Abstract
Shewanella species live widely in deep-sea and shallow-water areas, and thus grow piezophilically and piezosensitively. Piezophilic and psychrophilic Shewanella benthica cytochrome c 5 (SB cytc 5) was the most stable against guanidine hydrochloride (GdnHCl) and thermal denaturation, followed by less piezophilic but still psychrophilic Shewanella violacea cytochrome c 5 (SV cytc 5). These two were followed, as to stability level, by piezosensitive and mesophilic Shewanella amazonensis cytochrome c 5 (SA cytc 5), and piezosensitive and psychrophilic Shewanella livingstonensis cytochrome c 5 (SL cytc 5). The midpoint GdnHCl concentrations of SB cytc 5, SV cytc 5, SL cytc 5, and SA cytc 5 correlated with the optimal growth pressures of the species, the correlation coefficient value being 0.93. A similar trend was observed for thermal denaturation. Therefore, the stability of each cytochrome c 5 is related directly to its host’s optimal growth pressure. Phylogenetic analysis indicated that Lys-37, Ala-41, and Leu-50 conserved in piezosensitive SL cytc 5 and SA cytc 5 are ancestors of the corresponding residues in piezophilic SB cytc 5 and SV cytc 5, Gln, Thr, and Lys, respectively, which might have been introduced during evolution on adaption to environmental pressure. The monomeric Shewanella cytochromes c 5 are suitable tools for examining protein stability with regard to the optimal growth pressures of the source species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Shewanella species, gamma-proteobacteria, are distributed in nature from deep-sea to shallow-water areas (Hau and Gralnick 2007). A phylogenetic tree based on the Shewanella 16S rRNA gene sequence indicates that the species can be classified into two groups, Group 1 and Group 2 (Kato and Nogi 2001). Group 1 Shewanella species are mostly isolated from deep-sea, and Group 2 ones from shallow-water areas. For example, Group 1 Shewanella violacea DSS12 and Shewanella benthica DB6705 were obtained from the Ryukyu trench at a depth of 5,110 m (Nogi et al. 1998) and from the Japan Trench at a depth of 6,356 m (Kato et al. 1995), respectively. They show piezophilicity, growing optimally at 30 and 50 MPa, respectively (Table 1). On the other hand, Group 2 Shewanella livingstonensis Ac10 (Kulakova et al. 1999) and Shewanella amazonensis SB2B (Venkateswaran et al. 1998) were isolated at a depth of 0 m from the Antarctic Sea surface and Amazon River Delta, respectively. Although S. livingstonensis and S. amazonensis have not been examined as to growth pressure, it is likely that they optimally grow at 0.1 MPa (Table 1), because all the Group 2 Shewanella species examined were found to be piezosensitive (Kato and Nogi 2001).
Proteins from piezophilic Shewanella species remain active at high pressure, compared with the homologous counterparts from piezosensitive ones (Kato et al. 2008). For example, 3-isopropylmalate dehydrogenase from piezophilic S. benthica is more stable and active under high pressure conditions than the homologous enzyme from piezosensitive Shewanella oneidensis (De Poorter et al. 2004; Kasahara et al. 2009), indicating a stabilization mechanism against pressure in the former. Another comparative study has been performed on single-stranded DNA-binding proteins from piezophilic and piezosensitive Shewanella species (Chilukuri and Bartlett 1997; Chilukuri et al. 2002). However, these proteins are multimeric; thus their protein stability might involve both subunit assembly/dissociation and polypeptide folding/unfolding. In this context, monomeric proteins must provide substantial clues as to protein stability only in terms of folding/unfolding, which is important for understanding of the intrinsic properties of a polypeptide.
Cytochromes c are well-characterized monomeric proteins. It is known that pressure affects the expression levels (Yamada et al. 2000) and electron transfer activities (Ohke et al. 2013) of cytochromes c. Nevertheless, little study has been carried out on the stability-pressure relationship in terms of biophysics and structural biology. For this purpose, piezophilic Shewanella cytochromes c 5 appear to be suitable monomeric proteins. We have found that piezophilic S. violacea cytochrome c 5 (SV cytc 5) exhibits higher thermal stability than homologous cytochromes c 5 from piezosensitive S. livingstonensis (SL cytc 5) and S. amazonensis (SA cytc 5) (Ogawa et al. 2007; Takenaka et al. 2010; Masanari et al. 2011). These results indicate that the pressure adaptation of these Shewanella species reflects the differences in the thermal stability of their cytochromes c 5. However, the data obtained so far are too sparse to correlate Shewanella optimal growth pressure with cytochrome c 5 stability, because only two growth pressures have been examined (S. violacea, 30 MPa; S. livingstonensis and S. amazonensis, 0.1 MPa).
In this study, we introduced another piezophile, S. benthica, which grows optimally at 50 MPa, to our comparative study (Table 1). S. benthica cytochrome c 5 (SB cytc 5) exhibits 93, 80, and 84 % amino acid sequence identity with SV cytc 5, SL cytc 5, and SA cytc 5, respectively. Here we measured and compared the denaturant and thermal stabilities of the four Shewanella cytochromes c 5 in order to find any correlation between growth pressure and protein stability.
Materials and methods
Cloning and expression of the SB cytc 5 gene
S. benthica DB6705 cells were grown in a liquid medium comprising marine broth 2216 (Difco) at 10 °C and 50 MPa (Kato et al. 1995). Chromosomal DNA was extracted from the cells by the standard method, and used as a template to clone the SB cytc 5 gene through a PCR experiment. PCR primers were designed based on the genome sequence of S. benthica KT99 (Forward primer; 5′-GCGAATTCATGAAAAAACTGTTAGCTATG-3′, reverse primer; 5′-GCGTCGACTTACTTAGACATGAACTCG-3′) to amplify the gene encoding mature SB cytc 5 from the chromosomal DNA of S. benthica DB6705.
The amplified gene for the mature SB cytc 5 region was fused with the gene for the signal sequence of Pseudomonas aeruginosa cytc 551 (PA cytc 551) in the 5′ region, and then the fusion gene was inserted into the pKK223-3 vector (ampicillin resistance) under control of the tac promoter, as described previously (Ogawa et al. 2007). The resulting plasmid was designated as pKK-SB2. This was then used for expression of the SB cytc 5 gene in Escherichia coli JCB387. Plasmid pEC86 (chloramphenicol resistance), carrying the ccmABCDEFGH genes for cytochrome c maturation proteins, was co-transformed into E. coli JCB387 together with pKK-SB2.
Protein preparation
E. coli JCB387 cells carrying the pKK-SB2 and pEC86 plasmids were grown in 2 l of liquid Luria–Bertani medium containing 100 μg ml−1 ampicillin and 34 μg ml−1 chloramphenicol in 5 l flasks, which were then shaken aerobically at 37 °C for 20 h. A periplasmic extract of the resulting E. coli cells was obtained by the cold osmotic shock method, as described previously (Sambongi et al. 1996).
The SB cytc 5 protein was first purified by DEAE Toyopearl (3.5 × 10 cm; Tosoh, Tokyo) column chromatography, followed by Hi-Trap Q and SP column (1.6 × 2.5 cm; GE Healthcare, Tokyo), as described previously (Ogawa et al. 2007). Finally, gel-filtration chromatography was performed on a Superdex 75 column (1.6 × 60 cm; GE Healthcare), with equilibration and elution with 10 mM Tris–HCl buffer, pH 8.0, at 4 °C. The purified SB cytc 5 protein solution was subjected directly to N-terminal sequencing with an automatic peptide sequencer (Applied Biosystems, Tokyo). The protein concentrations were determined by the Bradford method using bovine serum albumin as a standard (Bradford 1976).
The SV cytc 5, SL cytc 5, and SA cytc 5 proteins were prepared by the methods described previously (Ogawa et al. 2007; Takenaka et al. 2010; Masanari et al. 2011).
Cyclic voltammetry
The cyclic voltammetry (CV) method was applied to the purified SB cytc 5, SV cytc 5, SL cytc 5, and SA cytc 5 proteins in order to determine their midpoint redox potential (E m) values relative to the standard hydrogen electrode (SHE) in 10 mM HEPES–NaOH (pH 7.0) at 25 °C. The experimental procedure and data analysis were described in detail previously (Takeda et al. 2009).
Denaturant stability
The stability of air-oxidized SB cytc 5, SV cytc 5, SL cytc 5, and SA cytc 5 against a chemical denaturant was assessed by means of guanidine hydrochloride (GdnHCl)-induced denaturation experiments. Absorption spectra (300 to 500 nm) of the protein samples were obtained using a 1-cm path length cuvette in a V-530 spectrophotometer (Jasco, Tokyo). The proteins (40 μM) were incubated in 20 mM sodium acetate buffer (pH 4.0) containing varying concentrations of GdnHCl at 25 °C for 2 h before the measurements in order to equilibrate the proteins with the denaturant. The absorbance at 396 nm of each protein solution was measured at 25 °C. The \(\varDelta G_{{{\text{H}}_{ 2} {\text{O}}}}\) (free energy change in water during GdnHCl denaturation) value and m (dependence of ΔG on the GdnHCl concentration) value were determined by least-square fitting of the data in the transition region using the equation \(\varDelta G = \varDelta G_{{{\text{H}}_{ 2} {\text{O}}}} - m\left[ {\text{GdnHCl}} \right]\) (Pace 1990). The C m (midpoint GdnHCl concentration of denaturation) value was the GdnHCl concentration at which the ΔG value became zero.
Thermal stability
Thermal denaturation experiments were performed by differential scanning calorimetry (DSC). A solution of an air-oxidized SB cytc 5 protein was dialyzed extensively against 50 mM sodium acetate buffer, pH 4.0, before the measurements. Under this pH conditions, thermal denaturation of the SB cytc 5 occurred in a reversible manner, providing equilibrium thermodynamic parameters. Hence, this pH was used to measure thermal stability. A degassed protein solution (0.5 mg ml−1) was loaded into a calorimeter cell, followed by heating from 10 to 110 °C at about 28 psi, at a heating rate of 1 °C min−1, with a calorimeter VP-DSC (Microcal, Piscataway). Buffer–buffer baselines were recorded at the same heating rate and then subtracted from the sample curves to obtain heat capacity (C p) curves. After fitting the data with MATHEMATICA 7.0, the transition temperature during thermal denaturation (T m) and the calorimetric enthalpy change (ΔH) at T m were obtained.
The heat capacity change accompanying the thermal denaturation, ΔC p, was estimated as a function of temperature, as described previously (Uchiyama et al. 2002). From these values, thermodynamic parameters (free energy change ΔG, enthalpy change ΔH, and entropy change ΔS) at a given temperature were calculated using the following equations:
These calculations facilitated comparison of thermodynamic protein stabilities, as described previously (Uchiyama et al. 2002; Ogawa et al. 2007). The resulting thermodynamic parameters for SB cytc 5 were compared with those for SV cytc 5, SL cytc 5, and SA cytc 5, which had been obtained under the same experimental conditions previously (Ogawa et al. 2007; Takenaka et al. 2010; Masanari et al. 2011).
Phylogenetic analysis of cytochromes c 5
The amino acid sequences of 16 mature cytochromes c 5 from Shewanella species and Pseudomonas aeruginosa, retrieved from the GenBank, were aligned by means of ClustalW using its default settings before tree building. The alignment was adjusted manually to correct for gaps. A total of 76 residue positions were selected to build a phylogenetic tree using the neighbor-joining method. In order to predict the amino acid sequence at each branch (node), ancestral sequences were inferred from the alignment and the molecular phylogenetic tree of cytochromes c 5 using the CODEML program of the PAML package (Ver. 4.7a) (Yang 1997) for the maximum likelihood method. The WAG substitution model was used as the substitution matrix for amino acid residues.
Results and discussion
Expression of SB cytc 5
In order to clone the SB cytc 5 gene from S. benthica DB6705, the PCR primers used in this study were based on the genome sequence of S. benthica KT99 that had been deposited in the GenBank. During the gene cloning, we realized that two amino acid residues differed in the mature SB cytc 5 sequences from the two strains; Thr-49 and Phe-56 in the KT99 strain were, respectively, replaced with Ala and Leu in the DB6705 strain. Hereafter, we adopted the DB6705 sequence, because the following experiments were carried out using the SB cytc 5 from this strain.
The recombinant SB cytc 5 expressed in E. coli JCB387 cells was recovered in the periplasmic extract after cold osmotic shock of the cells, but not in the membrane or cytoplasmic fractions. The N-terminal amino acid sequence of the recombinant SB cytc 5 expressed in the E. coli periplasm was determined to be Gln-Glu-Gly-Lys-Ala, which is identical to the putative sequence for the mature protein. This indicates that the signal peptide of PA cytc 551 artificially introduced to the present fusion protein was correctly processed in the E. coli cells.
Redox potential analysis
Electrochemical properties, i.e., the redox potential (E m) values of SB cytc 5, SV cytc 5, SL cytc 5, and SA cytc 5, were examined by CV. The E m values of SB cytc 5, SV cytc 5, SL cytc 5, and SA cytc 5 were 283.7 ± 3.7, 284.3 ± 3.6, 281.0 ± 3.6, and 283.3 ± 4.2 mV, respectively. These values did not differ significantly, indicating that these cytochromes c 5 have similar functions in the host cells. These four Shewanella cytochromes c 5 were then examined in order to find any difference in stability.
Denaturant stability
The denaturant stabilities of the four Shewanella cytochromes c 5 were compared. Absorption spectra (350–450 nm) of the air-oxidized SB cytc 5, SV cytc 5, SL cytc 5, and SA cytc 5 proteins in the presence of various GdnHCl concentrations at pH 4.0 and 25 °C were obtained (Fig. 1a–d). The Soret absorption peaks, which are specifically derived from the heme group, blue-shifted with an increase in the GdnHCl concentration. Cytochrome c usually has a hexacoordinate heme and thus shows a Soret absorption peak at 410 nm. The coordination state changes to a pentacoordinate one with GdnHCl denaturation, and the Soret absorption peak shifts to around 400 nm, as observed for the natively pentacoordinated cytochrome c´ (Inoue et al. 2011). This also occurred for the four Shewanella cytochrome c 5 proteins during GdnHCl denaturation, and the existence of isosbestic points (arrows in Fig. 1a–d) indicated that the observed GdnHCl denaturation can be described as a simple two-state process.
Representative GdnHCl-induced changes in absorption spectra of the four Shewanella cytochrome c 5 proteins. Changes in the absorption spectra of SB cytc 5 (a), SV cytc 5 (b), SL cytc 5 (c), and SA cytc 5 (d) are shown in the presence of various GdnHCl concentrations. The line width changes from thickest (0 M GdnHCl) to thinnest (higher concentration of GdnHCl). Arrows indicate the isosbestic points. Difference spectra of SB cytc 5 (e), SV cytc 5 (f), SL cytc 5 (g), and SA cytc 5 (h) are shown after subtraction of the spectra in the absence of GdnHCl from those in the presence of various GdnHCl concentrations
In order to further visualize the effect of GdnHCl, difference spectra were obtained by subtraction of the spectra in the absence of GdnHCl from those in the presence of various concentrations of GdnHCl. There were positive peaks at 396 nm and negative ones at 413 nm (Fig. 1e–h). The GdnHCl-induced denaturation was sharply reflected in the GdnHCl concentration-dependence at the 396-nm peak, which was used to monitor protein denaturation in the following analysis.
The difference spectra data obtained at 396 nm of the SB cytc 5, SV cytc 5, SL cytc 5, and SA cytc 5 proteins as a function of GdnHCl concentration (Fig. 1e–h) were normalized (Fig. 2a). The data adequately fitted to a two-state model for protein denaturation. For further thermodynamic analysis, ΔG values were plotted as a function of GdnHCl concentration (Fig. 2b). The m values, i.e., the slopes in Fig. 2b representing the dependence of ΔG on the GdnHCl concentration, for SB cytc 5, SV cytc 5, SL cytc 5, and SA cytc 5 were 9.23 ± 0.97, 9.10 ± 0.99, 9.76 ± 1.12, and 9.58 ± 0.86 kJ mol−1 M−1, respectively. Every two sample t test revealed that the m values of the four proteins were the same, indicating that the GdnHCl denaturation of these proteins occurred in a similar cooperative manner. Therefore, these proteins can be adequately compared with each other.
Analysis of GdnHCl-induced denaturation. Representative normalized denaturation curves (a). The fractions of the denatured proteins are plotted as a function of GdnHCl concentration for SB cytc 5 (red closed circles), SV cytc 5 (blue open circles), SL cytc 5 (black closed triangles), and SA cytc 5 (green open triangles) with a GdnHCl concentration interval of at least 0.2 M. Fitting curves obtained from the data with a concentration interval of at least 0.1 M for each experiment are also shown. Representative dependence of ΔG values on the GdnHCl concentration (b). Least-square fits of the data for the transition regions are shown. Correlations between optimal growth pressures of the Shewanella species and the denaturant stabilities of their cytochromes c 5 (c for C m value and panel d for \(\varDelta G_{{{\text{H}}_{ 2} {\text{O}}}}\) value). Data symbols used in b, c, and d are same as those in a. The data values are the averages for at least three independent measurements with standard deviations. Least-square fitting curves are also shown with the equations and correlation coefficient values (R 2)
The C m values, representing the GdnHCl concentration at which the fraction of the denatured protein is 0.5 in Fig. 2a, for SB cytc 5, SV cytc 5, SL cytc 5, and SA cytc 5 were 1.99 ± 0.08, 1.84 ± 0.06, 1.38 ± 0.06, and 1.54 ± 0.10 M, respectively. These values were significantly different; even the closest two, those for SB cytc 5 and SV cytc 5, exhibited P < 0.05 with the t test. In addition, these C m values correlated strongly with the optimal growth pressures of the source species, the correlation coefficient value being 0.93 (Fig. 2c). Previously, dihydrofolate reductases from various piezophilic and piezosensitive Shewanella species were examined as monomeric proteins in a similar manner to as in the present study (Murakami et al. 2011). However, their protein stabilities against chemical denaturation did not correlate with the optimal growth pressures of the sources. Therefore, the present results were the first observation of correlation between the protein stability against chemical denaturation and the optimal growth pressures of source organisms. Although both cytochrome c 5 and dihydrofolate reductase are monomeric proteins, their stability mechanisms differ, which might be due to differences in domain number, co-factor, and/or substrate. There is presently no explanation as to why the stability mechanisms are apparently different.
The \(\varDelta G_{{{\text{H}}_{ 2} {\text{O}}}}\) values, representing the conformational stability of a protein in water, obtained in the GdnHCl-induced denaturation experiments for SB cytc 5, SV cytc 5, SL cytc 5, and SA cytc 5 were 19.77 ± 2.62, 17.26 ± 2.09, 14.11 ± 1.05, and 16.05 ± 1.87 kJ mol−1, respectively. Although most similar two, those for SV cytc 5 and SA cytc 5 exhibited P > 0.18 with the t test indicating that they are the same, every other pair exhibited the significant difference, i.e., at most P < 0.05. Therefore, the resulting \(\varDelta G_{{{\text{H}}_{ 2} {\text{O}}}}\) values correlated with the optimal growth pressures of the source species, the correlation coefficient value being 0.87 (Fig. 2d). A similar trend as for the C m values was thus observed.
Thermal stability
The thermal stability of SB cytc 5 was measured and compared with those of the three other Shewanella cytochromes c 5 that had been published previously (Ogawa et al. 2007; Takenaka et al. 2010; Masanari et al. 2011). An excess molar heat capacity curve for the air-oxidized SB cytc 5 protein was obtained through DSC measurements at pH 4.0 (Fig. 3). At this pH value, equilibrium thermodynamic parameters were obtained because thermal denaturation reversibly occurred, similar to for the other three cytochromes c 5. Under the conditions used, the value for the transition temperature during thermal denaturation (T m) of SB cytc 5 was 85.3 °C. This was higher than those of SV cytc 5, SL cytc 5, and SA cytc 5 (Table 2), indicating the highest stability of the SB cytc 5 from the most piezophilic Shewanella strain among the four examined here.
From the curve for the observed excess molar heat capacity (C p; Fig. 3), we obtained temperature-dependent heat capacity changes (ΔC p) accompanying the thermal denaturation. Using the ΔC p value at the T m of the SL cytc 5 protein (72.6 °C, \(T_{\text{m}}^{*}\) at pH 4.0), \(\varDelta C_{\text{p}} \left( {T_{\text{m}}^{*} } \right)\), other thermodynamic parameters at 72.6 °C, \(\varDelta G\left( {T_{\text{m}}^{*} } \right)\), \(\varDelta H\left( {T_{\text{m}}^{*} } \right)\), and \(\varDelta S\left( {T_{\text{m}}^{*} } \right)\), could be compared among the four cytochrome c 5 proteins (Table 2). Since the T m value is defined as being equivalent to the temperature at which ΔG becomes zero, the \(\varDelta G\left( {T_{\text{m}}^{*} } \right)\) value of the SL cytc 5 protein was zero. At 72.6 °C, the \(\varDelta G\left( {T_{\text{m}}^{*} } \right)\) values of SB cytc 5, SV cytc 5, and SA cytc 5 were 12.6, 10.6, and 6.4 kJ mol−1, respectively (Table 2). These positive \(\varDelta G\left( {T_{\text{m}}^{*} } \right)\) values compared with that of SL cytc 5 indicate that the thermal stabilities of SB cytc 5, SV cytc 5, and SA cytc 5 are higher than that of SL cytc 5. Similar to the C m values for GdnHCl-induced denaturation, the T m and \(\varDelta G\left( {T_{\text{m}}^{*} } \right)\) values of the four Shewanella cytochromes c 5 differed significantly, i.e., at most P < 0.01. In addition the T m and \(\varDelta G\left( {T_{\text{m}}^{*} } \right)\) values correlated with the optimal growth pressures of the source species with correlation coefficient values of 0.69 and 0.76, respectively (Fig. 4a–b).
Relationship between optimal growth conditions of Shewanella species and the thermal stability of their cytochromes c 5. Correlation between optimal growth pressure and thermal stability (a for T m, b for \(\varDelta G\left( {T_{\text{m}}^{*} } \right)\), and c for \(\varDelta H\left( {T_{\text{m}}^{*} } \right)\) values). Data symbols used in this figure are same as those in a of Fig. 2. The data values are the averages for at least three independent measurements with standard deviations. Least-square fitting curves are also shown with the equations and correlation coefficient values (R 2). Relationship between optimal growth temperature and thermal stability (a for T m, b for \(\varDelta G\left( {T_{\text{m}}^{*} } \right)\), and c for \(\varDelta H\left( {T_{\text{m}}^{*} } \right)\) values). Data symbols used in this figure are same as those in a of Fig. 2
The \(\varDelta G\left( {T_{\text{m}}^{*} } \right)\) value can be further dissected into enthalpy (ΔH) and entropy (ΔS) terms, as shown by the equation, \(\varDelta G = \varDelta H - T\varDelta S\). The \(\varDelta H\left( {T_{\text{m}}^{*} } \right)\) values of SB cytc 5, SV cytc 5, and SA cytc 5 were, respectively, 353.2, 314.0, and 310.7 kJ mol−1, which were significantly higher than that of SL cytc 5, 272.7 kJ mol−1 (Table 2). At the same time, the \(\varDelta S\left( {T_{\text{m}}^{*} } \right)\) values of SB cytc 5, SV cytc 5, SL cytc 5, and SA cytc 5 were 0.99, 0.88, 0.79, and 0.88 kJ mol−1 K−1, respectively. These values show that the higher stabilities of SB cytc 5, SV cytc 5, and SA cytc 5 than that of SL cytc 5 can be attributed to \(\varDelta H\left( {T_{\text{m}}^{*} } \right)\) values, indicating an enthalpic stabilization mechanism. Similar to the T m and \(\varDelta G\left( {T_{\text{m}}^{*} } \right)\) values, the significantly different \(\varDelta H\left( {T_{\text{m}}^{*} } \right)\) values (P < 0.03) also correlated with the growth pressures, the correlation coefficient value being 0.73 (Fig. 4c).
Positive correlations between the optimal growth temperatures of the source organisms and the thermal stabilities of their proteins have already been observed (McFall-Ngai and Horwitz 1990; Gromiha et al. 1999; Akanuma et al. 2013). Moreover, cytochromes c from more thermophilic bacteria tend to be more stable than those from mesophiles (Sambongi et al. 2002; Nakamura et al. 2006). We then plotted the present thermal stability parameters, the T m, \(\varDelta G\left( {T_{\text{m}}^{*} } \right)\), and \(\varDelta H\left( {T_{\text{m}}^{*} } \right)\) values, of the four Shewanella cytochromes c 5 as a function of the optimal growth temperatures of the source species (Fig. 4d–f). Overall, the thermal stability was not dependent on the growth temperature, unlike in the case of the stability-pressure relationship. This was due that the thermal stability of SA cytc 5 from mesophilic and piezosentive S. amazonensis was lower than those of SB cytc 5 and SV cytc 5 from psychrophilic and piezophilic S. benthica and S. violacea, respectively. Although we do not know the pressure effect on the protein stability, SB cytc 5 and SV cytc 5 might be stabilized against high pressure, resulting in a thermal stability increase that exceeds that of mesophilic SA cytc 5.
Taken together, our results emphasize that the thermal stabilities of Shewanella cytochromes c 5 correlate with the optimal growth pressures of the source species. A similar conclusion was drawn in the previous study on monomeric G actins from deep-sea fishes, which are heat stable compared with those from mesophilic mammals (Swezey and Somero 1982; Somero 1992). However, these studies including the present one are the few examples, partly because most relevant studies have focused on subunit assembly/dissociation with multimeric proteins, not on polypeptide folding/unfolding with monomeric proteins.
Phylogenetic analysis of cytochromes c 5
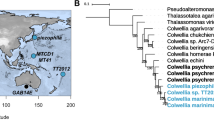
In order to determine the lineage of the four Shewanella cytochrome c 5 proteins examined here, phylogenetic analysis was carried out. The sequences of 16 cytochromes c 5 from Shewanella species, including those of the present four proteins, and mesophilic Pseudomonas aeruginosa were inspected through phylogenetic analysis. Neighbor-joining tree topology revealed that the Shewanella cytochrome c 5 sequences could be classified into the two groups, piezophilic Group 1 and piezosensitive Group 2 (Fig. 5a), consistent with a tree based on 16S rRNA gene sequences (Kato and Nogi 2001) and with a sequence comparison of dihydrofolate reductases (Murakami et al. 2011). These findings indicate that cytochromes c 5 together with other molecules separated according to the different pressure environments of the source species.
Sequence analysis of cytochromes c 5. Phylogenetic tree (a). Group 1 and Group 2 are indicated on the tree. Accession numbers are shown in parentheses. The numbers on the nodes represent bootstrap values with 1,000 replicates. The bar indicates 0.01 substitutions per amino acid position. Comparison of amino acid sequences of cytochromes c 5 (b). Identical amino acid residues with those in the SB cytc 5 sequence are indicated by dots. Identity to the SB cytc 5 sequence is also indicated. The amino acid numbers are shown above the SB cytc 5 sequence. Relevant amino acid residues mentioned in the text are boxed
An ancestral sequence is defined as the set of residues in which each residue has the greatest likelihood of existing at its associated position. The ancestral amino acid sequence at each node of the phylogenetic tree of Shewanella species was inferred using a statistical model of evolution. Each inferred ancestral sequence has several conserved residues in the two groups mentioned above. For example, Lys-37, Ala-41, and Leu-50 conserved in piezosensitive Group 2 Shewanella cytochromes c 5 including SL cytc 5 and SA cytc 5 were found to be ancestral to Gln, Thr, and Lys, respectively, which were conserved in SB cytc 5 and SV cytc 5 among the piezophilic Group 1 proteins (Fig. 5b). It is plausible that these three residues found in SB cytc 5 and SV cytc 5 were introduced during the evolutional time course of adaption to environmental pressure. Consistently, biogeographics and phylogenetics suggest that piezophilic Shewanella species might be derived from shallow-water piezosensitive ones (Lauro et al. 2007; Fang et al. 2010), although there is room for further research to verify this hypothesis.
Although the four cytochromes c 5 from Shewanella piezotolerans, loihica, pealeana, and halifaxensis are classified into piezophilic Group 1 (Fig. 5a), they have ancestral Lys-37, Ala-41, and Leu-50 (Fig. 5b, in which the cytochromes c 5 from S. piezotolerans and S. halifaxensis are shown, being abbreviated as SP cytc 5 and SH cytc 5, respectively). These four species were isolated from relatively “shallow” deep-sea areas compared with S. benthica and S. violacea. For example, S. piezotolerans was isolated at a depth of 1,914 m, consistently growing optimally at 20 MPa (Xiao et al. 2007), which is lower than the optimal growth pressures of S. benthica (50 MPa) and S. violacea (30 MPa). Stability experiments on SP cytc 5 would further strengthen the present conclusion of correlation between growth pressure and protein stability, but with only the present results it is possible to speculate that an optimal growth pressure of more than 30 MPa is necessary for the adoption of Gln-37, Thr-41, and Lys-50, as found in SB cytc 5 and SV cytc 5.
Molecular simulation revealed that the Lys-50 residue in SB cytc 5 and SV cytc 5 forms a hydrogen bond with the heme, while the corresponding Leu-50 in SL cytc 5 and SA cytc 5 hydrophobically interacts with amino acid residues near the heme propionate (Masanari et al. 2011). The hydrogen-bonding Lys-50 residue in cytochrome c 5 is more likely to contribute to the stability than the ancestral Leu-50 residue responsible for hydrophobic interactions. If this is the case, S. benthica and S. violacea adapted to deep-sea high pressure environments must have stabilized cytochromes c 5 with Lys at the 50th position.
Conclusion and perspectives
The denaturant and thermal stabilities of the monomeric cytochrome c 5 from most piezophilic S. benthica were the highest, followed by those of the other three proteins. The stabilities of the four Shewanella cytochromes c 5 correlate with the optimal growth pressures of the source species. In this study, we provide an insight into homologous proteins from piezophilic and piezosensitive organisms; positive correlation between growth pressure and protein stability.
Monomeric cytochromes c 5 from Shewanella are suitable tools for examining protein stability with regard to the optimal growth pressures of the source species. Not only between piezophilic proteins (SB cytc 5 and SV cytc 5) and piezosensitive ones (SL cytc 5 and SA cytc 5), as discussed here, but also comparison between piezophilic SB cytc 5 and SV cytc 5 will be of interest, because they exhibit an only five-residue difference, with a significant stability difference, as observed in this study.
Previous studies revealed that pressure has little effect on a protein’s three-dimensional structure, but that it can affect its quaternary structure in the pressure range of 0.1–140 MPa (Chilukuri et al. 2002; Kawano et al. 2004). It will be of interest to examine whether piezophilic and piezosensitive Shewanella cytochromes c 5 differ in stability against pressure. Pressure denaturation measurements of wild-type Shewanella cytochromes c 5 and a mutation study are now in progress, aiming at elucidation of the pressure stabilization mechanism of a protein, which will be basic for future biotechnology.
Abbreviations
- SA cytc 5 :
-
Shewanella amazonensis cytochrome c 5
- SB cytc 5 :
-
Shewanella benthica cytochrome c 5
- SL cytc 5 :
-
Shewanella livingstonensis cytochrome c 5
- SV cytc 5 :
-
Shewanella violacea cytochrome c 5
References
Akanuma S, Nakajima Y, Yokobori S, Kimura M, Nemoto N, Mase T, Miyazono K, Tanokura M, Yamagishi A (2013) Experimental evidence for the thermophilicty of ancestral life. Proc Natl Acad Sci USA 110:11067–11072
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chilukuri LN, Bartlett DH (1997) Isolation and characterization of the gene encoding single-stranded-DNA-binding protein (SSB) from four marine Shewanella strains that differ in their temperature and pressure optima for growth. Microbiology 143:1163–1174
Chilukuri LN, Bartlett DH, Fortes PAG (2002) Comparison of high pressure-induced dissociation of single-stranded DNA-binding protein (SSB) from high pressure-sensitive and high pressure-adapted marine Shewanella species. Extremophiles 6:377–383
De Poorter LMI, Suzaki Y, Sato T, Tamegai H, Kato C (2004) Effects of pressure on the structure and activity of isopropylmalate dehydrogenases from deep-sea Shewanella species. Mar Biotechnol 6:S190–S194
Fang J, Zhang l, Bazylinski DA (2010) Deep-sea piezosphere and piezophiles: geomicrobiology and biogeochemistry. Trends Microbiol 18:413–422
Gromiha MM, Oobatake M, Sarai A (1999) Important amino acid properties for enhanced thermostability from mesophilic to thermophilic proteins. Biophys Chem 82:51–67
Hau HH, Gralnick JA (2007) Ecology and biotechnology of the genus Shewanella. Annu Rev Microbiol 61:237–258
Inoue H, Wakai S, Nishihara H, Sambongi Y (2011) Heterologous sysnthesis of cytochrome c´ by Escherichia coli is not dependent on the System I cytochrome c biogenesis machinery. FEBS J 278:2341–2348
Kasahara R, Sato T, Tamegai H, Kato C (2009) Piezo-adapted 3-isopropylmalate dehydrogenase of the obligate piezophile Shewanella benthica DB21MT-2 isolate from the 11,000-m depth of the Mariana Trench. Biosci Biotechnol Biochem 73:2541–2543
Kato C, Nogi Y (2001) Correlation between phylogenetic structure and function: examples from deep-sea Shewanella. FEMS Microbiol Ecol 35:223–230
Kato C, Sato T, Horikoshi K (1995) Isolation and properties of barophilic and barotolerant bacteria from deep-sea mud samples. Biodivers Conserv 4:1–9
Kato C, Sato T, Abe F, Ohmae E, Tamegai H, Nakasone K, Siddiqui KS, Thomas T (2008) Protein adaptation to high-pressure environments. In: Siddiqui KS, Thomas T (eds) Protein adaptation in extremophiles. Nova Science Publisher, Hauppauge, pp 167–191
Kawano H, Nakasone K Matsumoto M, Yoshida Y, Usami R, Kato C, Abe F (2004) Differential pressure resistance in the activity of RNA polymerase isolated from Shewanella violacea and Escherichia coli. Extremophiles 8:367–375
Kulakova L, Galkin A, Kurihara T, Yoshimura T, Esaki N (1999) Cold-adaptive alkaline protease from the psychrotrophic bacterium Shewanella strain Ac10: Gene cloning and enzyme purification and characterization. Appl Environ Microbiol 65:611–617
Lauro FM, Chastain RA, Blankenship LE, Yananos AA, Bartlett DH (2007) The unique 16S rRNA genes of piezophiles reflect both phylogeny and adaptation. Appl Environ Microbiol 73:838–845
Masanari M, Wakai S, Tamegai H, Kurihara T, Kato C, Sambongi Y (2011) Thermal stability of cytochrome c 5 of pressure-sensitive Shewanella livingstonensis. Biosci Biotechnol Biochem 75:1859–1861
McFall-Ngai MJ, Horwitz J (1990) A comparative study of the thermal stability of the vertebrate eye lens: Antarctic ice fish to the desert iguana. Exp Eye Res 50:703–709
Murakami C, Ohmae E, Tate S, Gekko K, Nakasone K, Kato C (2011) Comparative study on dihydrofolate reductases from Shewanella species living in deep-sea and ambient atmospheric-pressure environments. Extremophiles 15:165–175
Nakamura S, Ichiki S, Takashima H, Uchiyama S, Hasegawa J, Kobayashi Y, Sambongi Y, Ohkubo T (2006) Structure of cytochrome c 552 from a moderate thermophilic bacterium, Hydrogenophilus thermoluteolus: comparative study on the thermostability of cytochrome c. Biochemistry 45:6115–6123
Nogi Y, Kato C, Horikoshi K (1998) Taxonomic studies of deep-sea barophilic Shewanella strains and description of Shewanella violacea sp. nov. Arch Microbiol 170:331–338
Ogawa K, Sonoyama T, Takeda T, Ichiki S, Nakamura S, Kobayashi Y, Uchiyama S, Nakasone K, Takayama SJ, Mita H, Yamamoto Y, Sambongi Y (2007) Roles of a short connecting disulfide bond in the stability and function of psychrophilic Shewanella violacea cytochrome c 5. Extremophiles 11:797–807
Ohke Y, Sakoda A, Kato C, Sambongi Y, Kawamoto J, Kurihara T, Tamegai H (2013) Regulation of cytochrome c- and quinol oxidases, and piezotolerance of their activities in the deep-sea piezophile Shewanella violacea DSS12 in response to growth conditions. Biosci Biotechnol Biochem 77:1522–1528
Pace CN (1990) Measuring and increasing protein stability. Trends Biotechnol 8:93–98
Sambongi Y, Stoll R, Ferguson SJ (1996) Alteration of heam-attachment and signal-cleavage sites for Paracoccus denitrificans cytochrome c 550 probes pathway of c-type cytochrome biogenesis in Escherichia coli. Mol Microbiol 19:1193–1204
Sambongi Y, Uchiyama S, Kobayashi Y, Igarashi Y, Hasegawa J (2002) Cytochrome c from a thermophilic bacterium has provided insights into the mechanisms of protein maturation, folding, and stability. Eur J Biochem 269:3355–3361
Somero GN (1992) Adaptations to high hydrostatic pressure. Annu Rev Physiol 54:557–577
Swezey RR, Somero GN (1982) Polymerization thermodynamics and structural stabilities of skeletal muscle actins from vertebrates adapted to different temperatures and hydrostatic pressures. Biochemistry 21:4496–4503
Takeda T, Sonoyama T, Takayama SJ, Mita H, Yamamoto Y, Sambongi Y (2009) Correlation between the stability and redox potential of three homologous cytochromes c form two thermophiles and one mesophile. Biosci Biotechnol Biochem 73:366–371
Takenaka S, Wakai S, Tamegai H, Uchiyama S, Sambongi Y (2010) Comparative analysis of highly homologous Shewanella cytochromes c 5 for stability and function. Biosci Biotechnol Biochem 74:1079–1083
Uchiyama S, Hasegawa J, Tanimoto Y, Moriguchi H, Mizutani M, Igarashi Y, Sambongi Y, Kobayashi Y (2002) Thermodynamic characterization of variants of mesophilic cytochrome c and its thermophilic counterpart. Protein Eng 15:455–461
Venkateswaran K, Dollhopf ME, Aller R, Stackebrandt E, Nealson KH (1998) Shewanella amazonensis sp. nov., a novel metal-reducing facultative anaerobe from Amazonian shelf muds. Int J Syst Bacteriol 48:965–972
Xiao X, Wang P, Seng X, Bartlett DH, Wang F (2007) Shewanella psychrophila sp. nov. and Shewanella piezotolerans sp. nov., isolated from west Pacific dee-sea sediment. Int J Syst Evol Microbiol 57:60–65
Yamada M, Nakasose K, Tamegai H, Kato C, Usami R, Horikoshi K (2000) Pressure regulation of soluble cytochromes c in a deep-sea piezophilic bacterium, Shewanella violacea. J Bacteriol 182:2945–2952
Yang Z (1997) PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci 13:555–556
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (No. 20118005) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by the Cooperative Research Program of the “Network of Joint Research Center for Materials and Devices”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Albers.
Rights and permissions
About this article
Cite this article
Masanari, M., Wakai, S., Ishida, M. et al. Correlation between the optimal growth pressures of four Shewanella species and the stabilities of their cytochromes c 5 . Extremophiles 18, 617–627 (2014). https://doi.org/10.1007/s00792-014-0644-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-014-0644-y