Abstract
The availability of microbiological and geochemical data from island-based and high-arsenic hydrothermal systems is limited. Here, the microbial diversity in island-based hot springs on Ambitle Island (Papua New Guinea) was investigated using culture-dependent and -independent methods. Waramung and Kapkai are alkaline springs high in sulfide and arsenic, related hydrologically to previously described hydrothermal vents in nearby Tutum Bay. Enrichments were carried out at 24 conditions with varying temperature (45, 80 °C), pH (6.5, 8.5), terminal electron acceptors (O2, SO4 2−, S0, NO3 −), and electron donors (organic carbon, H2, AsIII). Growth was observed in 20 of 72 tubes, with media targeting heterotrophic metabolisms the most successful. 16S ribosomal RNA gene surveys of environmental samples revealed representatives in 15 bacterial phyla and 8 archaeal orders. While the Kapkai 4 bacterial clone library is primarily made up of Thermodesulfobacteria (74 %), no bacterial taxon represents a majority in the Kapkai 3 and Waramung samples (40 % Proteobacteria and 39 % Aquificae, respectively). Deinococcus/Thermus and Thermotogae are observed in all samples. The Thermococcales dominate the archaeal clone libraries (65–85 %). Thermoproteales, Desulfurococcales, and uncultured Eury- and Crenarchaeota make up the remaining archaeal taxonomic diversity. The culturing and phylogenetic results are consistent with the geochemistry of the alkaline, saline, and sulfide-rich fluids. When compared to other alkaline, island-based, high-arsenic, or shallow-sea hydrothermal communities, the Ambitle Island archaeal communities are unique in geochemical conditions, and in taxonomic diversity, richness, and evenness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent studies have broadened the perspective of global microbial diversity in hydrothermal environments. Worldwide, these include deep-sea and continental hydrothermal systems, as well as the increasingly recognized shallow-sea hydrothermal vent systems. Diversity in hydrothermal ecosystems is linked directly to highly variable geochemistry, the corresponding array of potential metabolisms in the form of redox disequilibria, and input of nutrients from external sources (Shock et al. 2005, 2010; Spear et al. 2005, Swingley et al. 2012). The myriad geochemical environments created by water–gas–rock interactions inherent to hydrothermal systems provide energy, nutrients, and essential trace elements, as well as potentially toxic substances (Amend and Shock 2001; Meyer-Dombard et al. 2012a; Pol et al. 2013). For example, many terrestrial hot springs contain elevated concentrations of trace or toxic elements, including As (Aiuppa et al. 2003; Arnorsson 2003; Baba and Sözbilir 2012; Bundschuh et al. 2013; Landrum et al. 2009; Le Guern et al. 2003; Nordstrom et al. 2005; Planer-Friedrich et al. 2006; Price and Pichler 2005; Stauffer and Thompson 1984; Summers Engel et al. 2013). Relevant to the Ambitle Island hydrothermal systems, redox reactions involving arsenic have been shown to drive metabolic processes in continental hydrothermal ecosystems in Yellowstone National Park (Donahoe-Christiansen et al. 2004; Inskeep et al. 2004; Langner et al. 2001; Macur et al. 2004). In fact, the fastest rate of arsenic oxidation in a natural aquatic system was recorded in one Yellowstone National Park hot spring in the Norris Geyser Basin, where arsenite oxidation, ferrihydrite precipitation, and biogenesis of arsenate-rich hydrous ferric oxide (HFO) mats appear to be directly influenced by the presence of sulfide and its consumption by archaeal and bacterial taxa (Donahoe-Christiansen et al. 2004; Inskeep et al. 2004; Macur et al. 2004).
The volcanic Ambitle Island (Papua New Guinea) has received recent attention due to elevated concentrations of arsenic in hydrothermal fluids in Tutum Bay (Akerman et al. 2011; Meyer-Dombard et al. 2012b, 2013; Price and Pichler 2005; Price et al. 2007). Ambitle Island features shallow-sea hydrothermal vents in Tutum Bay and several on-land hot springs characterized by highly elevated levels of bioavailable arsenic that is released into the local ecosystem (Pichler and Dix 1996; Price and Pichler 2005; Price et al. 2013b). While Ambitle Island is sparely populated, the reported >2 ppm total As (two orders of magnitude above the EPA declared safe level for drinking water) may present acute health risk to the local inhabitants, who live in close proximity to the springs. In the shallow-sea sediments of Tutum Bay, diverse bacterial populations dominate the microbial communities, but metabolic functions are poorly constrained because most of the community members are only distantly related to characterized isolates (Meyer-Dombard et al. 2012b). Gene surveys of DNA extracted from biofilms and vent fluid, however, showed that microorganisms are likely involved in the oxidation of arsenite (AsIII) and ferrous iron (FeII) (Meyer-Dombard et al. 2013). Here, we extend the study of Ambitle Island hydrothermal ecosystems to include several terrestrial (on-land) hot springs that feature some of the highest reported arsenic concentrations in terrestrial hydrothermal systems, ~1000× higher than the above-mentioned Norris Geyser Basin system in Yellowstone National Park. We use culture-dependent and culture-independent approaches to assess the archaeal and bacterial diversity and metabolic function in two alkaline, high-arsenic springs, and interpret the findings within a geochemical context. These data add to the global inventory of data garnered from island-based hydrothermal ecosystems, and are compared to other previously published island-based datasets.
Methods
Sample collection
Sediments and associated biofilms were sampled with sterile technique at Kapkai and Waramung springs on Ambitle Island, Papua New Guinea during a one-time research expedition in 2005. Samples were placed in 15-mL Falcon tubes, and either stored at 4 °C (for future culturing) or frozen at −20 °C (for DNA extraction) on return to the lab several hours later. At Waramung Spring, the upper ~1 cm of a fine-grained, black mud was taken from the edge of a small pool. At Kapkai Spring, two locations in an outflow channel were sampled. The first (Kapkai 3) was a fine-grained, light gray sediment covered with a green biofilm, and the second (Kapkai 4) was a soft, light gray, gelatinous sediment capped with a ~2-mm hard siliceous crust. At each Kapkai location, the crust/biofilm and ~0.2 cm of underlying sediment were sampled and homogenized.
Enrichment culturing
Enrichment growth media were designed from available geochemical data for Waramung and Kapkai hot springs (Pichler 2005; Pichler et al. 1999a). A base solution contained (per liter of water): 21.1 g NaCl, 10.5 g Na2SO4, 2.83 g KCl, 0.848 g NaHCO3, 0.71 g Na2SiO3·5H2O, and 10 mL of a 100× trace element solution (containing per liter of water: 3.6 g NaBr, 28.4 g H3BO3, 2.86 g LiCl, 0.69 mg MnCl2·4H2O, 0.432 g RbCl, 0.72 g SrCl2·6H2O, 18.24 mg SbCl3, 0.3864 g NaAsO3, 2.14 mg TiCl, 63.12 mg CsCl, 0.712 g MgCl2·6H2O, 0.06 mg CoCl2, 3.3 mg ZnCl2, 7.7 mg CuCl2·2H2O, 1.32 g AlCl3·6H2O, 0.18 mg Na2MoO4·2H2O, 0.44 mg NiCl2·6H2O). From this base solution, six different media—each at two pHs (6.5 and 8.5)—were designed to target different metabolisms. The medium targeting anaerobic heterotrophy included per liter of base solution: 3 g yeast extract, 3 g peptone, 3.7 mL PNG N/P solution (per liter of water: 14.4 g NH4Cl and 3.6 g KH2PO4), 1.05 mL PNG NO3 − solution (per liter of water: 100 g NaNO3), 3 g PIPES (pH 6.5) or AMPSO (pH 8.5) pH buffer, and 0.5 mL 0.2 % resazurin as an oxygen indicator. The headspace gas was N2. The medium targeting aerobic heterotrophy was the same as the anaerobic heterotrophy medium, except that 1.5-mL filtered (0.2 μm) air was added to the headspace and resazurin was omitted. The sulfate-reduction medium targeted both heterotrophic and autotrophic sulfate reducers, and included per liter of base solution: 10 mg yeast extract, 3 g sodium lactate, 3 g sodium acetate, 7.5 mL PNG N/P solution, 3 g PIPES or AMPSO pH buffer, and 0.5 mL 0.2 % resazurin. The headspace gas was a mixture of H2:CO2 (80:20). The medium targeting aerobic arsenite oxidation included per liter of base solution: 10 mg yeast extract, 7.3 mL NO3/PO4 solution (per liter of water: 28.4 g NaNO3 and 3.6 g KH2PO4), 2 mL As(III) solution (per liter of water: 3.2 g NaAsO2), and 3 g PIPES or AMPSO pH buffer. The headspace gas was a mixture of N2:CO2 (70:30), with 1.5 mL filtered (0.2 μm) air. The medium targeting anaerobic chemolithoautotrophy contained per liter of base solution: 10 mg yeast extract, 3.7 mL PNG N/P solution, 1.05 mL PNG NO3 − solution, 3 g PIPES or AMPSO pH buffer, and 0.5 mL 0.2 % resazurin. The headspace gas was a mixture of H2:CO2 (80:20), and each tube was amended with elemental sulfur (see below). The medium targeting aerobic chemolithoautotrophy was the same as the anaerobic heterotrophy medium, except that 1.5 mL filtered (0.2 μm) air was added to the headspace and resazurin was omitted. The pH of each medium was adjusted prior to autoclaving. After autoclaving, the following filter-sterilized (0.2 μm) solutions were added to all media: 0.5 mL vitamins (per liter of water: 0.04 g biotin, 0.04 g folic acid, and 0.1 g each of pyridoxine–HCl, thiamine-HCl·2H2O, riboflavin, nicotinic acid, D-Ca-pantothenate, cobalamin, p-aminobenzoic acid and lipoic acid) (Robb and Place 1995), 1.0 mL Fe/EDTA solution (per liter of water: 1.54 g FeSO4·7H2O and 2.06 g Na2EDTA), and 10 mL CaCl2 solution (2.2 g CaCl2·2H2O per liter of water). The media were then heated to a low boil and degassed under a steady stream of the prescribed headspace. Ten milliliter of medium was then transferred to sterile, acid-washed 25-mL Balch tubes. Tubes for aerobic and anaerobic chemolithoautotrophy also contained ~0.3 g S0 (sterilized by heating to 98 °C in an oven for at least 3 days with occasional stirring). Tubes were capped with butyl-rubber stoppers and aluminum seals and then pressurized to 3 bar with the prescribed gas. If appropriate, filtered air or filtered 2.5 % Na2S solution (0.3 mL) was added, prior to inoculation.
Sediment samples from Kapkai and Waramung were mixed with a pH-balanced aqueous base solution to form a slurry for inoculations. Tubes were inoculated using 2 mL of the slurry and incubated at 45 and 80 °C.
Assessment of cell growth
Cells were regularly observed using epifluorescence microscopy at 1300× magnification using a Zeiss Axioskop2 microscope. Cultures (0.5 mL) were fixed with 0.25 mL 10 % formalin solution (Sigma-Aldrich, St. Louis, MO) and stained with 0.75 mL 10 ppm DAPI (4′, 6′ diamidino-2-phenylindole, Sigma) solution for 15 min in the dark. This was then filtered through 0.2 µm, 25-mm black polycarbonate filters (GE/Thermo-Fisher Scientific) and mounted on glass slides using paraffin oil. Cells were enumerated by microscopy to determine growth, and results were binned as follows: “no growth”, “minimal growth” (fewer than 2 doublings observed), “growth” (more than 2 doublings observed), and “growth and successful transfer” (one or more transfers of cells followed by growth as defined by more than 2 doublings).
DNA extraction and 16S rRNA gene amplification
DNA extraction was performed on cell pellets of selected pure cultures, and environmental samples (thawed to room temperature), followed by PCR amplification of both the archaeal and bacterial 16S rRNA gene. Three different extraction methods were used: bead-beating using the PowerSoil DNA Isolation Kit (MoBio Laboratories Inc., Carlsbad, CA), alternative extraction method using heat in the PowerSoil DNA Isolation Kit, and bead-beating using the FastDNA SPIN Kit for Soil (Qbiogene, Carlsbad, CA).
Polymerase chain reaction (PCR) of both archaeal and bacterial 16S rRNA genes was completed with archaeal (21F:5′-TTC CGG TTG TAC CYG CCG GA-3′, 1391R: 5′-GAC GGG CGG TGT GTR CA-3′), modified from DeLong (1992), and bacterial (27F: 5′-AGA GTT TGA TCC TGG CTC AG-3′, 1492F: 5′-GGT TAC CTT GTT ACG ACT T-3′) primers (Lane 1991). Several PCR conditions were used to maximize amplification using a Hybaid PCR Express thermal cycler. PCR for archaea consisted of an initial denaturing step at 95 °C for 5 min followed by 30 cycles of 95 °C for 1 min, 55 °C for 1 min, and 72 °C for 2 min. The final extension step was at 72 °C for 15 min. PCR for bacterial 16S rRNA was similar with an initial denaturing step at 95 °C for 5 min, but followed by 35 cycles of 95 °C for 1 min, 52 °C for 1 min, and 72 °C for 2 min. The extension step was at 72 °C for 5 min. Each 20-µL PCR reaction mixture consisted of 1.8–2.8 µL of 25 mM MgCl2 solution, 2 µL of 10 × PCR Buffer II (Applied Biosystems, Carlsbad, CA), 0.2 µL of 100 mM dNTP Mix (Bioline USA Inc., Taunton, MA), 0.25 µL of 5 U µL−1 AmpliTaq Gold DNA polymerase (Applied Biosystems), 0.5 µL each of forward and reverse primers (1 µM), and 1 µL template DNA. Some of the reactions contained 0.5 µg µL−1 BSA. Products were visualized on a 1.5 % agarose gel using SYBR Green I Nucleic Acid Gel Stain (Cambrex Bio Science Rockland, Inc., Rockland, ME) combined with the PCR product. Products were then cleaned using the Wizard SV Gel and PCR Clean-up System (Promega Corp., Madison, WI) using the manufacturer’s instructions.
16S rRNA gene cloning and sequencing
DNA extraction using the bead-beading method (MoBio PowerSoil kit) was the most successful, and PCR products were cloned and plated on LB agar plates using the Qiagen PCR Cloning Kit (Qiagen, Inc., Valencia, CA) according to manufacturer’s instructions. Colonies were randomly selected and incubated overnight in 3-mL Luria Broth medium with ampicillin. Plasmid DNA was extracted and purified using the QIAPrep Spin Miniprep Kit (Qiagen). Purified DNA concentrations were determined using spectrophotometry, and template DNA was sequenced at Polymorphic DNA Technologies, Inc. (Alameda, CA) and MCLAB (South San Francisco, CA) with either the T7 promoter and SP6 promoter or M13 forward and M13 reverse sequencing primers. Some plasmids were also sequenced using either 958R [5′-YCC GGC GTT GAM TCC AAT T-3′, (DeLong 1992)] for archaea or 907R [5′-CCG TCA ATT CCT TTG AGT TT-3′, (Lane 1991)] for bacteria for optimum overlapping in the middle of the 16S rRNA gene. The total number of bacterial clones sequenced was 358, with 113 for Kapkai 3, 46 for Kapkai 4, and 199 for Waramung. The total number of archaeal clones was 263, with 168 for Kapkai 3, 49 for Kapkai 4, and 46 for Waramung.
Phylogenetic analysis
Contiguous sequences were assembled using Sequencher v. 4.8 (Gene Codes Corporation, Ann Arbor, MI) and compared to the NCBI (National Center for Biotechnology Information) database using BLAST (Altschul et al. 1997) and the Ribosomal Database Project’s Classifier tool (Wang et al. 2007). All archaeal and bacterial sequences from each site were grouped based on 97 % similarity in Sequencher, and a representative from each group was used for phylogenetic analysis. Representative sequences were aligned using the Greengenes NAST server (DeSantis et al. 2006) and checked for chimeras using the Bellerophon v.3 server at Greengenes. For phylogenetic analysis, BLAST searches were used to find the closest relatives of the representative, non-chimeric sequences in the clone libraries from both archaea and bacteria at each site for use as reference sequences. The representative sequences and references sequences were manually aligned using the software BioEdit v. 7.5.0.3 (Hall 1999). Phylogenetic analysis of homologous positions in the alignment was performed using neighbor-joining (NJ) and maximum parsimony (MP) methods in the program PAUP v. 4b.10 (Sinhauer Associates, Sutherland, MA). MP analyses were performed with random addition of taxa (1000 replicates). All trees were evaluated using only unambiguously aligned nucleotides and bootstrapped (1000 replicates). Reference information concerning clonal taxonomic identity and abundance for these clone libraries can be found in Supplemental Table 1. The 16S rRNA sequences reported here have been deposited in the Genbank database (NCBI) under the accession numbers JF935152-JF935232.
Calculation of community diversity indices
Standard indices of diversity and evenness were calculated for all samples and reference datasets. These are Shannon-Weiner (H′), Chao1, and Pielou (J′) diversity indices, and Pielou evenness (E) (Shannon and Weaver 1949; Pielou 1969, 1977; Chao et al. 2005). The equations for (H′), (J′), and (E) are conveniently reviewed in Smith and Wilson 1996. The Chao1 index is calculated by;
where S obs is the number of observed taxa, n 1 is the number of observed taxa with a single representative (a “singlet”) and n 2 is the number of observed taxa with two representatives (a “doublet”). The Chao1 index is often utilized as a means of assessing whether the full taxonomic diversity of a site has been realized with the sampling/analysis effort.
Results and discussion
Site description
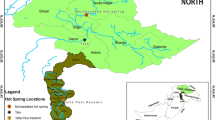
Ambitle Island (4.08°S, 153.62°E) is at the southern end of the Tabar-Feni island arc in eastern Papua New Guinea (PNG). It is part of a Quaternary stratovolcano atop Oligocene marine limestone (Wallace et al. 1983). Active thermal features are found primarily along the west coast, both above and below sea level (Pichler and Dix 1996). Off-shore, shallow-sea (5–10 m) venting (previously described) occurs in Tutum Bay (Pichler and Veizer 1999). Of particular note are the high levels of arsenic, which reach ~1000 ppb in the venting fluids (as arsenite, AsIII) and up to 76000 ppb adsorbed by FeIII-oxyhydroxides (as arsenate, AsV) directly next to the venting orifices (Pichler et al. 1999a, b, 2006).
The array of on-land hot mud pools, springs, and fumaroles on Ambitle Island reaches temperatures up to 100 °C and varies in pH from 1.9 to 9.1 (Licence et al. 1987; Wallace et al. 1983). The source springs of the two locations investigated in the present study (Kapkai and Waramung, see Fig. 1) are hot (>90 °C), and alkaline (pH 8.5)—in contrast to many other island-based hydrothermal systems which are often acidic. Additional geochemical data for Kapkai and Waramung are given in Table 1. The geochemistry of the Ambitle Island locations, both on-shore and shallow-submarine, is compared with the geochemistry of other representative hot springs for reference in Supplementary Table 2. These reference locations are used in the discussion below to provide context, and include other island-based, alkaline, and/or high-arsenic sites. Where possible, reference locations were chosen which also reported corresponding biological data.
Kapkai and Waramung springs undergo moderate water–rock interaction, based on [SiO2] and the ratio of Ca+2:Mg+2, similar to many other island-based, and shallow-sea springs (Fig. 2a). The Ambitle Island on-shore and off-shore sites have similar [SiO2], [Ca+2] and [Mg+2], indicating a likely shared source fluid (Pichler et al. 1999a). However, the [SO −24 ] and [Cl−] in the Kapkai and Waramung locations exceed nearly all other reference hydrothermal locations shown in Fig. 2b, including the Ambitle Island shallow-sea locations. As previous work has shown that the fluid in PNG springs is of meteoric and not marine origin (Pichler et al. 1999a), the elevated [SO −24 ] and [Cl−] may indicate a larger degree of water–gas interaction and longer fluid residence time as compared to the reference locations. Alternatively, a 1:1 correspondence in Fig. 2b indicates that the on-shore locations at Ambitle are proportionally higher in [SO −24 ] and [Cl−] than the shallow-sea vents, suggesting that while they share a common hydrothermal source, the on-shore locations are experiencing some degree of evaporation that has increased [SO −24 ] and [Cl−] during phase separation.
Site fluid geochemistry (SiO2, SO −24 , Cl−, Ca+2, Mg+2) shown with data from other hot spring and hydrothermal systems for reference. a [SiO2] and the molar ratio of Ca+2:Mg+2. b [SO −24 ] and [Cl−]. Key for both a and b gray circles = Island and shallow-sea hydrothermal vents (Amend et al. 2003; Dotsika 2012; Kaasalainen and Stefánsson 2012; Kim Phuong et al. 2012; Joseph et al. 2013; Price et al. 2013a); open squares = Continental systems, typically chosen from alkaline or high As sites (Ozler 2000; Langner et al. 2001; Tarcan and Gemici 2003; McCleskey et al. 2004; Shock et al. 2010; Baba and Sözbilir 2012; Guo and Wang 2012; Loiacono et al. 2012; Meyer-Dombard et al. 2012b; Swingley et al. 2012; Pürschel et al. 2013); blue circles = Waramung Spring (Pichler et al. 1999a); purple circles = Kapkai Spring (Pichler et al. 1999a); black circles = PNG shallow-sea vents; red and orange circles = PNG 4A03-2.5 0 cm and PNG 4A03-2.5 10 cm, respectively (Meyer-Dombard et al. 2012a)
While redox sensitive species, such as sulfide and dissolved oxygen, are rarely reported for hydrothermal systems, these are important components of microbial metabolism. A comparison of dissolved oxygen and total sulfide concentrations in the PNG systems with other available published data can be seen in Fig. 3a. Sulfide concentrations at Kapkai and Waramung are higher than at other terrestrial locations and the Tutum Bay shallow-sea vent 4A, but lower than in several shallow-sea locations in Sicily (Fig. 3a). Comparison with sulfide concentrations in other published island-based on-land hydrothermal systems (e.g., on Iceland, Java, Greece, and St. Lucia, supplemental Table 2) is not possible as sulfide is not reported for these sites. The high sulfide in the Kapkai and Waramung ecosystems likely plays an important role in microbial metabolism and the cycling of iron and arsenic, potentially interfering with microbial transformations of arsenic (Langner et al. 2001). Further, Kapkai and Waramung springs also contain some of the highest total arsenic concentrations reported in hydrothermal systems (Fig. 3b), including comparisons with other island locations, as well as shallow-sea and continental locations. Higher total arsenic in the on-land Ambitle Island locations relative to the vent fluid compositions may be partially due to an evaporative effect during phase separation of the source fluid at depth (Pichler et al. 1999a).
Redox sensitive chemical species in Kapkai and Waramung Springs, shown with data from other hot spring and hydrothermal systems for reference. a Sulfide and dissolved oxygen concentrations. Key for a black circles = PNG locations (this work); gray circles = Sicily and shallow-sea vent systems (Amend et al. 2003; Price et al. 2013a); open squares = Continental hydrothermal systems (Langner et al. 2001; McCleskey et al. 2004; Shock et al. 2010; Loiacono et al. 2012; Meyer-Dombard et al. 2012b; Swingley et al. 2012). b Total arsenic concentrations. Key is the same as in Fig. 2
Microbial diversity
The bacterial and archaeal diversity at Kapkai 3, Kapkai 4, and Waramung was assessed by full length 16S rRNA gene sequences and phylogenetic analysis with representative sequences (Fig. 4; Supplemental Table 1).
Bacteria
The bacterial diversity at Kapkai and Waramung springs is shown in Fig. 4, with 59 representative sequences, spread across 13 major bacterial phyla (identified using a 97 % similarity cutoff). The bacterial diversity of on-land Ambitle Island springs as a group is widely spread among many bacterial taxa, including clones that affiliate with Aquificae, Thermotogae, Thermodesulfobacteria, Deinococcus–Thermus, Deferribacteres, Spirochaetes, Planctomycetes, Firmicutes, Actinobacteria, Proteobacteria (α, β, γ, δ), Bacteroidetes–Chlorobi, and Fibrobacteres–Acidobacteria. In addition, several sequences formed a clade with sequences from the candidate group UB-40. The richness and evenness of Bacteria in Kapkai 3, Kapkai 4, and Waramung springs are readily observed in Fig. 5, and Supplemental Table 3.
Taxonomic diversity of Bacteria in PNG Kapkai and Waramung locations, and several additional published datasets for reference. Data are presented as percentages of the total reported dataset (values can be found in Supplemental Table 3). Shown are published submarine locations from Ambitle Island (Meyer-Dombard et al. 2012b, 2013) and Milos, Greece (Price et al. 2013a), several other island-based systems (Niederberger et al. 2008; Stout et al. 2009; Kato et al. 2011), and continental, alkaline and/or high-arsenic systems (Jackson et al. 2001; Meyer-Dombard et al. 2011; Coman et al. 2013; Hou et al. 2013 and pers. com.)
The clone library from Kapkai 3 featured nine different phyla with >2 clones. The dominant group within the clone library was the Proteobacteria (40 %), followed by Deinococcus/Thermus and Bacteroidetes/Chlorobi (both at 15 %) and Planctomycetes (12 %). Other taxa each accounted for only a few percent in the clone library; of note is UB-40 (6 %), which is the only clade at this site without any cultured representatives. Among the Proteobacteria at Kapkai 3, the subdivision levels were represented in the clone libraries as follows: Alphaproteobacteria (27 %), Betaproteobacteria (40 %), Gammaproteobacteria (9 %), and Deltaproteobacteria (24 %). Very few of the bacterial clones at Kapkai 3 fall into the typically thermophilic and hyperthermophilic phyla. This is consistent with the moderate temperature of 45 °C at this site.
In contrast, the bacterial diversity at the higher temperature Kapkai 4 site is low, represented by only four phyla. Here, the Thermodesulfobacteria dominate the clone library (74 %), followed by Deinococcus/Thermus (13 %) and Thermotogales (11 %). Note that the three dominant phyla are all typically thermophilic or hyperthermophilic, consistent with the elevated temperature (72 °C) at this location.
The Waramung clone library features nine bacterial phyla with >2 clones, dominated by the thermophilic Aquificales (39 %) and Thermodesulfobacteria (20 %). Two other identified groups—Deinococcus/Thermus (13 %) and Thermotogales (9 %)—also feature high temperature organisms. The temperature of the Waramung source pool was near boiling (98.6 °C). This explains the ~60 % Aquificales and Thermotogales in this clone library, the two bacterial phyla featuring hyperthermophilic strains with maximum growth temperatures up to and exceeding 90 °C (Huber et al. 1986; Jannasch et al. 1988; Huber et al. 1992, 1998). The abundance of Aquificales, specifically those closely related to strains of Persephonella, may also be related to the groups’ ability to oxidize sulfide.
The Proteobacteria, represented in the Kapkai 3 (45/113 clones) and Waramung (20/199 clones) libraries, are gram-negative and metabolically diverse. The majority are mesophilic, but some are moderately to extremely thermophilic. The Alphaproteobacteria at Kapkai and Waramung are most closely related to uncultured clones. However, three clones from Kapkai 3 share >99 % sequence identity with Albidovulum inexpectatum, an aerobic moderate thermophile isolated from a marine hot spring in the Azores (Albuquerque et al. 2002). In addition, three clones from Waramung are >97 % similar to Nitratireductor, a genus of mesophilic nitrate reducers with very broad pH (5.5–12) and salinity (0–8 % NaCl) tolerances (Kang et al. 2009; Kim et al. 2009; Labbé et al. 2004). Note also the high nitrate concentrations (~0.2 mM, Table 1) at Waramung and Kapkai, which could support nitrate reduction as a viable metabolic strategy. The Kapkai and Waramung clones identified with Betaproteobacteria fall into three operational taxonomic units (OTUs), one of which affiliates closely with the genus Tepidimonas, a group of moderate thermophiles found in circumneutral to slightly alkaline hot springs (Chen et al. 2006; Moreira et al. 2000). In addition, Waramung clone B31 is closely related to a Tutum Bay clone (PNG_TBSL_B73) indicating a possible link to the shallow-sea communities. The Gammaproteobacteria at Kapkai and Waramung affiliated closely (>98 %) with Thermomonas or Thioalkalivibrio. A few known Thermomonas strains are moderately thermophilic, including T. hydrothermalis isolated from a hot spring in Portugal that grows optimally at ~50 °C at circumneutral to slightly alkaline pH (Alves et al. 2003; Busse et al. 2002). The Deltaproteobacteria in the Kapkai 3 and Waramung clone libraries affiliated most often with uncultured clones, but one Kapkai 3 OTU was most similar (92 %) to Desulfovibrio, a cosmopolitan group of sulfate reducers that includes thermophiles and alkaliphiles.
OTUs affiliating with Bacteroidetes/Chlorobi, Deinococcus/Thermus, Thermodesulfobacteria, and Thermotogae were observed in all three clone libraries. However, only the Deinococcus/Thermus group accounted for >10 % of each library. Isolates of this phylum are predominantly from terrestrial hydrothermal areas where the pH ranges from mildly acidic to moderately alkaline (da Costa et al. 2006). The Thermodesulfobacteria account for a large fraction of the Kapkai 4 and Waramung libraries, with uncultured clones the closest relatives. Many Thermodesulfobacteria have been identified in and isolated from terrestrial and marine hydrothermal systems worldwide; they are obligately thermophilic anaerobes, and most respire sulfate (Alain et al. 2009; Jeanthon et al. 2002; Zeikus et al. 1983). Note that at 66–74 mM (Table 1), the sulfate levels at Kapkai and Waramung are about 2.5× seawater levels, providing an available oxidant in anaerobic portions of the pools.
The Thermotogae and Aquificae represent the deepest-branching phyla in the Bacteria (Winker and Woese 1991), and both feature numerous extreme thermophiles isolated from terrestrial and marine hydrothermal systems (Wagner and Wiegel 2008). Thermotogae were present at all three sites, with the closest relative (>99 % identity) of seventeen OTUs being Thermotoga neapolitana, an anaerobic, chemoheterotrophic hyperthermophile isolated from hot springs near Naples, Italy (Jannasch et al. 1988). Aquificae were only noted at Waramung, but there they accounted for 78 of the 199 clones, 74 of which were closely related (>97 % identity) to the genus Persephonella. The known strains of Persephonella are chemolithoautotrophic, microaerophilic thermophiles from deep-sea hydrothermal vent systems (Götz et al. 2002; Nakagawa et al. 2003). Waramung clone B70 is closely related to a clone (PNG_TBSL_B73) reported at the shallow-sea vent system in Tutum Bay, suggesting that subsurface contact between the on-land and shallow-submarine sites may exist.
Archaea
The archaeal diversity is shown in supplemental Table 1 as nearest neighbors (determined by BLAST) with twenty-one representative sequences (>97 % similarity) from Kapkai and Waramung. These sequences affiliate with several major taxonomic groups, including the Thermoproteales and Desulfurococcales among the Crenarchaeota, the Thermococcales among the Euryarchaeota, and the recently described Thaumarchaeota (Brochier-Armanet et al. 2008). However, a number of the representative sequences affiliate with uncultured Crenarchaeota and uncultured Euryarchaeota. Several uncultured Crenarchaeotal and Euryarchaeotal clones from Kapkai 3 and Kapkai 4 are closely related to archaeal clones reported in shallow-sea hydrothermal systems, such as Vulcano, Italy (Rogers and Amend 2005) and Tutum Bay, PNG (Akerman 2009), again suggesting that the on-land sites at Ambitle Island may share a subsurface biological reservoir with hydrothermal fluids emanating from the shallow-sea systems in the bay.
At all three sites, the (hyper)thermophilic Thermococcales are the dominant taxa—65 % at Kapkai 3, 86 % at Kapkai 4, and 74 % at Waramung, as shown in Fig. 6. Two sequences at Kapkai 3 are identified as Thaumarchaeota, a proposed third archaeal phylum of predominantly mesophiles, including Cenarchaeum symbiosum (Brochier-Armanet et al. 2008). Uncultured Euryarchaeota (18 % at Kapkai 3) and uncultured Crenarchaeota (10 % at Kapkai 3, 14 % at Kapkai 4) were the only other taxa of note.
Taxonomic diversity of Archaea in PNG Kapkai and Waramung locations, and several additional published datasets for reference. Data are presented as percentages of the total reported dataset (values can be found in Supplemental Table 3). Shown are published submarine locations from Ambitle Island (Meyer-Dombard et al. 2012b, 2013) and Milos, Greece (Price et al. 2013a), several other island-based systems (Niederberger et al. 2008; Stout et al. 2009; Kato et al. 2011), and continental, alkaline and/or high-arsenic systems (Jackson et al. 2001; Meyer-Dombard et al. 2011; Coman et al. 2013; Hou et al. 2013 and pers. com.)
Of the 186 Thermococcales clones in this study, 174 affiliated very closely (>99 % sequence identity) with strains of Thermococcus. This genus features strictly anaerobic moderate and extreme thermophiles that grow on complex organic matter. The numerous species (~30 according to Wagner and Wiegel 2008), have been isolated predominantly from terrestrial, shallow-sea, and deep-sea hydrothermal systems, and grow over broad temperature (40–103 °C), salinity (~0–8 % NaCl), and pH (3.5–10.5) ranges. The Thermoproteales, found at Kapkai 3, are most closely related to uncultured clones of the genus Thermoproteus, which includes obligately anaerobic, sulfur-respiring hyperthermophiles first isolated from terrestrial hot springs in Russia and Iceland (Bonch-Osmolovskaya et al. 1990; Zillig and Reysenbach 2001; Zillig et al. 1981). The typically sulfur-respiring Desulfurococcales are found only in the Waramung clone library, with >80 % of the clones affiliating most closely with Ignisphaera aggregans, an anaerobic chemoheterotrophic hyperthermophile isolated from terrestrial thermal areas in New Zealand (Niederberger and Gotz 2006). Both Thermoproteales and Desulfurococcales are common community members in many terrestrial hot spring systems, including other alkaline systems (Meyer-Dombard et al. 2005; Spear et al. 2005; Childs et al. 2008; Niederberger and Gotz 2006), and are often represented by novel, uncultured organisms.
Community diversity and evenness
The richness and evenness of microbial communities at Kapkai and Waramung are shown in Figs. 5 and 6 and Supplemental Table 3, with comparison to other reference sites calculated using the Chao1, Shannon, and Pielou diversity indices (Pielou 1977; Smith and Wilson 1996). These reference sites, a collection of data available from PNG, and other shallow-sea, island-based, high-arsenic, and alkaline continental systems, were chosen because they represent some of the few available published accounts with both sufficient geochemical and microbial diversity datasets, as well as their relevance to the on-land Ambitle Island systems investigated here. Of the three on-land PNG samples, Kapkai 3 is the most rich (both with respect to bacterial and archaeal communities) of the sample locations, and has the most even bacterial community of all sites shown in Fig. 5. Despite being nearly 34 °C warmer than the Kapkai 3 site, Waramung has one of the most diverse bacterial communities in Fig. 5, only marginally lower than that of site “PNG 4A05-0 rust,” a rust-colored biofilm from a shallow-sea vent site in Tutum Bay at Ambitle Island (Meyer-Dombard et al. 2012b). In general, all other reference sites are of lower bacterial diversity than the on-land and shallow-sea PNG sites. The on-land Ambitle Island locations show lower archaeal diversity than many of the Tutum Bay shallow-sea locations, but have higher or comparable diversity than the shown island-based and continental reference datasets.
In addition, it can be seen in Figs. 5 and 6 that the microbial communities of the on-land Ambitle Island sites at Kapkai and Waramung bear very little similarity to any of the reference sites shown. While most high temperature and alkaline locations shown (see Supplemental Table 2) are dominated by Aquificales, of the three on-land Ambitle Island locations, only Waramung supports a population of Aquificales. The Kapkai 4 and Waramung communities also host some of the largest populations of Thermotogales and Thermodesulfobacteriales. The on-land Ambitle Island sites are the only locations considered herein to support large proportions of Thermococcales. Hence, the archaeal communities are more similar to each other than to any other reference location.
Our data yield evenness (E) values (in order of decreasing evenness) for bacterial communities of 0.79 for Kapkai 3, 0.56 for Kapkai 4, and 0.55 for Waramung. For comparison, bacterial E values were also computed for the reference hydrothermal systems (Supplemental Table 3). The E values for the Tutum Bay shallow-sea bacterial communities were also high (0.60–0.77), while the evenness of continental and island-based systems ranged widely, but were typically <0.5. By comparison, most archaeal E values, including those at Kapkai 3, Kapkai 4, and Waramung, were low (<0.5), with exceptions at Milos and Romania (both 0.77), and Yellowstone (0.97).
In general, diversity metrics may be employed to describe the stability of a given community. Thus, these data suggest that the Kapkai 3 and Waramung microbial communities enjoy greater functional redundancy, implied by high species richness relative to communities in reference locations. Further, the evenness of the Kapkai 3 bacterial community suggests a reduced need for competition within the community, perhaps pointing to resource abundance in this system. Indeed, the lower temperature of the Kapkai 3 sample allows for both chemo- and phototrophic metabolic functions. While measurements of dissolved organic carbon were not made, it can be assumed that the dense vegetation surrounding the on-land sites, and the shallow marine ecosystem in the bay, both provide an abundance of nutrients to support the hydrothermal ecosystems, lending to community stability and less necessity for resource competition.
Enrichment of thermophiles
Six different growth media—each at pH 6.5 and 8.5—were inoculated with sediment slurries from Kapkai 3, Kapkai 4, and Waramung and incubated at two temperatures (45 and 80 °C). The media were designed to target the following metabolisms: anaerobic heterotrophy, aerobic heterotrophy, heterotrophic sulfate reduction, aerobic arsenite oxidation, anaerobic chemolithoautotrophy, and aerobic chemolithoautotrophy. The base medium and trace element solution were designed from chemical analyses given in Table 1 and data tabulated in Pichler et al. (1999a) and Pichler (2005).
Results can be found in Supplemental Table 4. Definitive growth (defined here as the categories ‘*’ and ‘+’ in Supplementary Table 4) was observed in 20 of 72 enrichment experiments; an additional 21 tubes showed minimal growth (‘◦’ in Supplementary Table 4). No growth (‘–’ in Supplementary Table 4) was observed in the other 31 tubes even following 3–4 months of incubation. The most successful media were those that targeted aerobic heterotrophy (definitive growth in 6/12 tubes) and anaerobic heterotrophy (5/12). These media yielded mixed cultures that supported various Bacillus, Anoxybacillus, and Thermococcus sp. (Supplemental Table 4). While Bacillus-type taxa were not retrieved in the Kapkai or Waramung libraries, Anoxybacillus and Thermococcus taxa were identified in the clone libraries built from the sediments (Figs. 4, 5; and Supplemental Table 1). No growth or minimal growth was observed in the aerobic arsenite oxidation medium—at either temperature or pH—despite the high arsenite concentrations found in the Waramung and Kapkai fluids (Pichler et al. 1999a).
In comparing culturing success among the three different inocula (see Supplementary Table 4), Kapkai 3 and Kapkai 4 each yielded more positive enrichments than Waramung. Kapkai 4 showed definitive growth (*, +) in 9 tubes, minimal growth (◦) in another 12 tubes, and no growth (–) in only 3 tubes. At Kapkai 3, the results were 8 (*, +), 5 (◦), and 11 (–), and at Waramung, they were 3 (*, +), 4 (◦), and 17 (–).
Understanding the chemical composition and setting of Kapkai and Waramung springs helps to interpret these culturing successes and failures. Ambitle Island is a heavily vegetated tropical forest averaging ~200 mm of precipitation a year. Both springs are located within the dense foliage, and therefore there are ample opportunities for input of exogenous organic carbon to enter the spring ecosystems, in the form of plants, soil, insects, or even occasionally mammals and birds. It is thus perhaps not surprising that heterotrophic growth media were the most successful of the metabolic strategies attempted. This interpretation is also supported by the calculations of richness and evenness for the Kapkai 3 and Waramung sites as discussed above. Further, while the sites contain some of the highest concentrations of total arsenic measured in hydrothermal systems, they also have high sulfide concentrations that may inhibit arsenite oxidation in these ecosystems. It is thus far unknown if the microbial communities at Kapkai and Waramung springs have the genetic capacity for arsenite oxidation, arsenate reduction, or arsenic detoxification strategies, as have been previously demonstrated in other hydrothermal systems (Macur et al. 2004; Meyer-Dombard et al. 2013; Price et al. 2013a; Summers Engel et al. 2013; Takai et al. 2003). However, given the high concentrations of sulfide and sulfate, and the potential for both aerobic and anaerobic microniches (as typically found in terrestrial hydrothermal systems), the heterotrophic and sulfur-respiring taxa identified in the Kapkai and Waramung clone libraries are likely driving the primary productivity of these springs.
Concluding remarks
Our global census of terrestrial hot spring geobiology is heavily weighted by studies from a few bonanza sites, especially at Yellowstone National Park (USA). A few recent investigations have drawn attention to lesser-known, but geochemically and microbiologically intriguing locales. The microbial diversity study at Kapkai and Waramung on Ambitle Island, Papua New Guinea adds fundamentally to this global effort. Certainly, the microbial community of a previously unknown environment cannot be definitively, or fully, characterized by culturing successes and/or clonal sequences. However, much can be learned from such data if they are interpreted in the context of detailed geochemical analyses.
The bulk of the archaeal (227/263) and bacterial (197/358) clones in the Kapkai 3, Kapkai 4, and Waramung libraries share >97 % 16S rRNA gene sequence identity with previously identified clones or cultured isolates in well-characterized groups. Many of these groups—Thermotogae, Aquificae, Deinococcus/Thermus, Thermodesulfobacteria, Thermococcales, Thermoproteales, Desulfurococcales—feature exclusively or predominantly (hyper)thermophiles. Further, the cultured representatives closely related to Ambitle Island clones were often isolated from terrestrial hot springs, not unlike those at Kapkai and Waramung. While some of these isolates can respire oxygen, others rely on sulfate, sulfur, or nitrate as terminal electron acceptors; the observed high levels of sulfate, nitrate, and sulfide (as a metabolic waste product) are consistent with these findings. Note also the culturing successes in diverse growth media (aerobic and anaerobic, lithoautotrophic and heterotrophic, circumneutral and alkaline, moderately thermophilic and hyperthermophilic), which are consistent with both our phylogenetic interpretations and geochemical data.
Based on evidence from cultured closest neighbors, our own culturing efforts, and the available geochemistry, we can characterize the ecosystems at Kapkai and Waramung as supporting an array of thermophilic heterotrophs, including those that may use sulfate or nitrate as electron acceptors. Enrichments targeting arsenic-based metabolisms were not successful, however, the high sulfide may suppress these functions in Kapkai and Waramung. Further, the presence of potential archaeal ammonia oxidizers (the Thaumarchaeota) in these and other PNG springs indicates a potentially important nitrogen cycling role in these ecosystems. These observations are supported by on-site observations and geochemistry, including great potential for exogenous carbon and nitrogen input, high sulfate, nitrate, sulfide, and arsenic concentrations, relative to other island-based and shallow-sea hydrothermal systems. The abundance of marine-related and sulfate-utilizing nearest relatives to Kapkai and Waramung clones in both the bacterial and archaeal libraries, including several examples of nearest neighbors from Tutum Bay samples, further suggests that there may be a subterranean hydrologic link between the shallow-sea venting and the on-land springs of Ambitle Island.
The on-land Ambitle Island hydrothermal springs are unique geochemically and microbiologically when compared to other (scantily) available reference datasets. While similar geochemically to the Ambitle Island shallow-sea locations, Kapkai and Waramung springs host microbial communities which bear minimal resemblance to their shallow-sea counterparts. Exceptions include a few shared taxa that are commonly associated with marine hydrothermal ecosystems. The Kapkai and Waramung sites are also diverse relative to available reference data from other island-based, alkaline, or high [As] systems. These hot springs represent a valuable new resource, adding to a currently small global dataset of island-based, high [As] hydrothermal ecosystems.
References
Aiuppa A, D’Alessandro W, Federico C, Palumbo B, Valenza M (2003) The aquatic geochemistry of arsenic in volcanic groundwaters from Southern Italy. Appl Geochem 18:1283–1296
Akerman, NH, 2009. Microbial Diversity and Geochemical Energy Sources of Tutum Bay, Ambitle Island, Papua New Guinea, and Arsenic-Rich, Shallow-Sea Hydrothermal System. Ph.D. Thesis, Washington University in St. Louis, St. Louis, MO
Akerman NH, Price RE, Pichler T, Amend JP (2011) Energy sources for chemolithotrophs in an arsenic- and iron-rich shallow-sea hydrothermal system. Geobiology 9:436–445
Alain K, Callac N, Guégan M, Lesongeur F, Crassous P, Cambon-Bonavita M-A, Querellou J, Prieur D (2009) Nautilia abyssi, sp. nov., a thermophilic, chemolithoautotrophic, sulfur-reducing bacterium isolated from an East Pacific Rise hydrothermal vent. Int J Syst Evol Microbiol 59:1310–1315
Albuquerque L, Santos J, Travassos P, Fernanda Nobre M, Rainey FA, Wait R, Empadinhas N, Silva MT, da Costa MS (2002) Albidovulum inexpectatum gen. nov., sp. nov., a nonphotosynthetic and slightly thermophilic bacterium from a marine hot spring that is very closely related to members of the photosynthetic genus Rhodovulum. Appl Environ Microbiol 68:4266–4273
Altschul S, Madden T, Schaffer A, Zhang J, Zhang Z, Miller W, Lipman D (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Alves MP, Rainey FA, Nobre MF, da Costa MS (2003) Thermomonas hydrothermalis sp. nov., a new slightly thermophilic γ-Proteobacterium isolated from a hot spring in central Portugal. Syst Appl Microbiol 26:70–75
Amend JP, Shock EL (2001) Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol Rev 25:175–243
Amend JP, Rogers KL, Shock EL, Inguaggiato S, Gurrieri S (2003) Energetics of chemolithoautotrophy in the hydrothermal system of Vulcano Island, southern Italy. Geobiology 1:37–58
Arnorsson S (2003) Arsenic in surface- and up to 90 °C ground waters in a basalt area, N-Iceland: processes controlling its mobility. Appl Geochem 18:1297–1312
Baba A, Sözbilir H (2012) Source of arsenic based on geological and hydrogeochemical properties of geothermal systems in Western Turkey. Chem Geol 334:364–377
Bonch-Osmolovskaya EA, Miroshnichenko ML, Kostrikina NA, Chernych NA, Zavarzin GA (1990) Thermoproteus uzoniensis sp. nov., a new extremely thermophilic archaebacterium from Kamchatka continental hot springs. Arch Microbiol 154:556–559
Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P (2008) Mesophilic crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nat Rev Microbiol 6:245–252
Bundschuh J, Maity JP, Nath B, Baba A, Gunduz O, Kulp TR, Jean J-S, Kar S, Yang H-J, Tseng Y-J, Bhattacharya P, Chen C-Y (2013) Naturally occurring arsenic in terrestrial geothermal systems of western Anatolia, Turkey: potential role in contamination of freshwater resources, J Hazard Mater 262:951–959
Busse H-J, Kämpfer P, Moore ERB, Nuutinen J, Tsitko IV, Denner EBM, Vauterin L, Valens M, Rosselló-Mora R, Salkinoja-Salonen MS (2002) Thermomonas haemolytica gen. nov., sp. nov., a γ-proteobacterium from kaolin slurry. Int J Syst Evol Microbiol 52:473–483
Chao A, Chazdon RL, Colwell RK, Shen T-J (2005) A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol Lett 8:148–159
Chen T-L, Chou Y-J, Chen W-M, Arun B, Young C-C (2006) Tepidimonas taiwanensis sp. nov., a novel alkaline-protease-producing bacterium isolated from a hot spring. Extremophiles 10:35–40
Childs AM, Mountain BW, O’Toole R, Stott MB (2008) Relating microbial community and physicochemical parameters ofa hot spring: Champagne Pool, Wai-o-tapu, New Zealand. Geomicrobiol J 25:441–453
Coman C, Drugă B, Hegedus A, Sicora C, Dragoş N (2013) Archaeal and bacterial diversity in two hot spring microbial mats from a geothermal region in Romania. Extremophiles 17:523–534
da Costa MS, Rainey FA, Nobre MF (2006) The genus Thermus and relatives. In: Stackebrandt E, Dworkin M, Falkow S, Rosenberg E, Schleifer K-H (eds) The Prokaryotes. Springer, New York, pp 797–812
DeLong EF (1992) Archaea in coastal marine environments. Proc Natl Acad Sci USA 89:5685–5689
DeSantis TZ, Hugenholtz P, Keller K, Brodie EL, Larsen N, Piceno YM, Phan R, Anderson GL (2006) NAST: a multiple sequence alignment server for comparative analysis of 16S rRNA genes. Nucleic Acids Res 34:W394–W399
Donahoe-Christiansen J, D’Imperio S, Jackson CR, Inskeep WP, McDermott TR (2004) Arsenite-oxidizing Hydrogenobaculum strain isolated form an acid-sulfate-chloride geothermal spring in Yellowstone National Park. Appl Environ Microbiol 70:1865–1868
Dotsika E (2012) Isotope and hydrochemical assessment of the Samothraki Island geothermal area, Greece. J Volcanol Geotherm Res 233–234:18–26
Götz D, Banta A, Beveridge TJ, Rushdi AI, Simoneit BRT, Reysenbach AL (2002) Persephonella marina gen. nov., sp. nov. and Persephonella guaymasensis sp. nov., two novel, thermophilic, hydrogen-oxidizing microaerophiles from deep-sea hydrothermal vents. Int J Syst Evol Microbiol 52:1349–1359
Guo Q, Wang Y (2012) Geochemistry of hot springs in the Tengchong hydrothermal areas, Southwestern China. J Volcanol Geotherm Res 215–216:61–73
Hall TA (1999) BIOEDIT: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Res 41:95–98
Hou W, Wang S, Dong H, Jiang H, Briggs BR, Peacock JP, Huang Q, Huang L, Wu G, Zhi X, Li W, Dodsworth JA, Hedlund BP, Zhang CL, Hartnett HE, Dijkstra P, Hungate B (2013) A comprehensive census of microbial diversity in hot springs of Tengchong, Yunnan Province China using 16S rRNA gene pyrosequencing. PLoS One 8(1):e53350
Huber R, Langworthy TA, König H, Thomm M, Woese CR, Sleytr UB, Stetter KO (1986) Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90 °C. Arch Microbiol 144:324–333
Huber R, Wilharm T, Huber D, Trincone A, Burggraf S, König H, Rachel R, Rockinger I, Fricke H, Stetter KO (1992) Aquifex pyrophilus gen. nov., sp. nov., represents a novel group of marine hyperthermophilic hydrogen-oxidizing bacteria. Syst Appl Microbiol 15:340–351
Huber R, Eder W, Heldwein S, Wanner G, Huber H, Rachel R, Stetter KO (1998) Thermocrinis ruber gen. nov., sp. nov., a pink-filament forming hyperthermophilic Bacterium isolated from Yellowstone National Park. Appl Environ Microbiol 64:3576–3583
Inskeep WP, Macur RE, Harrison G, Bostick BJ, Fendorf S (2004) Biomineralization of As(V)-hydrous ferric oxyhydroxide in microbial mats of an acid-sulfate-chloride geothermal spring, Yellowstone National Park. Geochim Cosmochim Acta 68:3141–3155
Jackson CR, Langner HW, Donahoe-Christiansen J, Inskeep WP, McDermott TR (2001) Molecular analysis of microbial community structure in an arsenite-oxidizing acidic thermal spring. Environ Microbiol 3:532–542
Jannasch HW, Huber R, Belkin S, Stetter KO (1988) Thermotoga neapolitana, sp. nov. of the extremely thermophilic, eubacterial genus Thermotoga. Arch Microbiol 150:103–104
Jeanthon C, L’Haridon S, Cueff V, Banta A, Reysenbach A-L, Prieur D (2002) Thermodesulfobacterium hydrogeniphilum sp. nov., a thermophilic, chemoautotrophic, sulfate-reducing bacterium isolated from a deep-sea hydrothermal vent at Guaymas Basin, and emendation of the genus Thermodesulfobacterium. Int J Syst Evol Microbiol 52:765–772
Joseph EP, Fournier N, Lindsay JM, Robertson R, Beckles DM (2013) Chemical and isotopic characteristics of geothermal fluids from Sulfur Springs, Saint Lucia. J Volcanol Geotherm Res 254:23–36
Kaasalainen H, Stefánsson A (2012) The chemistry of trace elements in surface geothermal waters and steam, Iceland. Chem Geol 330–331:60–85
Kang YS, Yang HL, Lee SD (2009) Nitratireductor kimnyeongensis sp. nov., isolated from seaweed. Int J Syst Evol Microbiol 59:1036–1039
Kato S, Itoh T, Yamagishi A (2011) Archaeal diversity in a terrestrial acidic spring field revealed by a novel PCR primer targeting archaeal 16S rRNA genes. FEMS Microbiol Res Lett 319:34–43
Kim Phuong N, Harijoko A, Itoi R, Unoki Y (2012) Water geochemistry and soil gas survey at Ungaran geothermal field, central Java, Indonesia. J Volcanol Geotherm Res 229–230:23–33
Kim K-H, Woon Roh S, Chang H-W, Nam Y-D, Yoon J-H, Jeon CO, Oh H-M, Bae J-W (2009) Nitratireductor basaltis sp. nov., isolated from black beach sand. Int J Syst Evol Microbiol 59:135–138
Labbé N, Parent S, Villemur R (2004) Nitratireductor aquibiodomus gen. nov., sp. nov., a novel α-proteobacterium from the marine denitrification system of the Montreal Biodome (Canada). Int J Syst Evol Microbiol 54:269–273
Landrum JT, Bennett PC, Summers Engel A, Alsina MA, Pasten PA, Milliken K (2009) Partitioning geochemistry of arsenic and antimony, El Tatio Geyser Field, Chile. Appl Geochem 24:664–676
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, Chichester, pp 115–175
Langner HW, Jackson CR, McDermott TR, Inskeep WP (2001) Rapid oxidation of arsenite in a hot spring ecosystem, Yellowstone National Park. Environ Sci Technol 35:3302–3309
Le Guern C, Baranger P, Crouzet C, Bodenan F, Conil P (2003) Arsenic trapping by iron oxyhydroxides and carbonates at hydrothermal spring outlets. Appl Geochem 18:1313–1323
Licence PS, Terrill JE, Fergusson LJ (1987) Epithermal gold mineralisation, Ambitle Island, Papua New Guinea, Pacific Rim Congress 87. The Australasian Institute of Mining and Metallurgy, Gold Coast, pp 273–278
Loiacono ST, Meyer-Dombard DR, Havig JR, Poret-Pederson A, Hartnett H, Shock EL (2012) Evidence for high-temperature in situ nifH transcription in an alkaline hot spring of Lower Geyser Basin, Yellowstone National Park. Environ Microbiol 14(5):1272–1283
Macur RE, Langner HW, Kocar BD, Inskeep WP (2004) Linking geochemical processes with microbial community analysis: successional dynamics in an arsenic-rich, acid-sulphate geothermal spring. Geobiology 2:163–177
McCleskey RB, Ball JW, Nordstrom DK, Holloway JM, Taylor HE (2004) Water-chemistry data for selected hot springs, geysers, and streams in Yellowstone National Park, Wyoming, 2001–2002, OFR 2004-1316, U.S. Geological Survey
Meyer-Dombard DR, Shock EL, Amend JP (2005) Archaeal and bacterial communities in geochemically diverse hot springs of Yellowstone National Park, USA. Geobiology 3:211–227
Meyer-Dombard DR, Swingley W, Raymond J, Havig J, Shock EL, Summons RE (2011) Hydrothermal ecotones and streamer biofilm communities in the Lower Geyser Basin, Yellowstone National Park. Environ Microbiol 13:2216–2231
Meyer-Dombard DR, Shock EL, Amend JP (2012a) Effects of trace element concentrations on culturing thermophiles. Extremophiles 16:317–331
Meyer-Dombard DR, Price RE, Pichler T, Amend JP (2012b) Prokaryotic populations in arsenic-rich shallow-sea hydrothermal sediments of Ambitle Island, Papua New Guinea. Geomicrobiol J 29:1–17
Meyer-Dombard DR, Amend JP, Osburn MR (2013) Microbial diversity and potential for arsenic and iron biogeochemical cycling at an arsenic rich, shallow-sea hydrothermal vent (Tutum Bay, Papua New Guinea). Chem Geol 348:37–47
Moreira C, Rainey FA, Nobre MF, da Silva MT, da Costa MS (2000) Tepidimonas ignava gen. nov., sp. nov., a new chemolithoheterotrophic and slightly thermophilic member of the β-Proteobacteria. Int J Syst Evol Microbiol 50:735–742
Nakagawa S, Takai K, Horikoshi K (2003) Persephonella hydrogenophila sp. nov., a novel thermophilic, hydrogen-oxidizing bacterium from a deep-sea hydrothermal vent chimney. Int J Syst Evol Microbiol 53:863–869
Niederberger TD, Gotz DK, McDonald IR et al (2006) Ignisphaera aggregans, gen. nov., sp. nov., a novel hyperthermophilic crenarchaeote isolated from hot springs in Rotorua and Tokaanu, New Zealand. Int J Syst Evol Microbiol 56:965–971
Niederberger TD, Ronimus RS, Morgan HW (2008) The microbial ecology of a high-temperature near-neutral spring situated in Rotorua, New Zealand. Microbiol Res 163:594–603
Nordstrom DK, Ball JW, McCleskey RB (2005) Ground water to surface water: chemistry of thermal outflows in Yellowstone National Park. In: Inskeep W, McDermott TR (eds) Geothermal biology and geochemistry in Yellowstone National Park. Montana State University, Thermal Biology Institute, Bozeman, pp 71–94
Ozler HM (2000) Hydrogeology and geochemistry in the Curuksu (Denizli) hydrothermal field, western Turkey. Environ Geol 39:1169–1180
Pichler T (2005) Stable and radiogenic isotopes as tracers for the origin, mixing and subsurface history of fluids in submarine shallow-water hydrothermal systems. J Volcanol Geotherm Res 139:211–226
Pichler T, Dix GR (1996) Hydrothermal venting within a coral reef ecosystem, Ambitle Island, Papua New Guinea. Geology 24:435–438
Pichler T, Veizer J (1999) Precipitation of Fe(III) oxyhydroxide deposits from shallow-water hydrothermal fluids in Tatum Bay, Ambitle Island, Papua New Guinea. Chem Geol 162:15–31
Pichler T, Veizer J, Hall GEM (1999a) The chemical composition of shallow-water hydrothermal fluids in Tutum Bay, Ambitle Island, Papua New Guinea and their effect on ambient seawater. Mar Chem 64:229–252
Pichler T, Veizer J, Hall GEM (1999b) Natural input of arsenic into a coral-reef ecosystem by hydrothermal fluids and its removal by Fe(III) oxyhydroxides. Environ Sci Technol 33:1373–1378
Pichler T, Amend JP, Garey JR, Hallock P, Hsia N, Karlen DJ, McCloskey BJ, Meyer-Dombard DR, Price RE (2006) A natural laboratory to study arsenic geobiocomplexity. EOS 87–23:221–225
Pielou EC (1969) An introduction to mathematical ecology. Wiley, New York
Pielou EC (1977) Mathematical ecology. Wiley, New York
Planer-Friedrich B, Lehr C, Matschullat J, Merkel BJ, Nordstrom K, Sandstrom MW (2006) Speciation of volatile arsenic at geothermal features in Yellowstone National Park. Geochim Cosmochim Acta 70:2480–2491
Pol A, Barends TRM, Dietl A, Khadem AF, Eygensteyn J, Jetten MSM, Op den Camp HJM (2013) Rare earth metals are essential for methanotrophic life in volcanic mudpots. Environ Microbiol. doi:10.1111/1462-2920.12249
Price RE, Pichler T (2005) Distribution, speciation and bioavailability of arsenic in a shallow-water submarine hydrothermal system, Tutum Bay, Ambitle Island, PNG. Chem Geol 224:122–135
Price RE, Amend JP, Pichler T (2007) Enhanced geochemical gradients in a marine shallow-water hydrothermal system: unusual arsenic speciation in vertical and horizontal pore water profiles. Appl Geochem 22:2595–2605
Price RE, Lesniewski R, Nitzsche KS, Meyerdierks A, Saltikov C, Pichler T, Amend JP (2013a) Archaeal and bacterial diversity in an arsenic-rich shallow-sea hydrothermal system undergoing phase separation. Front Microbiol. doi:10.3389/fmicb.2013.00158
Price RE, London J, Wallschlager D, Ruiz-Chancho MJ, Pichler T (2013b) Enhanced bioaccumulation and biotransformation of As in coral reef organisms surrounding a marine shallow-water hydrothermal vent system. Chem Geol 348:48–55
Pürschel M, Gloaguen R, Stadler S (2013) Geothermal activities in the main ethiopian rift: hydrogeochemical characterization of geothermal waters and geothermometry applications (Dofan-Fantale, Gergede-Sodere, Aluto-Langano). Geothermics 47:1–12
Robb FT, Place AR (eds) (1995) Thermophiles Archaea—a laboratory manual. Cold Spring Harbor Laboratory Press, Plainview, p 217
Rogers KL, Amend JP (2005) Archaeal diversity and geochemical energy yields in a geothermal well on Vulcano Island, Italy. Geobiology 3:319–332
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana
Shock EL, Holland M, Meyer-Dombard DR, Amend JP (2005) Geochemical sources of energy for microbial metabolism in hydrothermal ecosystems: obsidian pool, Yellowstone National Park. In: Inskeep WP, McDermott TR (eds) Geothermal biology and geochemistry of Yellowstone National Park. Thermal Biology Institute, Bozeman, pp 95–112
Shock EL, Holland ME, Meyer-Dombard DR, Amend JP, Osburn GR, Fisher T (2010) Quantifying inorganic sources of geochemical energy in hydrothermal ecosystems, Yellowstone National Park, USA. Geochim Cosmochim Acta 74:4005–4043
Smith B, Wilson JB (1996) A consumer’s guide to evenness indices. Oikos 76:70–82
Spear JR, Walker JJ, McCollom TM, Pace NR (2005) Hydrogen and bioenergetics in the Yellowstone geothermal ecosystem. Proc Natl Acad Sci 102:2555–2560
Stauffer RE, Thompson JM (1984) Arsenic and antimony in geothermal waters of Yellowstone National Park, Wyoming, USA. Geochim Cosmochim Acta 48:2547–2561
Stout L, Blake R, Greenwood J, Martini A, Rose E (2009) Microbial diversity of boron-rich volcanic hot springs of St. Lucia Lesser Antilles. FEMS Microbiol Ecol 70:402–412
Summers Engel A, Johnson LR, Porter ML (2013) Arsenite oxidase gene diversity among Chloroflexi and Proteobacteria from El Tatio Geyser Field, Chile. FEMS Microbiol Ecol 83:745–756
Swingley WD, Meyer-Dombard DR, Alsop EB, Falenski HD, Havig JR, Shock EL, Raymond J (2012) Coordinated environmental genomics and geochemistry reveals metabolic transitions in a hot spring ecosystem. PLoS One 7(6):e38108
Takai K, Kobayahi H, Nealson KH, Horikoshi K (2003) Deferribacter desulfuricans sp. nov., a novel sulfur-, nitrate- and arsenate-reducing thermophile isolated from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol 53:839–846
Tarcan G, Gemici U (2003) Water geochemistry of the Seferihisar geothermal area, Izmir, Turkey. J Volcanol Geotherm Res 126:225–242
Wagner ID, Wiegel J (2008) Diversity of thermophilic anaerobes. Ann N Y Acad Sci 1125:1–43
Wallace DA, Johnson RW, Chappell BW, Arculus RJ, Perfit MR, Crick IH (1983) Cainozoic volcanism of the Tabar, Lihir, Tanga, and Feni Islands, Papua New Guinea: Geology, whole-rock analyses, and rock-forming mineral compositions. Bur Miner Resour Aust Geol Geophys Rep 243:62
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73(16):5261–5267
Winker S, Woese CR (1991) A definition of the domains Archaea, Bacteria and Eucarya in terms of small subunit ribosomal RNA characteristics. Syst Appl Microbiol 14:305–310
Zeikus JG, Dawson MA, Thompson TE, Ingvorsen K, Hatchikian EC (1983) Microbial ecology of volcanic sulfidogenesis: isolation and characterization of Thermodesulfobacterium commune gen. nov. and sp. nov. J Gen Microbiol 129:1159–1169
Zillig W, Reysenbach A-L (2001) Genus I. Thermoproteus. In: Boone DR, Castenholz RW, Garrity GM (eds) Bergey’s manual of systematic bacteriology. Spring, New York, pp 171–173
Zillig W, Sterrer KO, Schäfer W, Janecovic D, Wunderl S, Holz I, Palm P (1981) Thermoproteales: a novel type of extremely thermoacidophilic anaerobic archaebacteria isolated from Icelandic solfataras. Zentralblatt für Bakteriologie Mikrobiol Hyg C2:205–227
Acknowledgments
Thanks are due Thomas Pichler and Roy Price, instrumental in the field work in Papua New Guinea. Lauren Burcea generated the phylogenetic trees and maintained the enrichment experiments. This work was supported by NSF grants BC/CBC 0221834 and EAR 0447231. A special thanks goes to Chief Philippe of Ambitle Island (Fig. 1b), without whose skilled machete work the sampling sites would have remained inaccessible to us.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Oren.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Meyer-Dombard, D.R., Amend, J.P. Geochemistry and microbial ecology in alkaline hot springs of Ambitle Island, Papua New Guinea. Extremophiles 18, 763–778 (2014). https://doi.org/10.1007/s00792-014-0657-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-014-0657-6