Abstract
Non-photochemical fluorescence quenching (NPQ) is mainly associated with the transthylakoid proton gradient (ΔpH) and xanthophyll cycle. However, the exact mechanism of NPQ is different in different oxygenic photosynthetic organisms. In this study, several inhibitors were used to study NPQ kinetics in the sea ice alga Chlamydomonas sp. ICE-L and to determine the functions of ΔpH and the xanthophyll cycle in the NPQ process. NH4Cl and nigericin, uncouplers of ΔpH, inhibited NPQ completely and zeaxanthin (Z) was not detected in 1 mM NH4Cl-treated samples. Moreover, Z and NPQ were increased in the samples containing N,N’-dicyclohexyl-carbodiimide (DCCD) under low light conditions. We conclude that ΔpH plays a major role in NPQ, and activation of the xanthophyll cycle is related to ΔpH. In dithiothreitol (DTT)-treated samples, no Z was observed and NPQ decreased. NPQ was completely inhibited when NH4Cl was added suggesting that part of the NPQ process is related to the xanthophyll cycle and the remainder depends on ΔpH. Moreover, lutein and β-carotene were also essential for NPQ. These results indicate that NPQ in the sea ice alga Chlamydomonas sp. ICE-L is mainly dependent on ΔpH which affects the protonation of PSII proteins and de-epoxidation of the xanthophyll cycle, and the transthylakoid proton gradient alone can induce NPQ.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Photosynthetic organisms have evolved various photoprotective mechanisms in the thylakoid membrane to prevent the damage caused by excess excitation (Bajkán et al. 2011). Under high light conditions a mechanism known as non-photochemical quenching (NPQ) is triggered to dissipate excess absorbed light energy within the photosystem II (PSII) antenna as heat, preventing photodamage to the reaction center (Johnson and Ruban 2011). This process is one of the most important photoprotection mechanisms in higher plants and algae (Müller et al. 2001; Horton and Ruban 2005). Three components contribute to the regulation of NPQ: first, the build up of a proton gradient (ΔH+ or ΔpH) across thylakoid membranes (Horton et al. 1996), which is generated by photosynthetic proton pumping; second, the activity of the xanthophyll or violaxanthin (V-) cycle with the corresponding formation of zeaxanthin (Z) (Demmig-Adams et al. 1990), and third, the protein PsbS/LhcSR, a protein homologous to antenna components (Demmig-Adams et al. 2006; Li et al. 2000).
The major component of NPQ is rapid reversible, ΔpH-dependent quenching, known as qE (Li et al. 2000). In addition to qE, a second component is photoinhibitory quenching ‘qI’, which is irreversible in the short-term, resulting in the sustained down-regulation of photochemical efficiency (Horton et al. 2008; García-Plazaola et al. 2012). Recently, a third component of NPQ, which is ΔpH independent and entirely dependent on the Z content, was termed ‘qZ’ (Dall’Osto et al. 2005; Nilkens et al. 2010). Carotenoids play fundamental roles in light harvesting and photoprotection (Demmig-Adams and Adams 1996). Exposure to different light conditions induces changes in carotenoid composition via the xanthophyll cycle (XC) (Li et al. 2009). The XC involves the reversible light-dependent conversion of violaxanthin (V) to zeaxanthin (Z) via antheraxanthin (A) intermediates. Z is involved in the NPQ response through either a direct or an indirect mechanism (Lepetit et al. 2012; Zhang et al. 2012). Following dark or low light conditions, zeaxanthin epoxidase (ZEP) converts Z back to V in a reverse reaction. Diatoms and other chromophytes display a simple XC, which comprises the one-step conversion of diadinoxanthin (Ddx) to diatoxanthin (Dtx) (Goss and Jakob 2010; Depauw et al. 2012).
The exact mechanisms of NPQ vary among the plant kingdom. In plants, NPQ is triggered by a change in thylakoid lumen pH under high light conditions, activating the XC and the protonation of PsbS protein (Li et al. 2000). Protonation of PSII proteins leads to their dissociation from PSII and their aggregation within the membrane (Betterle et al. 2009; Johnson et al. 2011). NPQ in green algae shows the same pH modulation as in plants, but relies on LHCSR proteins which are induced by high light in Chlamydomonas and correlate with the qE capacity (Peers et al. 2009). Interestingly, the moss, Physcomitrella patens, encodes both PsbS and LHCSR proteins (Alboresi et al. 2010), which are both active in NPQ. In diatoms, NPQ seems to depend on at least two factors: a specific XC and the activity of LHCSR-like effectors, with different efficiencies and pH requirements to those of terrestrial plants and green algae (Depauw et al. 2012). In the giant kelp, Macrocystis pyrifera, in contrast to higher plants, the degree of NPQ induction is related to the amount of Z synthesized in high light, and the transthylakoid proton gradient alone does not induce NPQ (García-Mendoza and Colombo-Pallotta 2007).
The Antarctic sea ice alga, Chlamydomonas sp. ICE-L, a typical species of green microalgae, can adapt to natural habitats in the polar region and similar extreme environments. It has been shown to undergo specific ecological and physiological adaptations to harsh habitats, including low temperature, intense solar irradiance, excessive UV radiation and nutrient depletion (Kan et al. 2006). Chlamydomonas sp. ICE-L must have several photoprotective mechanisms to survive in these harsh conditions. Similar to higher plants and Chlamydomonas reinhardtii, Chlamydomonas sp. ICE-L has a XC and a higher NPQ capacity under high light conditions. In this study, we determined NPQ kinetics in Chlamydomonas sp. ICE-L and the relationship between ΔpH and the XC in the NPQ process. Several specific inhibitors, namely NH4Cl, DTT, nigericin and DCCD, were used to characterize the nature of the quenching. In parallel, we also examined the changes in NPQ capacity and XC pigment contents.
Materials and methods
Culture conditions
The Antarctic sea ice alga, Chlamydomonas sp. ICE-L (Liu et al. 2006), was isolated from floating in sea ice near the Zhongshan Research Station in Antarctica (69.8°S, 77.8°E) during China’s 18th Antarctic Expedition (2001–2002). Algal cells were grown in sterile natural seawater Provasoli seawater medium (Provasoli 1968). This cultivation was conducted in 500 mL Erlenmeyer flasks containing 300 mL medium at 6 ± 1 °C. They were illuminated at a light intensity of 40 μmol photons m−2 s−1 under a 12 h/12 h light/dark photoperiod. All glassware and media used in the experiments were previously sterilized by autoclaving. Cells were harvested during the exponential phase of growth, centrifuged at 6000×g for 5 min and resuspended in their culture medium to a final concentration of OD 680 = 1.3. This concentrated suspension was used for the experiments.
The functions of ΔpH and the XC were characterized using the inhibitors NH4Cl, nigericin, DCCD and DTT. For each experiment, 2 ml of cell suspension was used. Sodium bicarbonate was added at a concentration of 4 mM from a freshly prepared 0.2 M stock water solution to prevent limitation of the photosynthetic rate due to carbon supply. When appropriate, NH4Cl (ammonium chloride), nigericin, DTT (dithiothreitol) or DCCD (N,N’-dicyclohexyl-carbodiimide) were added at the start of dark incubation. Stock solutions of NH4Cl (1 M in distilled water), DCCD (Sigma, 1 mM in absolute ethanol) and DTT (Sigma, 20 mM in distilled water) were freshly prepared.
Measurement of Chl fluorescence
To measure chlorophyll fluorescence, a fresh 2 mL sample was placed in a 5 mm quartz cuvette. The light responses of the Chl fluorescence parameters of dark-adapted algal cells were measured using a Dual-PAM fluorometer (Heinz Walz, Germany). Prior to measurement of fluorescence, samples cultivated under normal conditions were dark-adapted for 20 min (in triplicate). A pulse of saturating light (10000 μmol photon m−2 s−1 for 300 ms) was then applied to determine the maximum fluorescence (Fm). For cells cultured under different inhibitor conditions, actinic light was used at 830 μmol photons m−2 s−1. Once steady state fluorescence was achieved, saturating pulses were applied every 30 s to measure the Fm under actinic light (Fm′). Samples cultured under the induction conditions were analyzed directly in the Dual-PAM fluorometer. The energy dissipation that was not used for PSII photochemistry was determined based on the fluorescence quenching effect using the ratio NPQ = (Fm−Fm')/Fm' (Bilger and Björkman 1990). All experiments were conducted in triplicate. For NPQ kinetics, to minimize the contribution of the saturating light flashes (which are required to determine the NPQ parameter) to NPQ formation, the flashes were separated at 10 s intervals for the first 20 s of induction, 30 s intervals between 20 and 80, and 60 s intervals from 80 to 1400 s.
Pigment analysis
Culture samples (50 ml) were filtered through a grade GF/F filter (Whatman PLC, Kent, UK). The filter was wrapped in aluminum foil, immediately frozen in liquid nitrogen, and stored at −80 °C. The filtered cells were mechanically disrupted and the pigments were extracted in a tube by adding 5 ml of 85 % acetone. The tube was then placed in a beaker containing ice and water, which was placed in an ultrasonic bath for 5 min. Cellular debris was removed by centrifugation and the supernatants were filtered through a 0.45 μm syringe filter into amber high-pressure liquid chromatography (HPLC) vials. The vials were stored on ice in the dark prior to injection. All extraction procedures were carried out in a dimly lit room.
The concentrations of XC pigments were determined using reversed phase HPLC as described by Zapata et al. (2000) and spectrophotometrically detected by their absorbance at 450 nm. Pigment quantification was performed using calibration curves calculated from HPLC separations using purified pigment standards. The de-epoxidation state of the violaxanthin cycle was calculated as (Z + 0.5A)/(V + A + Z) (Casper-Lindley and Björkman 1998).
Results
Light responses of photosynthetic parameters
In order to determine the saturation light intensity of Chlamydomonas sp. ICE-L, we first measured chlorophyll a fluorescence quenching using different intensities of actinic light. The effects of these light intensities on photosynthetic electron transport rate (ETR) were investigated using different PSII fluorescence parameters calculated from the Chl a fluorescence curve. Figure 1 shows NPQ and ETR values in control cells measured at different actinic light intensities. The cells had a minimal NPQ value of about 0.13 when actinic light was 131 μmol photons m−2 s−1. As expected, NPQ and ETR were efficiently activated and their values increased with increasing irradiance. Actinic light intensities higher than 830 μmol photons m−2 s−1 did not induce a stronger final NPQ. We observed a slightly increased NPQ at higher light intensities, and ETR showed a similar relationship between light intensities. Actinic light was set to 830 μmol photons m−2 s−1, which was sufficient to ensure maximal NPQ activation in Chlamydomonas sp. ICE-L.
Effect of NH4Cl and nigericin on NPQ
The uncouplers of ΔpH, NH4Cl and nigericin were used to determine whether NPQ in Chlamydomonas sp. ICE-L was activated by ΔpH. NPQ induction kinetics were monitored in the algal cells incubated with each uncoupler for 10 min in darkness before high light treatment. Figure 2a shows the effects of different concentrations of NH4Cl on NPQ capacity in Chlamydomonas sp. ICE-L. The NPQ value in control samples was approximately 1.6, and NPQ decreased when NH4Cl concentration increased. When cultured in 1 mM NH4Cl, NPQ reached a stable level of about 0.17. NPQ was approximately 0.057 in the presence of 3 mM NH4Cl and when the NH4Cl concentration was increased this did not induce a smaller final NPQ. It was necessary to use NH4Cl at 3 mM to achieve complete inhibition of ΔpH, showing that NPQ was triggered by ΔpH. Figure 2b shows the NPQ kinetics in the samples cultured in the presence of NH4Cl and nigericin. Concentrations of 1 and 3 mM NH4Cl were used to study NPQ kinetics and NPQ values decreased significantly compared to the control cells. Nigericin also disrupted ΔpH and induced a reduction in NPQ values. Nigericin inhibited NPQ with greater potency than NH4Cl in Chlamydomonas sp. ICE-L as 10 μM nigericin inhibited NPQ completely. These data showed that ΔpH played a major role in NPQ capacity in Chlamydomonas sp. ICE-L.
Effect of NH4Cl and nigericin on NPQ in Chlamydomonas sp. ICE-L. a Increasing concentrations of NH4Cl. b NPQ kinetics in cells cultured in NH4Cl and nigericin. Before measuring NPQ values, cells were incubated in the dark for 10 min with NH4Cl and nigericin and then illuminated for 15 min at 400 μmol photons m−2 s−1. CL (control cells), NH4Cl-1 (1 mM NH4Cl), NH4Cl-3 (3 mM NH4Cl), nigericin (10 μM)
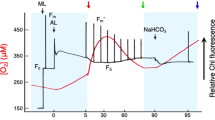
Effect of DCCD on NPQ kinetics
The relationship between ΔpH and NPQ was further investigated using DCCD to vary the extent of the light-dependent lumen acidification. Figure 3 shows NPQ kinetics in cells with and without 20 μM DCCD under low light (LL) and high light (HL) conditions. When dark-adapted cells were incubated with 20 μM DCCD; DCCD enhanced the extent of NPQ by about 0.5 at LL irradiance (131 μmol photons m−2 s−1), and low NPQ was generated in the absence of DCCD. In contrast, under HL conditions (830 μmol photons m−2 s−1) DCCD had a strong inhibitory effect on NPQ and decreased to 0.3 under higher illumination in the presence of DCCD (20 μM), as high concentrations of DCCD bind to carboxy amino residues located in the hydrophobic domains of the light harvesting antenna which can reverse acid-induced fluorescence quenching. These data indicated that ΔpH induced the protonation of PSII proteins under HL conditions and NPQ in Chlamydomonas sp. ICE-L was dependent on transthylakoid ΔpH.
Effect of DTT on NPQ
In order to verify the effects of Z, DTT-treated samples were used to measure NPQ capacity. NPQ values in DTT-treated and control samples were determined after 15 min of illumination (Fig. 4). The DTT-treated samples showed decreased NPQ in Chlamydomonas sp. ICE-L. NPQ capacity decreased rapidly from 1.27 to 0.6 when 1 mM DTT was added to the culture. NPQ decreased to about 0.5 in the 2 mM DTT-treated sample and subsequent increasing concentrations of DTT induced a small reduction in NPQ capacity, showing that this was sufficient to block the de-epoxidation reaction in Chlamydomonas sp. ICE-L. As shown in Fig. 4, NPQ was suppressed by DTT and was reduced by ~60 % which was related to an increase in A and Z due to V de-epoxidation after exposure to HL.
In order to determine the role of ΔpH and XC in NPQ, the effects of NH4Cl and DTT on NPQ induction were assayed in algal cells. Figure 5 shows the time course of NPQ kinetics induction in different incubated samples. In control cells, the NPQ value increased rapidly and reached 1.0 after 10 s, decreased to 0.5 at 140 s, and then reached a higher value of about 1.2 with prolonged illumination. In addition, NPQ in control samples decreased to a low level of about 0.1 after the addition of NH4Cl. In DTT-treated cells, NPQ decreased slightly at first and then reached a stable level of about 0.5 within a few seconds in saturating light and disappeared immediately after addition of the uncoupler NH4Cl. Therefore, the amount of NPQ suppressed by the application of DTT may be related to an increase in A and Z due to V de-epoxidation after exposure to saturating light. These results indicated that approximately 60 % of NPQ was suppressed by DTT in the second phase which was related to the XC and the remainder was dependent on ΔpH.
Time course of NPQ in Chlamydomonas sp. ICE-L exposed to 400 μmol photons m−2 s−1 for 15 min and kept in darkness after illumination. Cells incubated in the presence of 2 mM DTT for 20 min before light exposure are shown by solid square. Solid circle NPQ in untreated samples (control sample); solid square NPQ in cells incubated with DTT before high light exposure; open circle 1 mM NH4Cl was added after 440 s of illumination in control samples; open square 1 mM NH4Cl was added after 440 s of illumination in DTT-treated samples
Pigment compositions and xanthophyll cycle activities in different experimental cells
Pigment contents play a fundamental role in light harvesting and photoprotection. Chl a + b (Chls) contents and XC pigments measured under different light conditions and inhibitors are shown in Table 1. Under LL conditions, Z was not detected and the level of A was approximately 0.51. When the sample was induced under HL conditions, the ratio of Z to Chls content was 3.24 and the de-epoxidation ratio was 0.62, showing that the HL treatment was effective in inducing Z synthesis and the de-epoxidation index in the XC. When algal cells were cultured with DCCD, the Z ratio in the LL-DCCD and HL-DCCD samples were 0.56 and 2.43, respectively, which indicated that the level of ΔpH in DCCD-treated samples can affect activation of the XC, and under different light conditions DCCD had different effects on algal cells. No Z was observed in 1 mM NH4Cl-treated samples with the lowest NPQ values, showing that synthesis of Z is related to ΔpH. In the 2 mM DTT-treated sample, no Z was observed and the XC decreased to 0.06, showing that 2 mM DTT is necessary to block the conversion of V into A and Z under HL conditions. Furthermore, the induced samples were characterized by a slightly elevated Chl a/b ratio as compared to the LL sample (Table 1), and the content of both lutein and β-carotene increased. The size of the XC pool in other samples increased compared to the LL sample. However, there were no significant alterations in LL-DCCD-treated samples with regard to the ratio of β-carotenoid to Chl content, lutein content, the size of the XC pool and the de-epoxidation index of XC pigments. These data showed that the XC participates in the NPQ mechanism and ΔpH play an important role in xanthophyll conversion.
Discussion
Non-photochemical fluorescence quenching is a very complex and finely feed-back regulated process and its components play different roles in different oxygenic photosynthetic organisms. The discovery of novel regulators of light responses in photosynthetic organisms occurred during characterization of the NPQ process. In higher plants, the presence of a transthylakoid proton gradient (ΔpH) alone can induce NPQ and the obligatory role of de-epoxidized xanthophylls in NPQ remains controversial (Lavaud et al. 2002). In the green alga, C. reinhardtii, NPQ is essentially controlled by ΔpH and further depends on the action of xanthophylls. In the giant kelp, Macrocystis pyrifera, in contrast to higher plants, NPQ is activated by the amount of Z synthesized in HL conditions and the transthylakoid proton gradient seems to be related only to activation of violaxanthin de-epoxidase (García-Mendoza and Colombo-Pallotta 2007). In this study, NPQ kinetics in the sea ice alga, Chlamydomonas sp. ICE-L, were studied using several inhibitors to determine the function of ΔpH and the XC in the NPQ process.
The formation of a transthylakoid proton gradient is essential for NPQ, and disruption of this gradient immediately relaxes the fluorescence quenching formed in HL. NH4Cl and nigericin are well-known uncouplers which decrease ΔpH formed during illumination. Decreased NPQ values following the addition of these uncouplers were detected in Chlamydomonas sp. ICE-L, indicating that NH4Cl and nigericin disrupted ΔpH and induced a reduction in NPQ values. A large proportion of NPQ can be inhibited by 1 mM NH4Cl and Z was not detected in NH4Cl-cultured samples, suggesting that in Chlamydomonas sp. ICE-L NPQ is essentially controlled by ΔpH, and activation of the XC may be related to the formation of ΔpH.
At low concentrations, DCCD is an inhibitor of ATP synthase (Lavaud et al. 2002), which blocks the proton conductive channel, and its presence should increase ΔpH formed during low illumination. Low light intensity of 131 μmol photons m−2 s−1 did not induce high NPQ and Z synthesis in the absence of DCCD, while in the presence of DCCD, NPQ increased and Z was detected (Table 1). On the other hand, DCCD-treated cells under HL conditions inhibited the release of protons into the lumen (Fig. 3) as NPQ and Z content had decreased compared to HL-induced samples. These results showed that some PSII proteins are sensors of lumen acidification, as supported by the presence of protonatable DCCD-binding residues. In plants, pH transduction is operated by PsbS through the protonation of two glutamate residues (Li et al. 2004), and C. reinhardtii LhcSR3 has protonatable DCCD-binding sites which are induced by low lumen pH and are essential for energy quenching (qE) (Bonente et al. 2012). Two LHCSR proteins have been identified in Chlamydomonas sp. ICE-L (Mou et al. 2012) and may be sensors of lumen pH, which is a function of PsbS in plants. These results demonstrate that a build up of ΔpH induces the protonation of PSII proteins and NPQ is mainly dependent on ΔpH which may trigger de-epoxidation of the XC in the Antarctic sea ice alga Chlamydomonas sp. ICE-L.
Dithiothreitol has been used to detect Z-independent fluorescence quenching in higher plants and algae (Gilmore and Yamamoto 1991). In this study, algal cells were pre-incubated with increasing concentrations of DTT (up to 8 mM) and NPQ was determined at the end of the illumination period. As shown in Fig. 4, NPQ decreased when DTT concentrations increased. Z was not detected in 2 mM DTT-treated samples and NPQ decreased to 0.5. These results clearly demonstrated the obligatory presence of Z for NPQ in Chlamydomonas sp. ICE-L, and that NPQ can still be formed in the absence of Z. When DTT was incorporated with NH4Cl in cultured cells, NPQ kinetics decreased before NH4Cl was added and then decreased to approximately 0, showing that NPQ was partially suppressed by DTT which was related to the XC and the remainder was dependent on ΔpH. ΔpH affects not only the activation of NPQ, but also de-epoxidation of the XC in causing NPQ. We conclude that NPQ in Chlamydomonas sp. ICE-L is triggered by ΔpH and further depends on xanthophylls, and this mechanism is similar to that in higher plants.
The NPQ regulators in the Antarctic sea ice alga Chlamydomonas sp. ICE-L were identified in the present study. qE is mainly dependent on lumen pH in this alga, as clearly shown by its sensitivity to uncouplers and the inhibition of NPQ by DCCD. Chlamydomonas sp. ICE-L can regulate thermal dissipation of excess absorbed light energy in seconds to minutes. Furthermore, the xanthophyll pigments, lutein, and β-carotene are essential for NPQ. Therefore, the mechanism of NPQ in Chlamydomonas sp. ICE-L involves feedback energy dissipation which is triggered by low lumen pH which then triggers the protonation of PSII proteins and de-epoxidation of the XC, and then Z and other accumulated pigments are correlated with NPQ amplitude. This photoprotection process, similar to that in higher plants, may be related to the HL conditions found in the Antarctic region.
References
Alboresi A, Gerotto C, Giacometti GM, Bassia R, Morosinotto T (2010) Physcomitrella patens mutants affected on heat dissipation clarify the evolution of photoprotection mechanisms upon land colonization. PNAS 107(24):11128–11133
Bajkán S, Várkonyi Z, Lehoczki E (2011) Comparative study on energy partitioning in photosystem II of two Arabidopsis thaliana mutants with reduced non-photochemical quenching capacity. Acta Physiol Plant. doi:10.1007/s11738-011-0899-1
Betterle N, Ballottari M, Zorzan S, de Bianchi S, Cazzaniga S, Dall’Osto L, Morosinotto T, Bassi R (2009) Light-induced dissociation of an antenna hetero-oligomer is needed for non-photochemical quenching induction. J Biol Chem 284:15255–15266
Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25:173–185
Bonente G, Ballottari M, Truong TB, Morosinotto T, Ahn TK, Fleming GR, Niyogi KK, Bassi R (2012) Analysis of LhcSR3, a protein essential for feedback de-excitation in the green alga Chlamydomonas reinhardtii. PLoS Biol 9(1):e1000577
Casper-Lindley C, Björkman O (1998) Fluorescence quenching in four unicellular algae with different light-harvesting and xanthophyll-cycle pigments. Photosynth Res 56:277–289
Dall’Osto L, Caffari S, Bassi R (2005) A mechanism of non-photochemical energy dissipation, independent from PsbS, revealed by a conformational change in the antenna protein CP26. Plant Cell 17:1217–1232
Demmig-Adams B, Adams WW (1996) The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci 1:21–26
Demmig-Adams B, Adams WW III, Czygan F-C, Schreiber U, Lange OL (1990) Differences in the capacity for radiationless energy dissipation in green and blue-green algal lichens associated with differences in carotenoid composition. Planta 180:582–589
Demmig-Adams B, Ebbert V, Mellman DL, Mueh KE, Schaffer L, Funk C, Zarter CR, Adamska I, Jansson S, Adams III WW (2006) Modulation of PsbS and flexible vs sustained energy dissipation by light environment in different species. Physiol Plantarum 127:670–680
Depauw FA, Rogato A, d’Alcalá MR, Falciatore A (2012) Exploring the molecular basis of responses to light in marine diatoms. J Exp Bot 63(4):1575–1591
García-Mendoza E, Colombo-Pallotta MF (2007) The giant kelp Macrocystis pyrifera presents a different nonphotochemical quenching control than higher plants. New Phytol 173:526–536
García-Plazaola JI, Esteban R, Fernández-Marín B, Kranner I, Porcar-Castell A (2012) Thermal energy dissipation and xanthophyll cycles beyond the Arabidopsis model. Photosynth Res. doi:10.1007/s11120-012-9760-7
Gilmore AM, Yamamoto HY (1991) Zeaxanthin formation and energy-dependent fluorescence quenching in pea chloroplasts under artificially mediated linear and cyclic electron transport. Plant Physiol 96:635–643
Goss R, Jakob T (2010) Regulation and function of xanthophylls cycle-dependent photoprotection in algae. Photosynth Res 106:103–122
Horton P, Ruban AV (2005) Molecular design of the photosystem II light-harvesting antenna: photosynthesis and photoprotection. J Exp Bot 56(411):365–373
Horton P, Ruban AV, Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47:665–684
Horton P, Johnson MP, Perez-Bueno ML, Kiss AZ, Ruban AV (2008) Photosynthetic acclimation: does the dynamic structure and macro-organisation of photosystem II in higher plant grana membranes regulate light harvesting states? FEBS J 275:1069–1079
Johnson MP, Ruban AV (2011) Restoration of rapidly reversible photoprotective energy dissipation in the absence of PsbS protein by enhanced DeltapH. J Biol Chem 286(22):19973–19981
Johnson MP, Goral TK, DuVy CDP, Brain APR, Mullineaux CW, Ruban AV (2011) Photoprotective energy dissipation involves the reorganization of photosystem II light harvesting complexes in the grana membranes of higher plant chloroplasts. Plant Cell 23:1468–1479
Kan GF, Zheng Z, Jiang YH, Miao JL, Zhang BT, Li GY (2006) Salt resistance of Antarctic ice microalga Chlamydomonas sp. J Fish Sci China 13:73–78
Lavaud J, Rousseau B, Etienne AL (2002) In diatoms, a transthylakoid proton gradient alone is not sufficient to induce a non-photochemical fluorescence quenching. FEBS Lett 523:163–166
Lepetit B, Goss R, Jakob T, Wilhelm C (2012) Molecular dynamics of the diatom thylakoid membrane under different light conditions. Photosynth Res 111:245–257
Li XP, Bjorkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403:391–395
Li XP, Gilmore AM, Caffarri S, Bassi R, Golan T et al (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279:22866–22874
Li Z, Wakao S, Fischer BB, Niyogi KK (2009) Sensing and responding to excess light. Annu Rev Plant Biol 60:239–260
Liu C, Huang X, Wang X, Zhang X, Li G (2006) Phylogenetic studies on two strains of Antarctic ice algae based on morphological and molecular characteristics. Phycologia 45(2):190–198
Mou S, Zhang X, Ye N, Dong M, Liang C, Liang Q, Miao J, Xu D, Zheng Z (2012) Cloning and expression analysis of two different LhcSR genes involved in stress adaptation in an Antarctic microalga, Chlamydomonas sp. ICE-L. Extremophiles 16(2):193–203
Müller P, Li XP, Niyogi K (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Nilkens M, Kress E, Lambrev P, Miloslavina Y, Müller M, Holzwarth AR, Jahns P (2010) Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochim Biophys Acta 1797:466–475
Peers G, Truong TB, Ostendorf E, Busch A, Elrad D, Grossman AR, Hippler M, Niyogi KK (2009) An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462:518–521
Provasoli L (1968) Media and prospects for the cultivation ofmarine algae. In: Watanabe A, Hattori R (eds) Culture and collections of algae. Proceedings of US–Japan Conference, Hakone, Japan, pp 63–95
Zapata M, Rodríguez F, Garrido JL (2000) Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar Ecol Progr 195:29–45
Zhang QY, Wang LY, Kong FY, Deng YS, Li B, Meng QW (2012) Constitutive accumulation of zeaxanthin in tomato alleviates salt stress-induced photoinhibition and photooxidation. Physiol Plantarum 146:363–373
Acknowledgments
This work was supported by National Natural Science Foundation of China (31200187, 31200272, 41176153), Hi-Tech Research and Development Program (863) of China (2012AA052103), Shandong Science and Technology plan project (2011GHY11528), the Specialized Fund for the Basic Research Operating expenses Program (20603022012004), Natural Science Foundation of Shandong Province (2009ZRA02075), Qingdao Municipal Science and Technology plan project (11-3-1-5-hy), National Marine Public Welfare Research Project (200805069).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Driessen.
Rights and permissions
About this article
Cite this article
Mou, S., Zhang, X., Ye, N. et al. Analysis of ΔpH and the xanthophyll cycle in NPQ of the Antarctic sea ice alga Chlamydomonas sp. ICE-L. Extremophiles 17, 477–484 (2013). https://doi.org/10.1007/s00792-013-0532-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-013-0532-x