they show remarkably activity [3]. The defects of ceria nanoparticles and the metal–oxide interface are considered the active sites.
Precious metals have been used for a long time for CO oxidation [12,13,14], but, due to the high price and low abundance of the latter, catalysts of non-noble metal are attracting increasing interest. Especially, CuOx- and Cu-based catalysts are being recognised for their excellent activity [15,16,17]. CeO2 has been used widely as a support in conventional metal/CeO2 configurations for CO oxidation [18, 19]. And the inverse CeO2/metal catalysts have also been studied experimentally in great detail [3, 9, 20]. The CeO2–metal interaction generates active Ce3+ species in the oxide particle, which is related to the catalytic activity of the inverse system. This interaction was studied in situ by IRRAS and AP-XPS under WGS reaction (WGSR) conditions; the Cu–Ceria interaction caused the formation of Cu2+/Cu+(Cu0) and Ce4+/Ce3+ redox pairs [10]. For the CO oxidation reaction, the inverse CeOx/Cu(111) system also exhibited high activity [4, 20, 21], which was similar to, or even better than, that of catalysts based on noble metals. The special properties of ceria NPs provide high O2 dissociation activity, which is considered to be crucial for CO oxidation [21]. However, the identification of active sites on the surface structure of CeOx/Cu(111) remains a challenging task, due to the complexity of the inverse system under real working conditions.
In this work, we performed systematic DFT calculations of the surface structural properties of the inverse Ce6O12/Cu(111) surface and its activity to oxidize CO. The active sites of the ceria particle and the Cu–Ceria interface were studied in terms of oxygen activation and CO oxidation.
Computational details
All calculations were carried out using the Vienna ab initio simulation package (VASP) [22, 23], approached within the project-augmented wave (PAW) method [24, 25], and results were obtained using the MedeA® software environment [26]. The Perdew-Burke-Ernzerhof (PBE) [27] electron exchange-correlation functional based on the generalized gradient approximation (GGA) was employed. We treated the C 2 s, 2p, O 2 s, 2p, Cu 3d, 4 s, and Ce 4f, 5 s, 5p, 6d, 6 s as valence electrons. In order to correct for the description of the strong localization of the Ce 4f electrons, a Hubbard-like U term was used [28, 29], where U = 4.5 eV was applied to the Ce 4f states. This value was calculated self-consistently by Fabris et al. [30] for the cerium atoms in cerium oxides. The transition states in the reactions were determined by the climbing-image nudged elastic band (CI-NEB) method [31,32,33,34,35,36], and results obtained using MedeA® Transition State Search module. Vibrational analysis was also performed to insure the exact transition states were found. We then continued to calculate the electronic energies with zero-point energy (ZPE) correction [37]: \( \mathrm{ZPE}={\sum}_I\left(\raisebox{1ex}{$1$}\!\left/ \!\raisebox{-1ex}{$2$}\right.\right)h{\nu}_I \), where h is the Planck constant, and νI the calculated real frequencies of the system.
For the Cu(111) surface slab, we used a 6 × 6 supercell with five Cu layers. To avoid the interaction between neighboring slabs, a vacuum layer of 15 Å between slabs was built. The Ce6O12 NPs supported on Cu(111) (Ce6O12/Cu(111)) was shown in Fig. 1, which was described by previous literature reports [6, 9, 20]. For all structural optimizations, the bottom one layer was fixed, while the other slab atoms were allowed to move with a force threshold of 0.05 eV/Å. Spin polarization was considered in all the calculations. The energy cutoff of plane wave expansion was set to 400 eV and a Monkhorst-Pack [38] grid of 1 × 1 × 1 k-points mesh was used. For the gas-phase CO and O2 energies, we build a 10 × 10 × 10 Å3 lattice containing a CO/O2 molecule.
The adsorption energy of CO and O2 molecules is defined as:
Where E Ce6O12/Cu + mol is the total energy of CO/O2 adsorbed on the surface slab, E mol and E Ce6O12/Cu are energies of CO/O2 in the gas phase and the surface slab, respectively.
The formation energy of O vacancy (Ov) is defined as:
Where E Ce6O11/Cu, E Ce6O12/Cu and E O2 are the energies of Ce6O11/Cu(111), bulk Ce6O12/Cu(111), and gas-phase O2, respectively.
Results and discussion
Geometric and electronic properties
The Ce6O12/Cu(111) surface structure is illustrated in Fig. 1. The CeOx cluster with triangle geometry has been found as the most stable structure previously, both theoretically and experimentally [8, 9]. Accordingly, such triangle structure was considered here for Ce6O12. The Ce6O12 nanoparticles strongly interact with the support and the Cu(111) surface greatly stabilizes the oxide cluster. Both Ce and O ions are bound to Cu, which cause the structural change in the surface. One can see that the Cu(111) surface is rippled, as shown in Fig. 1b. Parts of the Cu atoms are pulled outward the surface and some are suppressed below, the distance between the vertical positions of the highest- and lowest-lying Cu atom in the top layer reached to 0.48 Å.
The Bader charge analyses (Table 1) showed that Cu(111) surface loses charge by as much as 1.8 e, and for all Ce atoms (Fig. 1a) of Ce6O12/Cu(111) are more positive than their counterparts in the Ce6O12 cluster, indicating reduction of the nanoparticle support on Cu(111) surface. Compared with the Bader charge of Ce3+ (9.9 e) in bulk Ce2O3, these Ce ions should be considered the Ce3+. A similar result was proposed by Graciani et al. [8], and they also proved that the strong interaction between the substrate and the supported nanoparticle overcame the loss of coordination and lowering of the Madelung electrostatic contribution in the ceria particles. Therefore, the special electronic and chemical properties of inverse Ce6O12/Cu(111) system provide good stability to support ceria and Ce3+ species.
O2 adsorption on Ce3+ and Ov sites
As shown in Fig. 2a, for the molecular adsorption of O2, significant relaxation occurred on the particles, and the O atoms moved into the interface, closer to the adsorbed O2. In our previous work [39], we calculated the relaxation energy (E relax) and bonding energy (E bond), which are proved to be two important components of adsorption energy (E ads). In this work, the E relax is the energy gained from the structural relaxation in the presence of the adsorbed O2, while the E bond is the energy required to remove the O2 with fixed structure of Ce6O12/Cu(111), and also the O2 molecule is in the same geometry as it adsorbed. The calculated O2 bonding energy was expressed as \( {\mathrm{E}}_{\mathrm{bond}}=-\left({\mathrm{E}}_{\mathrm{Ce}6\mathrm{O}12/\mathrm{Cu}+\mathrm{O}2}-{\mathrm{E}}_{\mathrm{O}2}^{\mathrm{fix}}-{\mathrm{E}}_{\mathrm{Ce}6\mathrm{O}12/\mathrm{Cu}}^{\mathrm{fix}}\right) \).
Calculated structures of O2 adsorption at the interface of Ce6O12/Cu(111). a, c Molecular and dissociative adsorptions, respectively; d, e, g Calculated structures of O vacancy and adsorbed O2; b, f isosurfaces (0.02 e/ Å3) of charge redistribution of a and d, respectively. The yellow and blue isosurfaces denote charge gain and loss, respectively. O atoms of O2 are in green
According to Table 2, the calculated adsorption energy and bonding energy of O2 at Ce3+ site is 2.09 eV and 0.71 eV, respectively. This clearly suggests that O2 can chemisorb on Ce3+ of ceria nanoparticles, and the structural relaxation can also reduce the energy of the adsorption structure. The strong bonding strength and relaxation gives rise to the high adsorption energy of O2. We then calculated the charge density difference, and also performed Bader charge analysis. As one can see from Fig. 2b, charge transfers from the substrate to the O atoms of adsorbed O2 molecule, by as much as 1.08 e (Table 2), and the distance between them is 1.44 Å (Table 2), nearly the same as that reported by Li [40] for the peroxide (O2 2−) species calculated on the defective CeO2(111) surface. This indicates that the adsorbed O2 at the interface of Ce6O12/Cu(111) is a peroxide (O2 2−), and that the surface is reoxidized.
The calculated structures of Ce6O12/Cu(111) with one Ov and O2 adsorption are shown in Fig. 2d,e, respectively. The corresponding energies are given in Table 2. The calculated O vacancy formation energy of the ceria nanoparticles is 1.74 eV, which is much lower than that in pure ceria [41], and O2 adsorption occurs at the Ov with relatively high stability (2.73 eV). As shown in Fig. 2f, electrons transfer from the surface to the adsorbed O2, by as much as 1.29 e (Table 2). The distance between O atoms of adsorbed O2 is 1.58 Å, longer than that of O2 adsorbed on Ce3+ (1.44 Å, Fig. 2a). These results show that the Cu(111) surface is able to promote the activity of the O atoms of ceria nanoparticles at the interface. Furthermore, O vacancy defects at the interface become active sites for O2 adsorption and activation.
We then calculated the reaction energy for adsorbed O2 dissociation as follows [2]: ΔE = 2*E(O/surface) − E(O2/surface) − E(surface). The dissociative O atoms at Ce3+ and Ov sites are represented as Odis (Fig. 2a) and Odis′ (Fig. 2e). The calculated structures of O2 dissociation at Ce3+ and Ov sites are shown in Fig. 2c,g, and the corresponding calculated ΔE is 5.61 eV and 4.95 eV (Table 2), respectively. One can see that the reactivity of O2 dissociation on Ov is higher than that at the Ce3+ site. It is worth mentioning that we also tested the gas-phase CO reaction with the adsorbed O2 at Ce3+ and Ov sites, and found that, during the static optimization, CO can combine with Odis′ to form CO2 spontaneously, even if the length of the OC–O bond was set to 1.72 Å in the initial structure. On the contrary, no CO2 formed at Ce3+ site though the gas-phase CO very closed to the Odis (1.34 Å). This indicates that the adsorbed O2 at Ov site is expected to be attacked directly by gas-phase CO molecules in the actual reaction conditions.
The reactions between gas-phase CO molecule and O2 2− species on Ov and Ce3+ sites are shown in Fig. 3. As we can see, the energy barrier of CO reacting with Odis is 1.17 eV, while it is only 0.13 eV for CO reacting with Odis′, in line with the results of reaction energy discussed above.
CO oxidation on interface
According to our calculations, CO molecules adsorb rather weakly on ceria nanoparticles. The calculated most stable CO adsorption structure is shown in Fig. 4a, as well as the coadsorption structure of CO and O2 molecules (Fig. 4b). As we can see, CO adsorbs onto the hollow site of the Cu(111) surface, and the adsorption energy is 1.13 eV. Both CO and O2 are stably adsorbed on the interface.
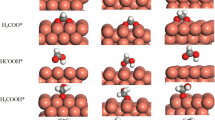
We then studied two different routes of CO oxidation: (1) the reaction between adsorbed CO and O2, and (2) CO oxidation with O atom of ceria nanoparticles at the interface. For the first reaction pathway, CO and O2 can adsorb on the interface, and the CO molecule reacts with one O atom of the adsorbed O2. Fig. 5a shows the transition state (TS) structure, the distance between the C atom of CO and the O atom of O2 decreased from 3.64 Å in coadsorption state to 2.05 Å, and the barrier was estimated to be 1.27 eV. After the TS, CO2 forms and desorbs, with a single Oad left on the surface. As presented in Fig. 5(b), this Oad can further react with another CO. The calculated adsorption energy for CO is 0.94 eV, and the reaction barrier is 0.91 eV. Therefore, it can be expected that this reaction path requires high activation energy, especially for the first step.
For the second pathway, the energy profile is depicted in Fig. 5c, from which we can see that the adsorbed CO molecule reacts directly with the O atom of ceria nanoparticles at the interface by overcoming a barrier of 0.7 eV. This indicates that the strong interaction between ceria nanoparticles and the Cu(111) surface activated the O atoms of the particle much more than the adsorbed O2 on it. With desorption of CO2, an O vacancy formed at the interface (Fig. 2d). Then the Ov can be filled easily by adsorbed O2. From our previous discussion, the adsorbed O2 is activated by Ov. It is then attacked directly by gas-phase CO molecules, and the energy barrier is only 0.13 eV. With desorption of CO2, a defect-free surface is left behind that can further oxidize CO.
Conclusions
Through DFT + U calculations, we have systematically studied the adsorption and reaction of CO and O2 at the interface of the inverse Ce6O12/Cu(111) surface. According to our calculation results, the strong interaction between the substrate and the supported ceria nanoparticles causes the reduction of Ce ions, and activates the O2 adsorbed on them. In particular, the O atoms of the supported particle at the interface proved to have higher reactivity with CO than that of adsorbed O2 on Ce3+, and the Ov defect can further active the O2 adsorbed on it. The Ov defects at the interface of inverse CeOx/Cu(111) surface are more likely to be the active sites for CO oxidation.
References
Senanayake SD, Stacchiola D, Evans J, Estrella M, Barrio L, Pérez M, Hrbek J, Rodriguez JA (2010) J Catal 271:392–400
Vidal AB, Liu P (2012) Phys Chem Chem Phys 14:16626–16632
Rodriguez JA, Ma S, Liu P, Hrbek J, Evans J, Pérez M (2007) Science 318:1757–1760
Ringleb F, Fujimori Y, Brown MA, Kaden WE, Calaza F, Kuhlenbeck H, Sterrer M, Freund HJ (2015) Catal Today 240:206–213
Kim HY, Liu P (2015) J Phys Chem C 119:22985–22991
Graciani J, Mudiyanselage K, Xu F, Baber AE, Evans J, Senanayake SD, Stacchiola DJ, Liu P, Hrbek J, Sanz JF, Rodriguez JA (2014) Science 345:546–550
Senanayake SD, Ramírez PJ, Waluyo I, Kundu S, Mudiyanselage K, Liu ZY, Liu Z, Axnanda S, Stacchiola DJ, Evans J, Rodriguez JA (2016) J Phys Chem C 120:1778–1784
Graciani J, Vidal AB, Rodriguez JA, Sanz JF (2014) J Phys Chem C 118:26931–26938
Rodriguez JA, Graciani J, Evans J, Park JB, Yang F, Stacchiola DJ, Senanayake SD, Ma S, Pérez M, Liu P (2009) Angew Chemie Int Ed 48:8047–8050
Mudiyanselage K, Senanayake SD, Feria L, Kundu AE, Baber J, Graciani J, Vidal AB, Agnoli S, Evans J, Chang R, Axnanda S, Liu Z, Sanz JF, Liu P, Rodriguez JA, Stacchiola DJ (2013) Angew Chemie Int Ed 52:5101–5105
Rodriguez JA, Hrbek J (2010) Surf Sci 604:241–244
Suchorski Y, Wrobel R, Becker S, Weiss H (2008) J Phys Chem C 112:20012–20017
Xu J, While T, Li P, He C, Yu J, Yuan W, Han YF (2010) J Am Chem Soc 132:10398–10406
Cheng XL, Zhao YY, Li F, Liu YJ (2015) J Mol Model 21:230
Snytnikov PV, Popova MM, Men Y, Rebrov EV, Kolb G, Hessel V, Schouten JC, Sobyanin VA (2008) Appl Catal A 350:53–62
Rao KN, Bharali P, Thrimurthulu G, Reddy B (2010) Catal Commun 11:863
Huang TJ, Tsai DH (2003) Catal Lett 87:173–178
Zhang C, Michaelides A, Jenkins SJ (2011) Phys Chem Chem Phys 13:22–33
Rodriguez JA, Liu P, Hrbek J, Evans J, Pérez M (2009) Angew Chemie Int Ed 46:1329–1332
Yang F, Graciani J, Evans J, Liu P, Hrbek J, Sanz JF, Rodriguez JA (2011) J Am Chem Soc 133:3444–3451
Senanayake SD, Stacchiola D, Rodriguez JA (2013) Acc Chem Res 46:1702–1711
Kresse G, Furthmüller J (1996) Comput Mater Sci 6:15–50
Kresse G, Furthmüller J (1996) Phys Rev B 54:11169–11186
Kresse G, Joubert D (1999) Phys Rev B 59:1758–1775
Blöchl PE (1994) Phys Rev B 50:17953–17979
(2013) MedeA® is a registered trademark of Materials Design, Santa Fe, NM
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865–3868
Dudarev SL, Botton GA, Savrasov SY, Humphreys CJ, Sutton AP (1998) Phys Rev B 57:1505–1509
Anisimov VI, Aryasetiawan F, Lichtenstein AI (1997) J Phys Condens Matter 9:767–808
Fabris S, Gironcoli DS, Baroni S, Vicario G, Balducci G (2005) Phys Rev B 72:237102
Jonsson H, Mills G, Jacobsen KW (1998) Nudged elastic band method for finding minimum energy paths of transitions. World Scientific, Singapore
Henkelman G, Jónsson H (2000) J Chem Phys 113:9978–9985
Henkelman G, Uberuaga BP, Jónsson H (2000) J Chem Phys 113:9901–9904
Sheppard D, Terrell R, Henkelman G (2008) J Chem Phys 128:134106
Sheppard D, Henkelman G (2011) J Comput Chem 32:1769–1771
Sheppard D, Xiao P, Chemelewski W, Johnson DD, Henkelman G (2012) J Chem Phys 136:074103
Wynne-Jones WFK, Eyring H (1935) J Chem Phys 3:492–502
Monkhorst HJ, Pack JD (1976) Phys Rev B 13:5188
Yang BX, Ye LP, Gu HJ, Huang JH, Li HY, Luo Y (2015) J Mol Model 21:195
Li HY, Wang HF, Gong XQ, Guo YL, Guo Y, Lu GZ, Hu P (2009) Phys Rev B 79:193401
Mayernick AD, Janik MJ (2008) J Phys Chem C 112:14955–14964
Acknowledgment
This work was supported by Natural Science Foundation of Shanghai (15ZR1421500).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, BX., Luo, Y. & Ye, LP. CO oxidation on inverse Ce6O12/Cu(111) catalyst: role of copper–ceria interactions. J Mol Model 24, 20 (2018). https://doi.org/10.1007/s00894-017-3567-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3567-6