Abstract
Numerous bacteria, fungi, yeasts and viruses have been exploited for biosynthesis of highly structured metal sulfide and metallic nanoparticles. Haloarchaea (salt-loving archaea) of the third domain of life Archaea, on the other hand have not yet been explored for nanoparticle synthesis. In this study, we report the intracellular synthesis of stable, mostly spherical silver nanoparticles (AgNPs) by the haloarchaeal isolate Halococcus salifodinae BK3. The culture on adaptation to silver nitrate exhibited growth kinetics similar to that of the control. NADH-dependent nitrate reductase was involved in silver tolerance, reduction, synthesis of AgNPs, and exhibited metal-dependent increase in enzyme activity. The AgNPs preparation was characterized using UV–visible spectroscopy, XRD, TEM and EDAX. The XRD analysis of the nanoparticles showed the characteristic Bragg peaks of face-centered cubic silver with crystallite domain size of 22 and 12 nm for AgNPs synthesized in NTYE and halophilic nitrate broth (HNB), respectively. The average particle size obtained from TEM analysis was 50.3 and 12 nm for AgNPs synthesized in NTYE and HNB, respectively. This is the first report on the synthesis of silver nanoparticles by haloarchaea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanotechnology is the design and manipulation of functional materials, devices and systems through control of matter at the nanometer scale (Salamanca-Buentello et al. 2005). Decreasing the dimensions of material to nano-dimensions has a pronounced effect on the physical, chemical, photo-electrochemical and electronic properties. Nanomaterials therefore find application in diverse areas such as electronics, optics, life sciences, medicine and coatings. Several physical and chemical methods have been employed for fabrication of nanomaterials. Chemical routes of synthesis allow for large quantities of nanoparticles to be synthesized in short periods of time, with a fairly good control of size and shape (Kowshik et al. 2003). However, contamination from pre-cursor chemicals, use of toxic solvents and generation of hazardous by-products are the major drawbacks of chemical synthesis (Thakkar et al. 2010). On the other hand physical methods of synthesis such as sputter deposition, laser ablation, attrition and pyrolysis require expensive equipment, are energy intensive (to maintain high temperature and pressure) and slow. Another problem encountered during nanomaterials synthesis is the high propensity of agglomeration of the synthesized materials due to their large surface energy. Additional treatments such as addition of stabilizing agents are required for maintaining the size of the nanoparticles (Li et al. 1999; Mallick et al. 2004). Biological synthesis is a clean, non-toxic and eco-friendly approach of nanoparticle synthesis that occurs at ambient/normal temperature and pressure, does not use toxic pre-cursors, and may result in nanoparticles capped with stabilizing cellular metabolites. The use of highly structured physical and biosynthetic activities of microbial cells for the synthesis of nanosized materials has emerged as a novel approach for the synthesis of metal-based nanoparticles (Gericke and Pinches 2006).
Metallic nanoparticles (MNPs) have been successfully synthesized by a plethora of micro-organisms with properties similar to chemically synthesized nanoparticles. Various fungi (Ahmad et al. 2003), yeast (Kowshik et al. 2003), bacteria (Lengke et al. 2006) and algae (Jianping et al. 2007) have been reported to biologically synthesize MNPs. Among the various MNPs, silver nanoparticles (AgNPs) have received considerable attention due to their attractive physicochemical properties. The large effective scattering cross section of individual AgNPs makes them ideal candidates for molecular labeling, where phenomena such as surface-enhanced Raman scattering can be exploited (Vo-Dinh 2008). Silver in its various chemical forms is known to be a potent antimicrobial agent (Catauro et al. 2004) and AgNPs have been shown to exhibit strong toxicity against microbes (Furno et al. 2004).
Here, we report for the first time MNP synthesis by Halophilic Archaea (Haloarchaea), member of the third domain of life, Archaea. Haloarchaea are the predominant population of thalassohaline and athalassohaline environments where salinity reaches up to 300 g/L (Zafrilla et al. 2010) and contribute to the red coloration of solar salt crystallizer. These organisms maintain osmotic balance with hypersaline surroundings by building up potassium ion concentration within their cells (Oren 2008). Haloarchaea are also known to encounter metals in their environment, but their metal tolerance has not been well documented (Srivastava and Kowshik 2013). Metal resistance genes have been annotated in model organism Halobacterium sp. strain NRC-1(Ng et al. 2000), but only arsenic resistance has been demonstrated experimentally (Wang et al. 2004). A system-level analysis of this organism has shown various strategies such as enhanced efflux and reduced influx that result in metal tolerance (Kaur et al. 2006).
The reduction of Ag+ to colloidal silver by microorganisms in aqueous solutions is a stepwise process. First various complexes of Ag+ are reduced to metallic silver atoms (Ag0). This is followed by the agglomeration of Ag0 into oligomeric clusters (Sharma et al. 2008). It is these clusters that eventually lead to the formation of colloidal Ag nanoparticles (Kapoor et al. 1994; Sharma et al. 2008). The low molecular weight peptide, glutathione (GSH) and proteins like metallothioneins and phytochelatins, enzymes such as oxidoreductases, NADH-dependent reductases, nitroreductases, NADH-dependent nitrate reductases (NRs) and cysteine desulfhydrases have been shown to be responsible for nanocrystal formation in yeast, bacteria, and fungi (Sweeney et al. 2004; Mokhtari et al. 2008; Bai et al. 2009; Ahmad et al. 2003; Shahverdi et al. 2007; Ramezani et al. 2010). In this study, we report the intracellular synthesis of silver nanoparticles by the haloarchaeon Halococcus salifodinae BK3. We demonstrate the involvement of NADH-dependent NRs in metal tolerance, silver reduction and nanoparticle synthesis.
Materials and methods
Materials
All the chemicals used for the present work were of certified A.R. grade and were bought from Himedia unless specified.
Organism and culture maintenance
H. salifodinae BK3 was used for the synthesis of AgNPs. The culture was previously isolated and characterized in the laboratory by Mani et al. (2012). It was maintained on NTYE (25 % NaCl, 0.5 % tryptone, 0.3 % yeast extract, 2 % MgSO4·7H2O and 0.5 % KCl, 2 % Agar) plates. It was also maintained in NTYE broth at 37 °C, 110 rpm and subcultured every 5 days.
Silver resistance and growth kinetic studies
The effect of silver on the growth of haloarchaeal isolate was determined in the presence of silver nitrate in the concentration range of 0.05–1 mM. Silver nitrate was prepared as 0.1 M stock solution and sterilized by filtration. The silver resistance studies were performed by growing H. salifodinae BK3 in the presence of 0.05 mM AgNO3. The culture was adapted to silver in NTYE by subculturing it in increasing concentrations of silver salt. To study the effect of metal on the growth kinetics of H. salifodinae BK3, 100 ml NTYE supplemented with 0.5 mM/1 mM silver nitrate was inoculated with 1 % culture adapted to silver and incubated at 37 °C, 110 rpm. A 1 ml aliquot of culture was withdrawn at intervals of 24 h and absorbance was measured at 600 nm on UV–Vis Double Beam spectrophotometer (Shimadzu, Japan, UV-2450). Medium without silver nitrate served as positive control and uninoculated medium with silver nitrate served as negative control. Similarly, the growth profile of the H. salifodinae BK3 in nitrate broth (0.5 % peptic digest of animal tissue, 0.3 % beef extract and 0.1 % potassium nitrate) amended with 25 % NaCl, 0.5 % KCl and 2 % MgSO4·7H2O (halophilic nitrate broth) was also determined. Specific growth rate was calculated according to Berney et al. (2006), and the lag time was calculated as per Breidt et al. (1994).
Biosynthesis of silver nanoparticles
H. salifodinae BK3 (2 %, OD = 0.6) was inoculated in NTYE medium with 0.5 mM AgNO3 and incubated at 37 °C, 110 rpm in the dark for nanoparticle synthesis. The accumulation and reduction of silver was followed by visual observation of biomass turning brown-black, which is a convenient indication of the formation of silver nanoparticles. The cells were harvested by centrifugation (10,000× g, 20 min). The pellet obtained was washed twice with sterile distilled water and dialyzed against deionized water for 24 h with change of water once every 2 h. The nanoparticle powder was obtained by drying the pellet at 60 °C for 12 h. Appropriate controls (uninoculated medium + silver nitrate and BK3 culture supernatant + silver nitrate) were run simultaneously. AgNPs were also synthesized in HNB following the same protocol.
Characterization
The excitation spectra of the AgNPs dried powder (prepared in NTYE and HNB) suspended in distilled water was recorded in the range of 200–800 nm using UV–Vis Double Beam spectrophotometer (Shimadzu, Japan, UV-2450) with distilled water as the blank. Crystallographic characterization was performed using the Rigaku Mini-Flex II powder x-ray diffractometer operated at 30 kV/15 mA with Cu Kα (1.54 Å) as radiation source and scanning mode of 2θ/θ continuous scanning. The crystallite domain size of nanoparticles was calculated by Debye–Scherrer formula: \( D = {\raise0.7ex\hbox{${k\lambda }$} \!\mathord{\left/ {\vphantom {{k\lambda } {\beta Cos\theta }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${\beta\, {\rm Cos}\theta }$}} \), where λ = wavelength of x-ray applied (1.54 Å), k = numerical constant for which the obtained value is 0.94, β 1/2 = full width (radians) at half maximum of the signal (1 1 1) and θ = Bragg angle for which 2θ is 38.08. The morphology of the nanoparticles powder was determined using transmission electron microscopy (TEM). The AgNPs synthesized in NTYE and HNB were dispersed in water by sonication (Microson™ Sonicator) at 0 °C for 15 min each at 3 RPS (40 W). The colloidal solution of AgNPs thus obtained was drop-coated on carbon-coated copper TEM grids. The TEM images were obtained using Philips (Model- CM200) transmission electron microscope (resolution 2.4 Å), which was operated at an accelerating voltage of 190 keV. Selected area electron diffraction studies were also carried out using the TEM. The elemental composition of the nanoparticles was determined by energy dispersive x-ray analysis (EDAX) using JEOL, JSM-5800LV scanning electron microscope equipped with EDAX and operated at 20 keV. The sample powders were deposited on a carbon tape before mounting on a sample holder and gold coated for EDAX.
Thiol assay
H. salifodinae BK3 cells (0.5 g wet weight) grown in the presence of 0.05 and 0.5 mM AgNO3 were suspended in TN buffer (50 mM Tris, 4 M NaCl). The suspension was sonicated (Microson™ Sonicator) at 0 °C for five cycles of 2 min each at three RPS (40 W). Cell debris was removed by centrifugation at 10,000g, at 4 °C for 20 min and the supernatant (Cell free extract- CFE) was stored at −20 °C for thiol assay. Cells grown in the absence of AgNO3 served as the negative control. Total thiol (T-SH), non-protein thiol (NP-SH) and protein-bound thiol (PB-SH) were estimated using 5, 5′-dithio-bis (2-nitrobenzoic acid) (DTNB; Sigma) according to Sedlak and Lindsay (1968). For determination of T-SH, 79 % methanol (Merck), 0.03 M Tris (pH 8.2) and 0.1 mM DTNB were added to 50 μl of CFE and incubated at RT for 15 min. The mixture was centrifuged at 1,700g, for 15 min and absorbance was recorded at 412 nm against a reagent blank. Reduced GSH (Sigma) was used as the standard. For determination of NP-SH, 500 μl of CFE, 400 μl of milli Q water and 5 % TCA were gently mixed for 12 min and centrifuged at 1,700g, for 15 min. Tris (0.16 M; pH 8.9) and DTNB (0.1 mM) were added to 200 μl of the supernatant and absorbance was measured at 412 nm within 3 min of addition of DTNB. PB-SH was determined by subtracting the NP-SH from the T-SH. The assay was carried out in triplicates.
Nitrate reductase assay
H. salifodinae BK3 was grown in HNB broth, with 0 (control), 0.05 and 0.5 mM KNO3 and 0.05 and 0.5 mM AgNO3 at 37 °C, 110 rpm for 10 days. Uninoculated medium with AgNO3-served as negative control for the assay and medium with KNO3 served as positive controls. Culture was centrifuged at 10,000g, for 20 min. To 300 μl of culture supernatant, 100 μl of Griess Ilosvays reagent and 2.6 ml distilled water were added and incubated at room temperature for 30 min. The concentration of nitrite was determined by recording absorbance at 548 nm using UV–Vis spectrophotometer. The standard curve was prepared using NaNO2.
The NR activity of H. salifodinae BK3 grown in HNB, with 0 (control), 0.05 and 0.5 mM KNO3 and 0.05 and 0.5 mM AgNO3 was determined using the method described by Harley (1993). The enzyme activity was measured by determining the amount of nitrate reduced within 2 min by the cell free extract on addition of substrate. The probes for the assay were taken on day 10. The substrate mixture comprised of 25 mM potassium phosphate buffer, 10 mM potassium nitrate, and 0.05 mM ethylene diamine tetra acetic acid (EDTA) (pH 7.3). CFE (100 μl) was added to substrate solution, along with 0.2 mM β-nicotinamide dinucleotide (reduced form; β-NADH). The reaction was allowed to proceed at 30 °C for 2 min and stopped by addition of 0.15 mM sulfanilamide solution in 3 mM HCl, followed by 0.19 mM N-(1-naphthyl) ethylene diamine hydrochloride (NED) solution, with proper mixing. Absorbance of the resultant colored products was recorded at 540 nm. Two sets of controls were run simultaneously. In one set of control, the substrate mixture was added after boiling the CFE and in the other set of control the reaction mix was boiled immediately on addition of the substrate to stop enzyme activity. The nitrite content of the control tubes was subtracted from that of the test, to give total amount of nitrite produced by the enzyme in 2 min. The assay was performed in triplicates.
Inhibitor study
Inhibitor studies were performed to determine the inhibitors of nanoparticle synthesis as well as to confirm the role of NR enzyme in nanoparticle synthesis. Inhibitors used were phenyl methane sulfonyl fluoride (PMSF), EDTA, sodium azide and iodoacetate (IAA). H. salifodinae BK3 was grown in absence of inhibitor (negative control), in the presence of 10 mM inhibitor only and in the presence of 10 mM inhibitor along with 0.5 mM AgNO3. Positive control for the study included culture grown in the presence of 0.5 mM AgNO3 in the absence of the inhibitor. The study was carried out in triplicates for each inhibitor. Further the NR activity of the CFE obtained from cells grown in the presence of 0.5 mM AgNO3 was determined in the presence of 10 mM inhibitor. The percentage of inhibition of NR enzyme activity was determined by the formula:
Antimicrobial effect of AgNPs
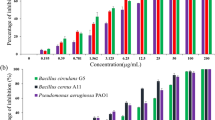
Disk diffusion method was employed to determine the antimicrobial activity of the as-synthesized nanoparticles against test organisms like Gram-negative Escherichia coli MTCC 2345, Pseudomonas aeruginosa MTCC 2582 and Gram-positive Staphylococcus aureus MTCC 737 and Micrococcus luteus. A stock solution of 20 mg/mL AgNPs synthesized in HNB was prepared in sterile distilled water and the suspension was sonicated for proper dispersal. Sterile disks of 6 mm diameter were loaded with AgNPs suspension to give a concentration of 10 and 50 μg/disc. Log-phase bacterial cultures (~106 CFU/mL) were spread plated on Mueller–Hinton agar, impregnated with the sample loaded disks and incubated at 37 °C for 18 h. The antibacterial activity was determined by measuring the zone of inhibition (ZoI), which is a fast and inexpensive method. The ZoI obtained due to AgNPs was compared to that obtained due to the antibiotic streptomycin (25 μg/disc) and gentamicin (30 μg/disc) which served as positive controls and saline served as negative control. The assay was performed in triplicates.
Results and discussion
Effect of silver on growth
Silver nitrate was found to be toxic to Halococcus salifodinae BK3 above concentrations of 0.5 mM. In the presence of 0.5 mM silver nitrate, growth of H. salifodinae BK3 was found to be slow, and the pigmentation of the culture changed from orange-red to brown-black, indicating the reduction of Ag+ to metallic silver (Ag0) (Fig. 1). During the first contact with silver nitrate, the culture in NTYE exhibited a retarded growth (Fig. 2). However, the silver-adapted H. salifodinae BK3 developed metal tolerance on continuous subculturing with increasing concentrations of silver nitrate. On adaptation to silver nitrate, achieved after three subcultures on media containing 0.5 mM silver nitrate, the growth profile of H. salifodinae BK3 (Fig. 2c) was similar to that of control (Fig. 2a). The specific growth rate (μ) of cells exposed to silver nitrate for the first time was 0.27 days−1, which increased to 0.67 days−1 for cells adapted to silver nitrate (supplementary Table SI). The lower growth rate and prolonged lag phase are indicative of longer adaptation periods required by H. salifodinae BK3 encountering silver nitrate for the first time. A similar tolerance to metal toxicity and metal uptake by adaptation was shown in the fungus Aspergillus foetidus (Ge et al. 2011).
The haloarchaeal isolate H. salifodinae BK3 was found to reduce toxic, colorless, soluble Ag+ to brown-black, elemental (Ag0) insoluble form as AgNPs. Various organisms have been shown to synthesize AgNPs which is accompanied by the brown-black coloration of the medium (Kowshik et al. 2003; Shankar et al. 2004; Basavaraja et al. 2008), that arises due to the excitation of surface plasmon vibrations typical of silver nanoparticles (Mulvaney 1996). Media controls without BK3 did not precipitate silver, thus confirming that synthesis of AgNPs was a culture-dependent phenomenon. Likewise, NP synthesis was not observed when silver nitrate was added to the supernatant of H. salifodinae BK3 grown both in the presence and absence of silver nitrate for 72 h. AgNPs synthesis occurred intracellularly and the biomass turned brown-black in color. Various organisms like yeast, bacteria and fungi have been shown to overcome metal stress and synthesize MNPs when the metal is introduced into the medium during the mid-log phase of growth (Kowshik et al. 2003; Bai et al. 2009; Hariharan et al. 2012). However, in the present study, the addition of silver nitrate during the mid-log phase was found to be inhibitory to the organism and did not result in AgNPs synthesis. A similar observation was made by Seshadri et al. (2012) in a study on the biosynthesis of silver nanoparticles by marine bacterium Idiomarina sp. PR58-8. The addition of silver nitrate at the time of inoculation eliminates the requirement of growth phase monitoring during AgNPs synthesis.
Mechanism of AgNPs synthesis
Organisms are known to tolerate metal stress by increasing the cellular pools of thiol compounds resulting in a thiolate–metal complex which neutralizes the toxicity of the metals and traps them inside the cells. These thiol compounds include the non-protein thiols (NP-SH) Glutathione (Avery 2001), and the protein-bound thiols (PB-SH) metallothioneins (Winge et al. 1985) and phytochelatins (Kneer et al. 1992). In certain fungi and yeast, heavy metal exposure results in an increased synthesis of phytochelatins or metallothioneins thereby exhibiting an increased PB-SH (Münger et al. 1987; Hayashi and Mutoh 1994; Borrelly et al. 2002). However, in some fungi and bacteria, exposure to heavy metal causes the sulfur in the proteome to be utilized for synthesis of GSH instead of sulfur-rich proteins (Speiser et al. 1992), thereby decreasing the levels of PB-SH and increasing the NP-SH levels in the cell (Guimaraes-Soares et al. 2007; Seshadri et al. 2011). Haloarchaea have γ-glutamyl cysteine (γGC) (Newton and Javor 1985; Malki et al. 2009) which is analogous to GSH and is involved in maintaining a reducing environment within the cell, overcoming oxidative and disulfide stress and detoxification of xenobiotics (Fahey 2001). However, its role in metal resistance of haloarchaea has not been investigated. To determine the role of thiols in conferring metal resistance in H. salifodinae, BK3 thiol assay was performed on cells grown in the presence and absence of AgNO3.
On exposure to AgNO3, H. salifodinae BK3 showed a concentration-dependent decrease in T-SH (Total thiol), NP-SH and PB-SH (supplementary Fig. S1). The thiol contents were normalized to the total protein content of the respective samples. In the presence of 0.05 mM AgNO3, a 60, 35 and 75 % decrease in concentration of T-SH, NP-SH and PB-SH, respectively, as compared to the control (cells grown in the absence of AgNO3), was observed. Similarly, in case of cells grown in 0.5 mM AgNO3 the concentration of T-SH, NP-SH PB-SH decreased by 81, 86 and 76 %, respectively. A similar observation of decrease in NP-SH and PB-SH in copper-exposed Articulospora tetracladia was made by Miersch et al. (2001), during studies on the influence of Cd2+, Cu2+, and Zn2+ on growth and thiol production in an aquatic hyphomycete (Articulospora tetracladia) and a zygomycete (Mucor racemosus). Here, the reduced levels of GSH and phytochelatins were attributed to the participation of Cu in Fenton reaction resulting in the oxidation of the thiols accompanied with imbalance in their synthesis/recycling and consumption/degradation. In haloarchaea, γGC is maintained in its reduced form by the enzyme bis-γ glutamyl cysteine reductase (GCR), which is analogous to glutathione reductase (Sundquist and Fahey 1989). Heavy metal ions of Zn2+, Cu2+, Hg2+ and arsenite have been shown to inhibit GCR in Halobacterium halobium (Sundquist and Fahey 1989). A similar inhibition of GCR by Ag+ may be responsible for a concentration-dependent decrease in the NP-SH and T-SH in H. salifodinae BK3. Thus, no involvement of thiol compounds was observed during AgNPs synthesis and metal resistance in this organism.
The reduction of silver ions to its metallic form in bacterial and fungal systems has also been shown to involve enzymatic detoxification by enzymes like nitrate reductase (NR), where toxic metal form is converted to non-toxic nanoparticulate form (Ahmad et al. 2003; Durán et al. 2005; Kalimuthu et al. 2008; Vaidyanathan et al. 2010). NR is normally involved in the reduction of nitrate to nitrite using NADH as the electron donor, however, in the presence of silver the electron is shuttled from NADH to reduction of silver ions (Deepak et al. 2011). The haloarchaeal isolate H. salifodinae BK3 exhibited NR activity as indicated by the pink color development on addition of Griess Ilosvays reagent. The assay was performed at two different time points, during active synthesis of AgNPs (day 5 after inoculation) and on completion of nanoparticle synthesis (day 10 after inoculation) (Fig. 3). The nitrite concentration within the cell at the time of inoculation was 0.68 nM. On day 5, the nitrite concentration for cells grown in the presence of 0.05 and 0.5 mM AgNO3 decreased by 18 and 49 %, respectively, as compared to the control (cells grown in the absence of AgNO3). This may be attributed to the shuttling of electrons for reduction of silver ions, instead of nitrate. However, after complete reduction of silver (day 10), the nitrite concentration of cells grown in the presence of 0.05 and 0.5 mM AgNO3 increased by 22 and 52 %, respectively, with respect to the control (cells grown in the absence of AgNO3). Thus, it may be postulated that after complete reduction of silver, the NR activity resumes and increases with increase in the metal concentration. This was confirmed by estimation of NR enzyme activity (Fig. 4) which exhibited a twofold (9.6 nmol nitrite/min/ml) and threefold (13.6 nmol nitrite/min/ml) increase at 0.05 and 0.5 mM AgNO3, respectively, as compared to the control (0 mM AgNO3; 5.4 nmol nitrite/min/ml). A similar upregulation of NADH-dependent NR during silver nitrate treatment was reported by Babu et al. (2011) in a global transcriptomic study of Bacillus cereus ATCC 14579 in response to silver nitrate stress. To verify whether the upregulation in the activity of NR was due to the silver ions or the nitrate component of AgNO3, the nitrate reductase enzyme assay was performed by spiking the growth medium of H. salifodinae BK3 with 0.05 and 0.5 mM KNO3. The NR enzyme activity of these cells was 1.7 times lower (5.7 and 7.9 nmol nitrite/min/ml for 0.05 and 0.5 mM KNO3, respectively) as compared to the activity of the cells grown in the presence of 0.05 and 0.5 mM AgNO3. The enzyme activity of the cells grown in the presence of 0.05 mM KNO3 was found to be similar to that of control (cells grown in the absence of AgNO3), which may be explained by the negligible increase in the overall nitrate concentration of the medium. The higher NR enzyme activity exhibited by the cells grown in the presence of 0.05 and 0.5 mM AgNO3 suggest that the upregulation is due to the silver component of AgNO3 and not the nitrate. Similarly, only a negligible increase in the nitrite content of the cells was observed for cells grown in the presence of KNO3 (Fig. 3). Thus, the enzymatic detoxification by NADH-dependent NR may be responsible for silver resistance and AgNPs synthesis in H. salifodinae BK3.
Effect of silver nitrate addition on the nitrate reductase activity of H. salifodinae BK3 during AgNPs synthesis (day 5) and after completion of AgNPs synthesis (day 10). On day 5 when silver reduction is not complete, a decrease in nitrite was observed, while on day 10, after silver reduction was complete, an increase in nitrite concentration was obtained. Control = 0 mM AgNO3. Values are mean ± SD (error bars) for three experiments
The role of NR in metal resistance and nanoparticle synthesis was further confirmed by enzyme inhibitor studies using sodium azide, PMSF, EDTA and IAA. In the absence of any metal stress, H. salifodinae BK3 was able to grow in the presence of 10 mM concentration of all the above inhibitors, growth being slightly delayed only in case of IAA. However, AgNPs synthesis was affected during growth in the presence of the inhibitor. Sodium azide exhibited the maximal inhibition followed by EDTA and IAA. Similar observations were made by Vaidyanathan et al. (2010) during studies on silver nanoparticle synthesis by Bacillus licheniformis. They postulated the involvement of enzyme NR in AgNPs synthesis based upon the inhibition of nanoparticle synthesis exhibited by the known inhibitor of NR, sodium azide. The NR activity of the CFE of H. salifodinae BK3 cells in the presence of 10 mM inhibitor and 0.5 mM AgNO3 was found to be 0.3 nmol nitrite/min/ml (for sodium azide), 10.2 nmol nitrite/min/ml (for IAA), 13.3 nmol nitrite/min/ml (for PMSF) and 9.5 nmol nitrite/min/ml (for EDTA), as compared to the control (cells grown in the presence of 0.5 mM AgNO3 and with no inhibitor) which exhibited a NR activity of 13.6 nmol nitrite/min/ml. Figure 5 shows the data which is the enzyme activity in the presence of inhibitor as a percentage of that in controls where inhibitor was not added but silver nitrate was present. The NR activity of CFE of cells grown in the absence of AgNO3 and any inhibitor was found to be 5.4 nmol nitrite/min/ml. Sodium azide is a known inhibitor of NR. Although the involvement of other factors (or enzymes etc.) cannot be ruled out, our results suggest the involvement of the enzyme NR in nanoparticle synthesis and silver nitrate tolerance. Therefore, the NR-mediated tolerance and detoxification of silver in the haloarchaeon H. salifodinae BK3 may represent a metal resistance mechanism that has not been exhibited earlier in haloarchaea.
The effect of various inhibitors like PMSF, IAA, EDTA and sodium azide on nitrate reductase enzyme activity of H. salifodinae BK3. Data are the enzyme activity in the presence of inhibitor as a percentage of that in controls where inhibitor was not added in the presence of 0.5 mM AgNO3. The CFE of H. salifodinae BK3 grown in the presence of 0.5 mM AgNO3 exhibited a NR enzyme activity of 13.574 nmol nitrite/min/ml. Values are mean ± SD (error bars) for three experiments
AgNPs synthesis by H. salifodinae BK3 was also obtained using nitrate broth amended with 25 % NaCl, 0.5 % KCl, and 2 % MgSO4 (HNB) as the growth medium. Growth kinetics studies using this medium (supplementary Fig. S2) showed that although there was a decrease in the growth rate of H. salifodinae BK3 in the presence of silver nitrate, the effect was lower as compared to growth in NTYE (supplementary Table SI). The doubling time of silver-adapted H. salifodinae BK3 in the presence of 0.5 mM AgNO3 in HNB was found to be 1.3 times (19.2 h) lower than the doubling time in NTYE (25.1 h). The specific growth rate (0.87 day−1) taken together with the lag phase (11.52 h) and doubling time of silver-adapted H. salifodinae BK3 thus indicate the HNB to be a medium for faster growth of the organism in the presence of silver nitrate and production of AgNPs. The AgNPs synthesis in HNB was intracellular and a similar brown coloration was obtained. Media controls without culture did not precipitate silver indicating that AgNPs synthesis was a culture-dependent phenomenon.
Nanoparticle characterization
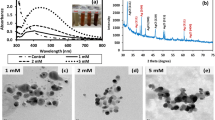
Silver nanoparticles exhibit localized surface plasmon resonances at visible and near-infrared frequencies leading to sharp peaks in their spectral extinction (Hutter and Fendler 2004). The frequency (i.e., absorption maxima or color) and intensity of the surface plasmon absorption bands are characteristic of the type of material and are highly sensitive to the size, shape and size distribution of the nanostructures as well as the environment that surrounds them (Underwood and Mulvaney 1994). The extinction is the result of collective excitation of conducting electrons due to strong interaction between the metallic nanoparticles and the incident electromagnetic radiation (Mie 1908). The absorbance scan of the AgNPs synthesized by H. salifodinae BK3 in NTYE as well as HNB exhibited strong, albeit broad surface plasmon peaks at around 440 nm (supplementary Fig. S3a). The absorption band was found to be slightly asymmetrical which indicates the presence of an additional weaker component at about 380 nm. This may be due to some anisotropy in the shape of the as-synthesized AgNPs. Shankar et al. (2004) also made similar observations for AgNPs formed on reduction with geranium root broth. A long tailing on the large-wavelength side may be due to small amount of particle aggregation. The nanoparticles synthesized by H. salifodinae BK3 were found to be stable in solution as suspension even after 6 months of their synthesis, as was evident from the absorbance profile (supplementary Fig. S3b).
Powder XRD was carried out for the as-synthesized AgNPs to determine the crystalline nature of the particles. The XRD profile of AgNPs synthesized in NTYE (supplementary Fig. S4a) shows intense peaks at 2θ values of 38.08, 44.18, 64.78, and 77.44 corresponding to (1 1 1), (2 0 0), (2 2 0) and (3 1 1) sets of lattice planes for crystalline fcc structures of silver (ICDD card/file No. 04-0783). The AgNPs prepared in HNB exhibited similar characteristic Bragg’s peaks at 38.08, 44.20, 64.38 and 77.34 with fcc crystalline structure (supplementary Fig. S4b). The crystallite domain size was calculated using line width of the (1 1 1) plane and was found to be 22 and 12 nm for AgNPs synthesized in NTYE and HNB, respectively.
TEM analysis of the dry powder of AgNPs synthesized by H. salifodinae BK3 in NTYE and HNB was carried out to further understand the morphology and the size distribution. The TEM images are shown in Fig. 6. The AgNPs were predominantly spherical in shape (Fig. 6) with a few being triangular, prismatic, or disc like (supplementary Fig. S5). Contrast was observed within the physical boundaries of majority of the AgNPs in both cases due to twined or multiply-twinned nanocrystals. Multiply-twinned particles formation is widespread in transition metals having fcc crystal lattice upon size reduction in nanometer scale (Marks 1994) and is a result of joining along twin boundaries of tetrahedral subunits sharing axis of fivefold symmetry (Hofmeister 1999; Nepijko et al. 2000). Presence of flat disc-like particles may be responsible for the out-of-plane SPR excitation which can be assigned to 380 nm of UV–Vis spectra. Similar observations have been made by Shankar et al. (2004). Average particle size for AgNPs synthesized in NTYE was found to be around 50.3 nm, and the range was 20–100 nm (Fig. 6a). The particle size of AgNPs synthesized in HNB was much smaller and was in the size range of 2–20 nm, with an average particle size of 12 nm. Very few particles of ~40 nm were observed (Fig. 6b). The micrographs also exhibit that as-synthesized AgNPs are well dispersed with no copious agglomeration. The EDAX spectrum of AgNPs synthesized in NTYE and HNB exhibited a peak corresponding to silver at around 3.4 keV (supplementary Fig. S6). The maximum located on the left of the spectrum at 0.2 keV corresponds to carbon. Besides Ag as major component, peaks of copper (Cu) and gold (Au) were also obtained which are contributions of the sample holder and gold coating, respectively.
Antimicrobial activity
The extensive use of antibiotics as antimicrobial agents has resulted in organisms acquiring resistance against them, causing the clinical strains to become more potent infectious agents (Farrar 1985; Monroe and Polk 2000). Therefore, there is need for producing antimicrobial agents that overcome this obstacle and efficiently kill bacteria. AgNPs have been shown to be more efficient than silver salts in mediating antimicrobial activity with lower propensity to induce microbial resistance (Kim et al. 2007; Rai et al. 2009). The exact mechanism of action of AgNPs though is still not fully understood. Some studies suggest the nanoparticulate form of AgNPs to be responsible for the antibacterial action (Hwang et al. 2008; Sondi and Salopek-Sondi 2004; Danilcauk et al. 2006; Kim et al. 2007), whilst others attribute the antimicrobial activity of the AgNPs to the release of Ag+ ions that result in damage to DNA, and proteins (Feng et al. 2008; Matsumura et al. 2003; Morones et al. 2005).
In this study, the antimicrobial effect of the AgNPs synthesized in HNB was determined by disk diffusion method. The AgNPs diffuse into the agar medium and are responsible for growth inhibition of the test organisms. A dose-dependent increase in sensitivity toward AgNPs was observed for all the four test organisms (Fig. 7). The ZoI were correlated to the antibiotic activity (streptomycin and gentamicin). Thus, the as-synthesized AgNPs were found to have antibacterial activity against both Gram-positive and Gram-negative bacteria and therefore are promising candidates for antimicrobial agents. Similar antimicrobial activity of biologically synthesized AgNPs against Gram-positive and Gram-negative bacteria has been reported by Furno et al. (2004), Morones et al. (2005) and Kim et al. (2011).
Conclusion
We have successfully synthesized silver nanoparticles using the Haloarchaeal Halococcus salifodinae BK3. The AgNPs were around 50 and 10 nm when synthesized in NTYE and HNB. In both cases, the NPs had fcc crystalline structure with spherical morphology. The nanoparticle preparations were found to be stable with almost negligible aggregation even after 6 months of synthesis. The concentration-dependent increase in NR activity in the presence of silver nitrate and the negligible NR enzyme activity along with inability of the haloarchaeal isolate to grow in the presence of NR inhibitor, sodium azide, confirms the involvement of the enzyme in metal resistance and silver nanoparticle synthesis. The obtained in vivo reduction of silver ions and subsequent formation of silver nanoparticles speaks in favor of environment friendly synthetic protocol for the synthesis and stabilization of metal nanoparticles. Thus, this strain can be used for nanoparticle synthesis as well as bioremediation of heavy metal polluted environments, because the nanoparticle synthesis is intracellular. To the best of our knowledge, this is the first report on nanoparticle synthesis by haloarchaea.
Abbreviations
- NTYE:
-
NaCl, tryptone, yeast extract
- AgNPs:
-
Silver nanoparticles
- MNPs:
-
Metallic nanoparticles
- HNB:
-
Halophilic nitrate broth
- XRD:
-
X-ray diffraction
- TEM:
-
Transmission electron microscopy
- EDAX:
-
Energy dispersive x-ray analysis
- SAED:
-
Selected area electron diffraction
- NR:
-
Nitrate reductase
- fcc:
-
Face-centered cubic
- EDTA:
-
Ethylene diamine tetra acetic acid
- PMSF:
-
Phenyl methane sulfonyl fluoride
- IAA:
-
Iodoacetate
- NED:
-
N-(1-Naphthyl) ethylene diamine hydrochloride
- DTNB:
-
5,5′-Dithio-bis (2-nitrobenzoic acid)
- T-SH:
-
Total thiol
- NP-SH:
-
Non-protein thiol
- PB-SH:
-
Protein-bound thiol
- CFE:
-
Cell-free extract
- ZoI:
-
Zone of inhibition
- GSH:
-
Glutathione
- γGC:
-
Gamma glutamyl cysteine
- GCR:
-
bis-γ-Glutamyl cysteine reductase
- MTP:
-
Multiply-twinned particles
References
Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R (2003) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B 28:313–318
Avery SV (2001) Metal toxicity in yeast and the role of oxidative stress. Adv Appl Microbiol 49:111–142
Babu MMG, Sridhar J, Gunasekaran P (2011) Global transcriptome analysis of Bacillus cereus ATCC 14579 in response to silver nitrate stress. J Nanobiotechnol 9:49. doi:10.1186/1477-3155-9-49
Bai HJ, Zhang ZM, Guo Y, Yang GE (2009) Biosynthesis of cadmium sulfide nanoparticles by photosynthetic bacteria Rhodopseudomonas palustris. Colloids Surf B 70:142–146
Basavaraja S, Balaji SD, Lagashetty A, Rajasab AH, Venkataraman A (2008) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater Res Bull 43:1164–1170
Berney M, Weilenmann HU, Ihssen J, Bassin C, Egli T (2006) Specific growth rate determines the sensitivity of Escherichia coli to thermal, UVA, and solar disinfection. Appl Environ Microbiol 72:2586–2593
Borrelly GPM, Harrison MD, Robinson AK, Cox SG, Robinson NJ, Whitehall SK (2002) Surplus zinc is handled by Zym1 metallothionein and Zhf endoplasmic reticulum transporter in Schizosaccharomyces pombe. J Biol Chem 277:30394–30400
Breidt F, Romick TL, Fleming HF (1994) Rapid method for the determination of bacterial growth kinetics. J Rapid Methods Autom Microbiol 3:59–68
Catauro M, Raucci MG, De Gaetano F, Marotta A (2004) Antibacterial and bioactive silver-containing Na2O × CaO × SiO2 glass prepared by sol-gel method. J Mater Sci Mater Med 15:831–837
Danilcauk M, Lund A, Saldo J, Yamada H, Michalik J (2006) Conduction electron spin resonance of small silver particles. Spectrochimaca Acta Part A 63:189–191
Deepak V, Kalishwaralal K, Ram Kumar Pandian S, Gurunathan S (2011) An insight into the bacterial biogenesis of silver nanoparticles, industrial production and scale-up. In: Rai M, Durán N (eds) Metal nanoparticles in microbiology. Springer, Berlin, pp 17–35
Durán N, Marcato PD, Alves O, Souza G (2005) Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Nanobiotechnol 3:8. doi:10.1186/1477-3155-3-8
Fahey RC (2001) Novel thiols of prokaryotes. Annu Rev Microbiol 55:333–356
Farrar WE (1985) Antibiotic resistance in developing countries. J Infect Dis 152:1103–1106
Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO (2008) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 52:662–668
Furno F, Morley KS, Wong B, Sharp BL, Arnold PL, Howdle SM, Bayston R, Brown PD, Winship PD (2004) Silver nanoparticles and polymeric medical devices: a new approach to prevention of infection? J Antimicrob Chemother 54:1019–1024
Ge W, Zamri D, Mineyama H, Valix M (2011) Bioaccumulation of heavy metals on adapted Aspergillus foetidus. Adsorption 17:901–910
Gericke M, Pinches A (2006) Biological synthesis of metal nanoparticles. Hydrometallurgy 83:132–140
Guimaraes-Soares L, Pascoal C, Cassio F (2007) Effects of heavy metals on the production of thiol compounds by the aquatic fungi Fontanospora fusiramosa and Flagellospor acurta. Ecotoxicol Environ Saf 66:36–43
Hariharan H, Al-dhabi NA, Karuppiah P, Rajaram SK (2012) Microbial synthesis of selenium nanocomposite using Saccharomyces cerevisiae and its antimicrobial activity against pathogens causing nosocomial infection. Chalcogenide Lett 9:509–515
Harley SM (1993) Use of a simple, colorimetric assay to demonstrate conditions for induction of nitrate reductase in plants. Am Biol Teach 55:161–164
Hayashi Y, Mutoh N (1994) Cadystin (phytochelatin) in fungi. In: Winkelmann G, Winge DR (eds) Metal ions in fungi. Marcel Dekker, New York, pp 311–337
Hofmeister H (1999) Fivefold twinning in nanosized particles and nanocrystalline thin films—ubiquitous metastable structures. Mater Sci Forum 325:312–314
Hutter E, Fendler JH (2004) Exploitation of localized surface plasmon resonance. Adv Mater 16:1685–1706
Hwang ET, Lee JH, Chae YJ, Kim YS, Kim BC, Sang B-I, Gu MB (2008) Analysis of the toxic mode of action of silver nanoparticles using stress-specific bioluminescent bacteria. Small 4:746–750
Jianping X, Jim YL, Daniel ICW, Yen PT (2007) Identification of active biomolecules in the high-yield synthesis of single-crystalline gold nanoplates in algal solutions. Small 3:668–672
Kalimuthu K, Suresh Babu R, Venkataraman D, Bilal M, Gurunathan S (2008) Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B 65:150–153
Kapoor S, Lawless D, Kennepohl P, Meisel D, Serpone N (1994) Reduction and aggregation of silver ions in aqueous gelatine solutions. Langmuir 10:3018–3022
Kaur A, Pan M, Meislin M, Facciotti MT, El-Gewely R, Baliga NS (2006) A systems view of haloarchaeal strategies to with stand stress from transition metals. Genome Res 16:841–854
Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH (2007) Antimicrobial effects of silver nanoparticles. Nanomedicine 3:95–101
Kim SH, Lee HS, Ryu DS, Choi SJ, Lee DS (2011) Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J Microbiol Biotechnol 39:77–85
Kneer R, Kutchan TM, Hochberger A, Zenk MH (1992) Saccharomyces cerevisiae and Neurospora crassa contain heavy metal sequestering phytochelatin. Arch Microbiol 157:305–310
Kowshik M, Ashtaputre S, Kharrazi S, Vogel W, Urban J, Kulkarni SK, Paknikar KM (2003) Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 14:95–100
Lengke M, Fleet M, Southam G (2006) Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver (I) nitrate complex. Langmuir 10:1021–1030
Li Y, Duan X, Qian Y, Yang L, Liao H (1999) Nanocrystalline silver particles: synthesis, agglomeration, and sputtering induced by electron beam. J Colloid Interface Sci 209:347–349
Malki L, Yanku M, Borovok I, Cohen G, Mevarech M, Aharonowitz Y (2009) Identification and characterization of gshA, a gene encoding the glutamate-cysteine ligase in the halophilicarchaeon Haloferax volcanii. J Bacteriol 191:5196–5204
Mallick K, Witcomb MJ, Scurell MS (2004) Polymer stabilized silver nanoparticles: a photochemical synthesis route. J Mater Sci 39:4459–4463
Mani K, Salgaonkar BB, Braganca JM (2012) Culturable halophilic archaea at the initial and crystallization stages of salt production in a natural solar saltern of Goa, India. Aquat Biosyst 8:15. doi:10.1186/2046-9063-8-15
Marks LD (1994) Experimental studies of small particle structures. Rep Prog Phys 57:603–649
Matsumura Y, Yoshikata K, Kunisaki S, Tsuchido T (2003) Mode of bacterial action of silver zeolite and its comparison with that of silver nitrate. Appl Environ Microbiol 69:4278–4281
Mie G (1908) Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen.Ann Phys 25:377-445 American translation at http://diogenes.iwt.uni-bremen.de/vt/laser/papers/SAND78-6018-Mie-1908-translation.pdf
Miersch J, Tschimedbalshir M, Barlocher F, Grams Y, Pierau B, Schierhorn A, Krauss GJ (2001) Heavy metals and thiol compounds in Mucor racemosus and Articulospora tetracladia. Mycol Res 105:883–889
Mokhtari N, Daneshpajouh S, Atashdehghan R, Seyedbagheri S, Abdi K, Sarkar S, Minaian S, Shahverdi HR, Shahverdi AR (2008) Biological synthesis of very small silver nanoparticles by culture supernatant of Klebsiella pneumonia: the effects of visible-light irradiation and the liquid mixing process. Mater Res Bull 44:1415–1421
Monroe S, Polk R (2000) Antimicrobial use and bacterial resistance. Curr Opin Microbiol 3:496–501
Morones JB, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JP, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346–2353
Mulvaney P (1996) Surface plasmon spectroscopy of nanosized metal particles. Langmuir 12:788–800
Münger K, Germann UA, Lerch K (1987) The Neurospora crassa metallothionein gene. Regulation of expression and chromosomal location. J Biol Chem 262:7363–7367
Nepijko SA, Hofmeister H, Sack-Kongehl H, Schlogl R (2000) Multiply twinned particles beyond the icosahedron. J Cryst Growth 213:129–134
Newton GL, Javor B (1985) Gamma-Glutamyl cysteine and thiosulfate are the major low-molecular-weight thiols in halobacteria. J Bacteriol 161:438–441
Ng WV, Kennedy SP, Mahairas GG, Berquist B, Pan M, Shukla HD, Lasky SR, Baliga NS, Thorsson V, Sbrogna J, Swartzell S, Weir D, Hall J, Dahl TA, Welti R, Goo YA, Leithauser B, Keller K, Cruz R, Danson MJ, Hough DW, Maddocks DG, Jablonski PE, Krebs MP, Angevine CM, Dale H, Isenbarger TA, Peck RF, Pohlschroder M, Spudich JL, Jung KW, Alam M, Freitas T, Hou S, Daniels CJ, Dennis PP, Omer AD, Ebhardt H, Lowe TM, Liang P, Riley M, Hood L, DasSarma S (2000) Genome Sequence of Halobacterium species NRC-1. PNAS 97:12176–12181
Oren A (2008) Microbial life at high salt concentrations: phylogenetic and metabolic diversity. Saline Syst 4:2. doi:10.1186/1746-1448-4-2
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83
Ramezani F, Ramezani M, Talebi S (2010) Mechanistic aspects of biosynthesis of nanoparticles by several microbes. Nanocon 10:12–14
Salamanca-Buentello F, Persad DL, Court EB, Martin DK, Daar AS, Singer PA (2005) Nanotechnology and the developing world. PLoS Med 2:e97
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Elman’s reagent. Anal Biochem 25:192–205
Seshadri S, Saranya K, Kowshik M (2011) Green synthesis of lead sulfide nanoparticles by the lead resistant marine yeast, Rhodosporidium diobovatum. Biotechnol Prog 27:1464–1469
Seshadri S, Prakash A, Kowshik M (2012) Biosynthesis of silver nanoparticles by marine bacterium Idiomarina sp. PR58-8. Bull Mater Sci 35:1201–1205
Shahverdi AR, Minaian S, Shahverdi HR, Jamalifar H, Nohi A (2007) Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: a novel biological approach. Process Biochem 42:919–923
Shankar SS, Rai A, Ahmad A, Sastry M (2004) Biosynthesis of silver and gold nanoparticles from extracts of different parts of the geranium plant. Appl Nanosci 1:69–77
Sharma VK, Yngard RA, Lin Y (2008) Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci 145:83–96
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci 275:177–182
Speiser DM, Ortiz DF, Kreppel L, Scheel G, McDonald G, Ow DW (1992) Purine biosynthetic genes are required for cadmium tolerance in Schizosaccharomyces pombe. Mol Cell Biol 12:5301–5310
Srivastava P, Kowshik M (2013) Mechanisms of metal resistance and homeostasis in haloarchaea. Archaea 16. doi:10.1155/2013/732864
Sundquist AR, Fahey RC (1989) The function of γ-glutamylcysteine and bis-γ-glutamylcysteine reductase in Halobacterium halobium. J Biol Chem 264:719–725
Sweeney RY, Mao C, Gao X, Burt JL, Belcher AM, Georgiou G, Iverson BL (2004) Bacterial biosynthesis of cadmium sulphide nanocrystals. Chem Biol 11:1553–1559
Thakkar KN, Mhatre SS, Parikh YR (2010) Biological synthesis of metallic nanoparticles. Nanomed NBM 6:262–275
Underwood S, Mulvaney P (1994) Effect of the solution refractive index on the color of gold colloids. Langmuir 10:3427–3430
Vaidyanathan R, Gopalram S, Kalishwaralal K, Deepak V, Pandian SR, Gurunathan S (2010) Enhanced silver nanoparticle synthesis by optimization of nitrate reductase activity. Colloids Surf B 75:335–341
Vo-Dinh T (2008) Nano biosensing using plasmonic nanoprobes. IEEE J Sel Topics Quantum Electron 14:198–205
Wang G, Kennedy SP, Fasiludeen S, Rensing C, DasSarma S (2004) Arsenic resistance in Halobacterium sp. strain NRC-1 examined by using an improved gene knockout system. J Bacteriol 186:3187–3194
Winge DR, Nielson KB, Gray WR, Hamer DH (1985) Yeast metallothionein sequence and metal binding properties. J Biol Chem 260:14464–14470
Zafrilla B, Martínez-Espinosa RM, Alonso MA, Bonete MJ (2010) Biodiversity of Archaea and floral of two inland saltern ecosystems in the Alto Vinalopó Valley, Spain. Saline Syst 6:10. doi:10.1186/1746-1448-6-10
Acknowledgments
We thank Ministry of Earth Science (MoES), Government of India for their funding of the project MoES/11-MRDF/1/38/P/10-PC-III. We would like to thank Dr. Neha Hebalkar at ARCI, Hyderabad and the SAIF at IIT-Bombay for their help with TEM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Atomi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
792_2013_563_MOESM2_ESM.tif
Supplemnetary Fig. S1 Effect of silver nitrate on the concentrations of total (T-SH), non-protein (NP-SH) and protein-bound (PB-SH) thiols in H. salifodinae BK3 exposed to 0.05 and 0.5 mM AgNO3. Control = 0 mM AgNO3. Values are mean ± SD for three experiments. (TIFF 8499 kb)
792_2013_563_MOESM3_ESM.tif
Supplementary Fig. S2 Effect of silver nitrate on growth profiles of H. salifodinae BK3 a in HNB without AgNO3 (control); b upon first exposure to AgNO3 by addition of 0.5 mM AgNO3 in HNB; c for cells adapted to AgNO3 upon addition of 0.5 mM AgNO3 in HNB. Values are mean ± SD (error bars) for three experiments. (TIFF 11254 kb)
792_2013_563_MOESM4_ESM.tif
Supplementary Fig. S3 UV–visible absorbance spectrum of the AgNPs synthesized by H. salifodinae BK3. a The spectra of AgNPs synthesized in NTYE (black) and in HNB (red); b Comparison of the UV–Visible profile of AgNPs prepared in HNB immediately after synthesis (blue) and after 6 months of storage (green). (TIFF 7995 kb)
792_2013_563_MOESM5_ESM.tif
Supplementary Fig. S4 X-ray diffraction pattern of AgNPs synthesized by H. salifodinae BK3 in a NTYE and b HNB. (TIFF 7995 kb)
792_2013_563_MOESM6_ESM.tif
Supplementary Fig. S5 Representative TEM micrographs showing triangular and disc like morphology of the AgNPs synthesized by H. salifodinae BK3 in the presence of 0.5 mM AgNO3. (TIFF 1256 kb)
Rights and permissions
About this article
Cite this article
Srivastava, P., Bragança, J., Ramanan, S.R. et al. Synthesis of silver nanoparticles using haloarchaeal isolate Halococcus salifodinae BK3 . Extremophiles 17, 821–831 (2013). https://doi.org/10.1007/s00792-013-0563-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-013-0563-3