Abstract
The present study investigated whether a water-soluble extract from the culture medium of Ganoderma lucidum mycelia (Japanese: Reishi or Mannentake) (designated as MAK) exerted a protective effect against induction of aberrant crypt foci (ACF) by azoxymethane (AOM) and small-intestinal damage induced by the anticancer drug 5-FU. Six-week-old male F344 rats were fed a basic diet (MF), either alone or containing 2.5 % MAK, beginning 1 week before treatment with AOM. The rats were then given subcutaneous injections of AOM (15 mg/kg body weight) once in a week for 3 weeks. Next, beginning 1 day after the final AOM treatment, 25 or 80 mg/kg 5-FU was injected intraperitoneally three times at 5-day intervals. Finally, the rats were killed 3.5 days after the last injection of 5-FU. The large and small intestines were removed, and tissue specimens were examined for both ACF in the large intestine and regeneration of small-intestinal crypts. The number of ACF was significantly decreased by treatment with 25 mg 5-FU and further decreased by 25 mg 5-FU + MAK in comparison with 5-FU alone. Moreover, there was a greater degree of recovery from small-intestinal damage in the 5-FU + MAK groups than in rats that had received 5-FU alone. The present results indicate that MAK ameliorates the colon precancerous lesions induced by AOM and the small-intestinal injury caused by 5-FU, suggesting that MAK could have potential as a preventive agent against colonic precancer, which is a common adverse effect of chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ganoderma lucidum (Fr.) Karst. (Polyporaceae) is a medicinal mushroom known to the Japanese as ‘Reishi’ or ‘Mannentake,’ or to the Chinese as ‘Lingzhi.’ Its fruiting bodies have been used for their medicinal properties in traditional Chinese medicine for more than 2,000 years. The use of this mushroom for promotion of vitality and as an anti-aging agent was described in detail in the classic compendium of traditional Chinese medicine Shen Nung Ben Cao Jin (dated 206 BC–8 AD), and G. lucidum has been used more recently in China and other oriental countries for the treatment of debility and weakness, hypertension, cardiovascular disease, bronchitis, arthritis, neurasthenia, insomnia, hepatopathy, chronic hepatitis, nephritis, gastric ulcer, asthma, diabetes, altitude sickness, acquired immunodeficiency syndrome (AIDS), and cancer [1–3]. Of particular interest among the reported biological/pharmacological properties of G. lucidum are its antitumor activities, including cell-cycle arrest, induction of apoptosis, inhibition of motility, anti-angiogenesis, and anti-mutagenesis [3–9]. The fruiting bodies and mycelium of the mushroom have very similar composition, but as the mycelium contains several additional nutrients and components it is considered the “essence” of the mushroom organism responsible for its beneficial functions. The use of Ganoderma mycelia as a “designer food” means that culture techniques for this organism have been well established.
A water-soluble extract from the culture medium of G. lucidum mycelia (designated as MAK) after fermentation on a solid medium containing bagasse contains various substances such as polysaccharides, proteins, water-soluble lignin, and triterpenes. Previously, we have reported that MAK exerts preventive effects against the development of chemical carcinogen-induced aberrant crypt foci (ACF), colon adenomas, colon adenocarcinomas, and pulmonary adenocarcinomas in rats [10–13], and against X-ray irradiation-induced small-intestinal injury in mice [14]. Chemotherapy is highly cytotoxic, causing a number of severe adverse effects such as nausea and vomiting. Recently, we reported that MAK was able to protect against small-intestinal injury after chemotherapy [15]. Many previous studies using animals have investigated the effects of various agents against implanted tumors, such as sarcoma 180 [16–18]. So far, however, no studies employing tumors induced by chemical agents have been reported.
The present study was conducted to assess the effects of MAK on ACF and small-intestinal injury induced in rats by azoxymethane after administration of the anticancer agent fluorouracil (5-FU).
Materials and methods
Animals
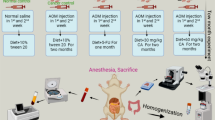
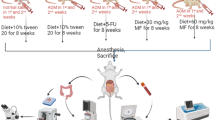
Fifty-one male F344/DuCrj rats, 6 weeks of age at study commencement, were purchased from Charles River Japan (Hino, Japan). They were housed 5 to a polycarbonate cage and kept under constant conditions of temperature (24° ± 2 °C) and relative humidity (55 ± 10 %) with a 12 h light:12 h dark cycle. The animals were maintained in accordance with the “Guidelines for the Care and Use of Laboratory Animals” and fed a commercial diet (MF; Oriental Yeast, Tokyo, Japan), either alone or supplemented with 2.5 % (w/w) MAK. Tap water was also provided ad libitum.
MAK
MAK was prepared by Noda Shokkin-Kogyo (Chiba, Japan). In brief, G. lucidum mycelia were cultured in a solid medium composed mainly of sugar cane bagasse for 3 months, and then the entire medium containing the mycelia was extracted with hot water. The extract was filtered and spray-dried to obtain MAK.
Azoxymethane
Azoxymethane (AOM) was purchased from Nard Institute (Hyogo, Japan). Starting at 7 weeks of age, the rats were given a subcutaneous injection of AOM (15 mg/kg body wt) weekly for 3 weeks to induce ACF.
5-FU
5-FU was obtained from Kyowa Hakko (Tokyo, Japan) (5-FU Injection 250 Kyowa). Starting 1 day after the final AOM treatment, 25 or 80 mg/kg 5-FU was injected intraperitoneally three times at 5-day intervals.
Autopsy
Rats were killed 3.5 days after the last injection of 5-FU (Fig. 1). Immediately after death, segments of the jejunum from the ileal junction were removed and fixed in Carnoy’s solution. The segments were cut into several pieces, bundled together, embedded in paraffin, sectioned at a thickness of 3 μm, and stained with hematoxylin and eosin. To quantify regenerating crypts, the number of crypts per circumference was determined in cross-sections. In each rat, the number of regenerative crypts in ten gut cross-sections was scored. Immunohistochemistry was performed using an anti-proliferating cell nuclear antigen (PCNA) antibody (Dako, Kyoto, Japan) by the avidin–biotin complex method. Tissue sections were deparaffinized with xylene, rehydrated through a graded ethanol series, and incubated with 0.3 % hydrogen peroxide for 30 min to block endogenous peroxidase activity. They were then incubated with 10 % normal horse serum at room temperature for 30 min to block background staining, and then reacted with the anti-PCNA antibody.
Each colon was removed, flushed with saline, slit open longitudinally from the cecum to the anus, placed on a paper towel, and fixed in 10 % buffered formalin for 24 h. Following the protocol reported by Magnuson et al. [19], the fixed colons were stained with 0.5 % methylene blue for 30 min, and then placed on a glass slide with the luminal side up. The stained colons were viewed under a microscope at a magnification of 20× to 30× and assessed for the presence of ACF. The numbers of ACF per colon, the numbers of AC (aberrant crypt) per colon, and the numbers of AC per focus were determined. Segments from the proximal to the descending colon were cut, and sections of paraffin-embedded tissue were stained with hematoxylin and eosin, Alcian blue–periodic acid–Schiff, and also for PCNA.
Cell counting
PCNA-positive cells in the small-intestinal crypts were counted in single vertically sectioned crypts from the bed of the crypt to the top, and crypt length was also measured using an image analyzer.
The PCNA positivity index and germinal region in the large intestine were measured by the method reported previously [10]. The PCNA-positive cells nearest to and furthest from the crypt bed were defined as the base and the top, respectively, and the distance between them (germinal region) was measured. The numbers of cells observed in single crypts, and from the crypt bed at the top, were counted and converted into percentage control values for half crypts. The numbers of positively stained nuclei were counted and divided by the total number of nuclei to give the PCNA index (%).
Statistical analysis
Statistical significance was determined by Dunnett’s method for multiple comparisons.
Results
Administration of AOM led to a significant decrease in body weight as compared with nontreated animals. After the first and second administrations of 80 mg 5-FU, body weight was significantly decreased as compared with animals receiving AOM, and body weight loss was especially evident in the AOM+5FU80+MAK group (Fig. 2).
The histological appearance of ACF is shown in Fig. 3. Many mitoses were evident in the crypts, and the mucin components revealed by Alcian blue–PAS staining differed from those in normal crypts. PCNA-positive cells were found in abnormal crypts. Table 1 summarizes the data for ACF, total numbers of AC, and numbers of AC per ACF. Numbers of ACF, total numbers of AC, and mean numbers of AC per ACF in the AOM + 5-FU and AOM + 5-FU + MAK groups were significantly decreased in comparison to rats that had received AOM alone, and the numbers of ACF and total numbers of AC in the AOM + 5-FU25 + MAK group were significantly decreased in comparison to the AOM + 5-FU25 group. In the 5-FU80 groups, the numbers of ACF and total numbers of AC were significantly decreased as compared to rats that had received AOM alone. However, the differences between the AOM + 5-FU80 and AOM + 5-FU + MAK groups were not significant.
The PCNA positivity index and the height of the germinal region in the AOM + 5-FU groups were significantly increased as compared with the MAK groups (Fig. 4; Table 2).
Tissue specimens from the small intestine were prepared and the numbers of regenerative crypts in them were counted (Table 3). Regenerated crypts in the AOM + 5-FU25 + MAK group (168 ± 130) tended to be increased in comparison with the AOM + 5-FU25 group (145 ± 71, p > 0.05). Histologically evident crypt damage in the AOM + 5-FU80 group was more severe than that in the AOM + 5-FU25 group, and the regenerative crypts in the AOM + 5-FU80 + MAK group were longer than those in the AOM + 5-FU group, which still retained damaged villi (Fig. 5). Crypt length and the number of PCNA-positive cells per crypt in the MAK groups (Fig. 6) were significantly increased as compared with the 5-FU groups (Table 4), and were significantly increased in the AOM + 5-FU80 + MAK group relative to the AOM + 5-FU25 + MAK group.
Discussion
In this study, the presence of ACF, which is a precancerous state, as induced by AOM was shown to be decreased by 5-FU and MAK. Many previous studies of cancer prevention have used transplanted tumors, such as sarcoma 180 [16–18], whereas here we developed a new assay model for evaluation of cancer prevention using tumors induced in a short time by AOM.
We found that 5-FU decreased the formation of ACF induced by AOM, and that combination with MAK further decreased the numbers of ACF. At a high dose of 5-FU (80 mg), AOM-induced ACFs were decreased (to 61.1) by 5-FU alone, and to 9.8 and 8.1, respectively, when MAK was used in combination. The decrease of ACFs following treatment with 5-FU was marked, but the effect of the combination treatment was not clear. Most of the intestinal mucosa containing ACFs could have been ablated as a result of the cytotoxic effect of 5-FU alone. By contrast, with a low dose of 5-FU (25 mg), the decrease of ACFs resulting from treatment with 5-FU alone was moderate (to 46.9), and an additional decrease of ACFs (to 26.4) was clearly observed when 5-FU was combined with MAK. Previously we reported that dietary application of MAK clearly inhibited the induction of ACF and adenoma in F344 rats [10]. Recently, using follow-up colonoscopy, Oka et al. [20] reported that the number and total size of adenomas in humans to whom MAK was administered were significantly decreased from the baseline. It is suggested that MAK suppresses the development of colorectal adenomas in the large bowel, and that MAK might decrease the effects of tumor prevention by 5-FU. Moreover, in the present study, 5-FU decreased ACF induced by AOM, and the decrease induced by 5-FU + MAK was greater than that with 5-FU alone. This finding suggests that MAK might decrease ACF without interfering with the effects of 5-FU. In fact, the two agents appeared to produce an additive antitumor effect, suggesting that MAK used in combination with chemotherapeutic drugs might improve the effectiveness of cancer treatment. Wang et al. [21] have reported that a G. lucidum extract ameliorated cisplatin (CDDP)-induced nausea and vomiting, and improved food intake, in a concentration-dependent manner in a rat pica model based on measurement of kaolin intake. In a clinical study reported by Zhuang et al. [22], a Chinese medicinal herb complex containing G. lucidum extract decreased leucopenia and neutropenia induced by chemotherapy. Nonaka et al. [23] recently reported that an antlered form of G. lucidum (Rokkaku-reishi) relieved cyclophosphamide (CPA)-induced weight loss and suggested that G. lucidum might be useful for reducing the adverse effects of anticancer drugs. Furthermore, Nonaka et al. [18] have reported that G. lucidum inhibited the growth of transplanted tumors and prolonged survival when administered orally to mice, as well as exerting antitumor activity when administered after tumor inoculation.
In the present study, MAK prevented small-intestinal injury by increasing the number of regenerative crypts induced by 5-FU. We reported previously that MAK ameliorated the small-intestinal injury caused by 5-FU, CDDP, CPA, and gefitinib [15]. We suggest that MAK may reduce the gastrointestinal adverse effects of anticancer drugs without attenuating their beneficial antitumor activity. Further studies are required to elucidate any differences in effects between the mycelia and fruiting bodies of G. lucidum.
In this experiment, the PCNA positivity index and the height of the germinal region in the AOM + 5-FU + MAK group were significantly decreased in comparison with the AOM + 5-FU group. Previously, we reported that MAK acted as a preventive agent against colon carcinogenesis by suppressing cell proliferation [10] and that MAK was able to immediately promote cell growth for repair of acute small-intestinal injury caused by X-irradiation [14] or administration of chemotherapeutic drugs [15]. Oral administration of dextran sulfate sodium also caused colon mucosal injury, which was rapidly ameliorated by application of MAK [24]. It is considered that MAK might have two contrasting actions: in the case of abnormal cell growth, renewal may be decreased, thus inhibiting tumor growth, whereas in the case of damage, cell proliferation may be increased to effect emergency repair. Similar results were obtained in the present study. Further studies are required to clarify how the switching of these different actions occurs.
In conclusion, our results suggest that 5-FU decreases the formation of ACF induced by AOM, and that combination with MAK further decreases the number of ACF and the degree of acute small-intestinal damage induced by 5-FU. Further longer-term studies are required to assess the value of MAK in the treatment of other adverse effects of anticancer drugs, such as organ injury and alopecia.
References
Lin ZB (2001) Pharmacological functions of Ganoderma lucidum. In: Lin ZB (ed) Modern research of Ganoderma lucidum, 2nd edn. Beijing Medical University Press, Beijing, pp 284–309
Wasser SP, Weis AL (1999) Medicinal properties of substances occurring in higher basidiomycetes mushrooms: current perspective. Int J Med Mushrooms 1:31–62
Shiao MS (2003) Natural products of the medicinal fungus Ganoderma lucidum: occurrence, biological activities, and pharmacological functions. Chem Rec 3:172–180
Hu H, Ahn NS, Yang X, Lee YS, Kang KS (2002) Ganoderma lucidum extract induces cell cycle arrest and apoptosis in MCF-7 human breast cancer cell. Int J Cancer 102:250–253
Sliva D, Labarrere C, Slivova V, Sedlak M, Lloyd FP Jr, Ho NW (2002) Ganoderma lucidum suppresses motility of highly invasive breast and prostate cancer cells. Biochem Biophys Res Commun 298:603–612
Ghafar MA, Golliday E, Bingham J, Mansukhani MM, Anastasiadis AG, Katz AE (2002) Regression of prostate cancer following administration of genistein combined polysaccharide (GCP™), a nutritional supplement: a case report. J Altern Complement Med 8:493–497
Gao Y, Zhou S, Jiang W, Huang M, Dai X (2003) Effects of ganopoly (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol Invest 32:201–215
Song YS, Kim SH, Sa JH, Jin C, Lim CJ, Park EH (2004) Anti-angiogenic and inhibitory activity on inducible nitric oxide production of the mushroom Ganoderma lucidum. J Ethnopharmacol 90:17–20
Lakshmi B, Ajith TA, Sheena N, Gunapalan N, Janardhanan KK (2003) Antiperoxidative, anti-inflammatory, and antimutagenic activities of ethanol extract of the mycelium of Ganoderma lucidum occurring in South India. Teratog Carcinog Mutagen 1:85–97
Lu H, Uesaka T, Katoh O, Kyo E, Watanabe H (2001) Prevention of the development of preneoplastic lesions, aberrant crypt foci, by a water-soluble extract from cultured medium of Ganoderma lucidum (Rei-shi) mycelia in male F344 rats. Oncol Rep 8:1341–1345
Lu H, Kyo E, Uesaka T, Katoh O, Watanabe H (2002) Prevention of development of N, N’-dimethylhydrazine-induced colon tumors by a water-soluble extract from cultured medium of Ganoderma lucidum (Rei-shi) mycelia in male ICR mice. Int J Mol Med 9:113–117
Lu H, Kyo E, Uesaka T, Katoh O, Watanabe H (2003) A water-soluble extract from cultured medium of Ganoderma lucidum (Rei-shi) mycelia suppresses azoxymethane-induction of colon cancers in male F344 rats. Oncol Rep 10:375–379
Kashimoto N, Hayama M, Kamiya K, Watanabe H (2006) Inhibitory effect of a water-soluble extract from the culture medium of Ganoderma lucidum (Rei-shi) mycelia on the development of pulmonary adenocarcinoma induced by N-nitrosobis (2-hydroxypropyl) amine in Wistar rats. Oncol Rep 16:1181–1187
Kubo N, Myojin Y, Shimamoto F, Kashimoto N, Kyo E, Kamiya K, Watanabe H (2005) Protective effects of a water-soluble extract from cultured medium of Ganoderma lucidum (Rei-shi) mycelia and Agaricus blazei Murrill against X-irradiation in B6C3F1 mice: increased small intestinal crypt survival and prolongation of average time to animal death. Int J Mol Med 15:401–406
Kashimoto N, Ishii S, Myojin Y, Ushijima M, Hayama M, Watanabe H (2010) A water-soluble extract from cultured media of Ganoderma lucidum (Reishi) mycelia attenuates the small intestinal injury induced by anti-cancer drugs. Oncol Lett 1:63–68
Wang J, Zhang L, Yu Y, Cheung PC (2009) Enhancement of antitumor activities in sulfated and carboxymethylated polysaccharides of Ganoderma lucidum. J Agric Food Chem 57:10565–10572
Nonaka Y, Ishibashi H, Nakai M, Shibata H, Kiso Y, Abe S (2008) Effects of the antlered form of Ganoderma lucidum on tumor growth and metastasis in cyclophosphamide-treated mice. Biosci Biotechnol Biochem 72:1399–1408
Nonaka Y, Shibata H, Nakai M, Kurihara H, Ishibashi H, Kiso Y, Tanaka T, Yamaguchi H, Abe S (2006) Anti-tumor activities of the antlered form of Ganoderma lucidum in allogeneic and syngeneic tumor-bearing mice. Biosci Biotechnol Biochem 70:2028–2034
Magnuson BA, Carr I, Bird RP (1993) Ability of aberrant crypt foci characteristics to predict colonic tumor incidence in rats fed cholic acid. Cancer Res 53:4499–4504
Oka S, Tanaka S, Yoshida S, Hiyama T, Ueno Y, Ito M, Kitadai Y, Yoshihara M, Chayama K (2010) A water-soluble extract from culture medium of Ganoderma lucidum mycelia suppresses the development of colorectal adenomas. Hiroshima J Med Sci 59:1–6
Wang CZ, Basila D, Aung HH, Mehendale SR, Chang WT, McEntee E, Guan X, Yuan CS (2005) Effects of Ganoderma lucidum extract on chemotherapy-induced nausea and vomiting in a rat model. Am J Chin Med 33:807–815
Zhuang SR, Chen SL, Tsai JH, Huang CC, Wu TC, Liu WS, Tseng HC, Lee HS, Huang MC, Shane GT, Yang CH, Shen YC, Yan YY, Wang CK (2009) Effect of citronellol and the Chinese medical herb complex on cellular immunity of cancer patients receiving chemotherapy/radiotherapy. Phytother Res 23:785–790
Nonaka Y, Ishibashi H, Nakai M, Shibata H, Kiso Y, Abe S (2005) Soothing effect of Ganoderma lucidum antlered form on cyclophosphamide-induced adverse reaction (in Japanese). Jpn J Cancer Chemother 32:1586–1588
Kashimoto N, Ueno T, Kyo E, Tanaka S, Watanabe H (2005) A water-soluble extract of Ganoderma lucidum mycelia (MAK) suppresses the ulcerative colitis induced by dextran sulfate sodium (in Japanese). J Jpn Mibyou Syst Assoc 11:153–156
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watanabe, H., Kashimoto, N., Ushijima, M. et al. Effects of a water-soluble extract of Ganoderma lucidum mycelia on aberrant crypt foci induced by azoxymethane and small-intestinal injury by 5-FU in F344 rats. Med Mol Morphol 46, 97–103 (2013). https://doi.org/10.1007/s00795-013-0012-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-013-0012-5