Abstract
Three strains were isolated from hydrocarbon-polluted alpine habitats and were representatives of Cryptococcus terreus (strain PB4) and Rhodotorula creatinivora (strains PB7, PB12). All three strains synthesized and accumulated glycogen (both acid- and alkali-soluble) and trehalose during growth in complex medium containing glucose as carbon source and in minimal salt medium (MSM) with phenol as sole carbon and energy source. C. terreus strain PB4 showed a lower total accumulation level of storage compounds and a lower extracellular polysaccharides (EPS) production than the two R. creatinivora strains, PB7 and PB12. Biofilm formation and phenol degradation by yeast strains attached to solid carriers of zeolite or filter sand were studied at 10°C. Phenol degradation by immobilized yeast strains was always higher on zeolite compared with filter sand under normal osmotic growth conditions. The transfer of cells immobilized on both solid supports to a high osmotic environment decreased phenol degradation activity by all strains. However, both R. creatinivora PB7 and PB12 strains maintained higher ability to degrade phenol compared with C. terreus strain PB4, which almost completely lost its phenol degradation activity. Moreover, R. creatinivora strain PB7 showed the highest ability to form biofilm on both carriers under high osmotic conditions of cultivation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenol is the most common representative of aromatic toxic environmental pollutants in a wide variety of wastewaters from different chemical industries. Among different methods for the removal of phenol and its homologues from polluted environments, biological treatment is generally preferable due to its effectiveness and the production of innocuous end products (Chang et al. 1998; Aleksieva et al. 2002). Some members of various yeast genera that can metabolize phenol as a sole energy and carbon source have been described in literature (Kurtz et al. 1997; Sampaio 1999; Juares-Ramirez et al. 2001). However, at high concentrations phenol inhibits microbial growth (Wase and Hough 1966; Zaicev 1995; Ruiz-Ordaz et.al. 1998). Immobilization of microorganisms and microbial biofilm formation on solid support can protect microorganisms from being damaged and maintain continuous cell growth and biodegradation. Contact of microbial cells to solid surfaces resulted in stimulation of exopolymers production by means of membrane-embedded signal-transmuting proteins, which are important components of the regulatory circuits controlling exopolymer synthesis as shown for several bacteria (Coplin and Cook 1990; Vandevivere and Kirchman 1993). Microbial extracellular polysaccharides (EPS) build up the biofilm matrix that can serve as protective barrier in which immediate direct contact between the highly toxic organic compound and the cells is minimized (Ehrhardt and Rehm 1985; Keweloh et al. 1989; Juares-Ramirez et al. 2001). Immobilized C. tropicalis cells were shown to have better phenol degradation rates and could be exposed without loss of viability to higher amounts of phenol compared with free cells (Juares-Ramirez et al. 2001).
The ability of microbial cells to accumulate carbon and energy reserve compounds (glycogen, polyhydroxyalkanoates and trehalose) also helps to cope with large fluctuation in external growth conditions. It is well known that in yeasts under conditions of nutrient starvation or entering into the stationary phase of growth, high temperature, osmotic and oxidative stress, glycogen and trehalose accumulation occurred as result in the activation of general stress response genes encoding, besides others, the proteins involved in regulation of glycogen metabolism and trehalose synthesis (Parrou et al. 1997; Hohmann 1997). For yeasts it has been shown that trehalose behaves as an important stress protectant against different stress factors during growth on non-fermentable carbon sources in the absence of glucose repression (Pardo et al. 1991; van Dijck et al. 1995; Garcia et al. 1997). Moreover, during subsequent oxidative growth trehalose could be utilized as an energy and carbon source for cell growth (Panek 1963; Thevelein 1984).
The progressive salinization of irrigated lands in the most productive areas of our planet made the understanding of the mechanism of salt tolerance utilized by yeasts rather important among other extreme environmental conditions. However, the effect of salt stress on yeast strains able to grow and multiply at low temperatures is poorly documented. Low temperature conditions greatly influence microbial conversion and degradation rates. Biodegradation in cold environments requires the activity of cold-adapted microorganisms (Margesin et al. 2003). The isolation of phenol-degrading yeast C. tropicalis and bacterium Alcaligenes faecalis using co-adaptation to salt stress resulted in higher resistance of yeast cells to phenol and salt concentration (16 mM and 15%, respectively) compared with bacterial cells (12 mM and 5.6%, respectively) (Bastos et al. 2000).
This paper reports on the ability of three phenol-degrading cold-tolerant yeast strains isolated from hydrocarbon-polluted alpine habitats to accumulate storage compounds and to form biofilm under salt stress conditions. The impact of high osmotic conditions on the phenol-degrading activity of suspended cells and cells attached to the surface of solid carriers was examined together with the ability of the yeast cells to form biofilm and to produce intracellular storage compounds and exopolysaccharides.
Materials and methods
Yeast strains and culture conditions
The yeast strains used in this study (Table 1) were isolated as described from an alpine oil-shale mine in Seefeld, Austria and a railway area at the Brenner pass in Austria (Bergauer et al. 2005). Mean annual air temperatures at sampling sites were 2.8–4.3°C. Isolation and enrichment procedure were carried out at 10°C. Assimilation tests and sequencing of the ribosomal ITS region revealed that all three strains were basidiomycetous yeasts. Strain PB4 was identified as C. terreus, whereas strains PB7 and PB12 were identified as R. creatinivora (Bergauer et al. 2005). All three strains were able to grow from 1 to 25°C in complex medium and in minimal salt medium (MSM) supplemented with phenol, and could thus be classified as cold-tolerant (facultatively psychrophilic). They utilized a number of monoaromatic compounds as the sole carbon source, such as phenol, catechol, resorcinol, hydroquinone, benzoate, salicylate (200 mg l−1). Guaiacol, o-cresol, o-nitrophenol, ethylbenzene and all three xylene isomers were toxic. The strains were maintained at 4°C on nutrient broth agar plates.
Effect of phenol on storage compounds accumulation
The effect of phenol on storage compounds accumulation was determined under aerobic conditions using two-stage batch cultivation of the yeast strains in 250-ml Erlenmeyer flasks containing 100 ml of culture medium on a rotary shake at 180 rev/min. The first stage cultivation was carried out over 48 h at 20°C in Rider medium with glucose (20 g l−1). For the second stage, biomass obtained from the first stage and washed with sterile 0.9% NaCl was cultivated for 24 h at 20°C in MSM medium containing phenol (1,000 mg l−1) as sole carbon and energy source.
Rider medium contained (g l−1): (NH4)2SO4 (3.3), MgSO4 7H2O (0.7), KH2PO4 (1), K2HPO4 (0.13), NaCl (0.5), yeast extract 0.3%, pH 5.5. MSM contained (g l−1): NaNO3 (3), MgSO4 7H20 (0.5), K2HPO4 (1), KCl (0.5), FeSO4 7H2O (0.01), pH 7.0. Both culture media were sterilised at 121°C for 20 min and afterwards supplemented with sterile-filtered glucose or phenol stock solutions, respectively.
At the end of cultivation the cells were harvested by centrifugation (4,000g, 10 min, 4°C) and washed twice with ice-cold 0.9% NaCl solution. The final pellet was frozen and stored at −20°C until analysed for trehalose and glycogen contents in yeast cells.
Precipitation of EPS was made from the cell-free supernatant of liquid cultures and after detachment from the surface of immobilized cells with two volumes of ice-cold ethanol overnight at 4°C.
Cell immobilization procedures
Biofilm formation and phenol degradation ability of the yeast strains were studied after attachment of the cells to the surface of sterile solid carriers differing in pore volume and material density (Table 2). Cultivation was performed at 10°C, the optimal temperature for efficient growth and phenol biodegradation by yeast strains isolated from alpine cold environments (Margesin et al. 2003; Bergauer et al. 2005).
Cells were immobilized on the surface of solid carriers by incubation of 1 ml of cell suspension (OD600 nm=0.65) with the carrier materials in 50 ml Falcon tubes during 3 h at room temperature. Removal of non-adherent cells was made by rinsing with phosphate-saline buffer (PSB). PSB contained (g l−1): KH2PO4 (0.34), Na2 HPO4 10 H2O (1.84), NaCl (8.5), pH=6.35. Yeast cells attached to the surface of both carriers were incubated at 10°C in Falcon tubes filled with MSM supplemented with phenol (200 mg l−1) as sole carbon source in a volume to carrier material ratio of 10:1. Biofilm formation was observed during incubation of yeast strains under normal (a w =0.998) and high osmotic stress conditions (a w =0.975 in presence of 0.7 M NaCl) in the same medium, whereby the MSM supplemented with 200 mg l−1 of phenol was renewed every 4 days, and the residual phenol concentration and viable cell numbers were quantified in the removed medium. After 2 weeks of incubation biofilm formation was visualized by scanning electron microscopy (SEM; Savenkova et al. 2002) and evaluated according to EPS formation by attached and suspended cells.
Extraction of extra- and intracellular metabolites
The EPS produced by free (floating) cells were precipitated from the cell-free supernatant of liquid cultures with two volumes of cold ethanol at 4°C overnight. The obtained EPS fraction was sedimented (9,000g, 10 min, at 4°C), the pellet was quantitatively transferred in Eppendorf tubes with a small amount of ethanol, centrifuged (6,000g, 3 min) and washed once with ethanol. The weight of the obtained EPS fraction was expressed as mg/ml of EPS wet weight and recalculated as milligram wet weight/gram dry weight of attached or suspended cells.
The EPS produced by cells attached to the surface of solid carrier were recovered by vortexing (2,400 rev/min) with 5 ml of PSB buffer for 1 min. After cooling in an ice bath, the vortex procedure was repeated, the carrier was rinsed with 1 ml of PSB buffer, and both volumes were combined. The EPS covalently bounded on cell surface were detached by sonication for 20 s at 62.5 W/cm2 after cells recovering from the surface of solid carriers by vortex procedure, sedimentation (4,000g, 10 min, 4°C), and suspension in 5 ml of PSB buffer. Total biofilm production was characterized as total values of the exopolymers attached to the surface of cells and capsular exopolymers.
For extraction of trehalose, fraction of yeast carbohydrates, 5.0 ml of yeast cell suspension in water containing 20–50 mg (w/w) of cell material was incubated with an equal volume of ice-cold 1 M TCA at 0°C for 60 min with occasional shaking according to Trevelyan and Harrison (1956). After sedimentation, the pellet was washed once with 3 ml of distilled water, both supernatants were combined, and the volume was made up to 10 ml by distilled water.
Glycogen was extracted according to the method of Francois et al. (1991). A suspension of yeast cells corresponding to 50 mg of wet weight was sedimented at 4,000g during 10 min and washed once with ice-cold 0.85% NaCl solution. The cell sediment was suspended in 1 ml of 0.25 M Na2CO3 and incubated for 2 h at 90°C with occasional shaking. This resulted in the extraction of alkali-soluble glycogen. Additional extraction of acid-soluble glycogen was made by treatment with 0.5 M HClO4 for 30 min at 100°C. Glycogen production was expressed as the sum of both alkali- and acid-soluble glycogen fractions. All metabolite extracts were stored at −20°C until analysed.
Analytical procedures
The number of attached cells was monitored by colony-forming units (CFUs) on nutrient agar using dilute plate counts. Portions (100 μl) of cell suspensions (detached from the surface of carriers) in sterile PSB buffer were appropriately diluted and spread onto R2A agar plates. The plates were incubated at room temperature over 48 h and CFUs were counted.
Phenol concentration was determined quantitatively by a colorimetric method, using 4-aminoantipyrine as colour reagent. The analysis was performed according to the procedure described by Grinberg et al. (1992).
The EPS precipitated from growth medium and removed from the surface of yeast cells were determined by the total carbohydrate content using the phenol sulphuric method (Herbert et al. 1971). Total carbohydrate content was calculated on the base of a glucose standard curve and expressed as glucose equivalents.
Intracellular glycogen and trehalose contents were determined by measuring the glucose content liberated during extraction according to the anthrone method (Stewart 1975). Glucose standard was used for the anthrone test, and the contents of extracted trehalose and glycogen were expressed as glucose equivalents. Total glycogen was expressed as sum of alkali- and HClO4-soluble forms of glycogen.
Water activity was calculated according to Gerhardt (1981) after measurement of osmomolarity of growth medium solution with a micro-osmometer (Type 5R, Herman Roebling, Germany).
Reproducibility of results
Experiments were generally performed at least in duplicate or triplicate with consistent, highly reproducible results, the standard deviations obtained were ≤10%. The mean values from replicate experiments are presented in this study.
Results
Storage compounds accumulation and EPS content in yeast cells after growth in Rider medium with glucose as carbon source
The ability of three cold-adapted yeast strains for storage products accumulation and EPS production (Table 3) was examined at 20°C in complex Rider medium containing glucose (20 g 1−1) after cells had reached the late stationary phase of growth. For this cultivation stage, a submaximal growth temperature and a carbon source that can be easily metabolized by yeasts were chosen in order to create favourable conditions for intracellular accumulation of storage compounds.
The results obtained showed that all studied yeast strains were able to synthesize and accumulate glycogen (both acid- and alkali-soluble) and trehalose during growth in complex medium. The level of accumulation of the two storage compounds varied between the strains. The lowest trehalose production was shown for C. terreus strain PB4; the two R. creatinivora strains PB7 and PB12 contained about two- to fourfold higher amount of this compound. Fast growing R. creatinivora strain PB7 presented the lowest level of glycogen. C. terreus strain PB4 and R. creatinivora strain PB12 showed a considerably higher accumulation level of glycogen compared with R. creatinivora strain PB7 (Table 3). R. creatinivora strain PB12 presented also the highest intracellular content of both storage compounds. The production of EPS was highest by R. creatinivora strain PB7 and lowest by C. terreus strain PB4. Thus, a lower total accumulation level of storage compounds and a lower EPS production than the two R. creatinivora strains characterized C. terreus strain PB4. A correlation between EPS production and the accumulation of storage compounds by the three yeast strains was not detected. However, for R. creatinivora strain PB7 maximal EPS production correlated with high biomass yield. Of course, EPS quantification as indicated in Table 3 is not a precise characteristic because the ratio of milligram wet weight of EPS produced by 1 g of dry cells is affected by the polysaccharide chemical structure, chain length, degree of hydration and so on.
Storage compounds accumulation and EPS content in yeast cells after growth in MSM with phenol as sole carbon source
In this set of experiments the influence of phenol as sole carbon source on the level of intracellular storage compounds and EPS production was evaluated. Transition of the yeast cells from first stage of cultivation to MSM with a phenol/biomass ratio of about 100 mg phenol g−1 of dry weight of biomass (second stage of cultivation) resulted in an increase of the intracellular content of glycogen for all three strains, with a more than threefold increase for R. creatinivora strain PB7. The intracellular trehalose level was considerably decreased for both R. creatinivora strains PB7 and PB12, since values of 52 and 29%, respectively, were obtained as compared with the first stage of cultivation. On the contrary, C. terreus strain PB4 showed an increase in intracellular trehalose content by 59% compared with the first stage of incubation (Table 4).
The EPS formation was remarkably decreased for all three strains after the change of the carbon source, especially for strains R. creatinivora PB7 and C. terreus PB4, their EPS formation was decreased by 89 and 75%, respectively. Contrary to the first stage of cultivation, the tendency of an inverse relation between EPS production and intracellular content of trehalose was observed when comparing both parameters for the three strains, that is, strains producing high amounts of EPS accumulated low amounts of trehalose and vice versa. During 24 h of incubation, all three strains showed complete phenol removal from the growth medium.
Growth and phenol removal by immobilized yeast cells
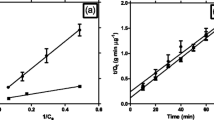
Yeast cells were immobilized on the surface of two solid carriers, filter sand and zeolite, differing in particle size and pore volume. The effect of cell immobilization on the surface of the two carriers on growth and phenol degradation was investigated under normal (a w =0.998) or high osmotic conditions (a w =0.975). The immobilized cells were incubated in MSM with 200 mg phenol l−1 over a total period of 12 days at 10°C, whereby the medium with phenol was renewed every 4 days, and the residual phenol concentration and viable cell numbers were determined in the removed medium. Thus, Figs. 1 and 2 show data obtained after first (0–4 days), second (4–8 days) and third (8–12 days) supplementations with fresh medium.
Phenol removal and yeast cells growth in MSM with 200 mg phenol l−1 phenol under high osmotic conditions after immobilization on the surface of zeolite (a) and filter sand (b). The medium was renewed every 4 days, and the residual phenol concentration and viable cell numbers were determined in the removed medium
All three studied strains possessed different levels of growth and phenol degradation under normal osmotic conditions of incubation (Fig. 1). Phenol degradation was always higher when cells were immobilized on zeolite compared with filter sand. Increasing of CFUs of viable cell numbers was only observed during the first and second supplementation with phenol for cells attached to both carriers. After the third supplementation (8–12 days), the cell numbers tended to decrease, which also resulted in lower phenol removal (Figs. 1, 2). These results showed that both the peculiarities of each strain and the nature of carriers affected phenol removal and the ability of the cells to grow and produce biomass.
The transfer of yeasts to a more saline environment gradually decreased phenol removal by all three strains immobilized on both carriers (Fig. 2). In spite of osmotic stress conditions and low uptake of carbon source, the CFUs for all strains immobilized on zeolite and R. creatinovora PB7 immobilized on filter sand increased at least during the first two supplementations with fresh medium (0–8 days of incubation). Longer incubation resulted in a decrease of cell numbers with exception of C. terreus strain PB4 on zeolite, where cell numbers even increased over the whole 12-day incubation period. As already observed under normal conditions, the decrease of viable cells number resulted in decreased phenol removal with time. After the third supplementation with phenol, phenol removal efficiency was generally lower than after the first and second supplementations.
The absence of a correlation between phenol removal from the incubation medium and microbial cell numbers could be explained by the ability of the cells to utilize intracellular storage compounds as carbon and energy source for biomass formation, especially under high osmotic conditions where the uptake of phenol was extremely low.
Biofilm formation was also examined by SEM microscopic examination, which revealed differences not only in the structure of the surface of carriers used for immobilization, but also in the ability of yeast strains to form a biofilm. The SEM image demonstrated a more intensive biofilm formation for two selected strains (C. terreus strain PB4 and R. creatinivora strain PB7) on the surface of zeolite compared with sand. Both strains presented a high ability to form cells surrounding extracellular polymeric substance (results not shown).
EPS production by immobilized yeast cells
The EPS fraction from the biofilm was extracted and quantitatively determined for all three investigated yeast strains (Fig. 3). The EPS production by attached cells was always higher on zeolite as a solid support compared with filter sand under normal osmotic growth conditions. The transfer of yeasts to a more saline environment resulted in a different response depending on the nature of the solid carrier, cells state (attached or free floating) and strains peculiarities. Decreased EPS formation by cells immobilized on zeolite was only observed for C. terreus strain PB4, whereas EPS production by the two R. creatinivora strains remained unchanged or was slightly increased. The immobilization on filter sand under high osmotic conditions resulted in an increase of EPS synthesis only for R. creatinivora strain PB7, whereas the EPS level of the other two strains was decreased.
The highest ability to form biofilm according to total EPS production was shown for C. terreus strain PB4 under normal conditions of cultivation on zeolite, whereas the two R. creatinivora strains PB7 and PB12 showed a better resistance to high osmotic conditions.
Discussion
In this study, we investigated the relationship between biofilm formation and storage compounds accumulation by cold-tolerant yeast cells under normal and unfavourable high osmotic environmental conditions, and the ability of the yeast strains to degrade phenol, which is an aromatic toxic environmental pollutant occurring in most waste waters from different chemical industries.
One of the more convenient ways to overcome the influence of unfavourable environmental factors is the isolation and optimization of selected indigenous microbial strains suitable for selected conditions of application. The studied yeast strains originated from high alpine environments that are characterized by dramatic seasonal shifts in climatic conditions, physical and biochemical properties. Due to the source of isolation (railway area, oil-shale mine) the strains were adapted to phenol contamination and were able to utilize phenol aerobically as the sole carbon and energy source for growth (Bergauer et al. 2005). Microorganisms originating from cold habitats possess unique genes for the metabolization of different toxic environmental pollutants, including phenol, at low temperatures (Meyer et al. 2004; Margesin et al. 2005).
The data obtained in this study indicate that all tested yeast strains, representatives of C. terreus and R. creatinivora, were able to accumulate both trehalose and glycogen during growth on glucose or phenol as carbon and energy sources. Trehalose and glycogen are storage compounds, and can represent less than 1% or more than 23% of the dry weight of the cells, depending on the environmental conditions and the stage of the life cycle. Yeast cells with high intracellular concentration of reserve carbohydrate, for example trehalose, are tolerant to adverse environmental conditions (Wiemken 1990). Trehalose plays an important role for the preservation of yeast cells under extreme environmental conditions, such as high temperature, dehydration and high osmotic conditions. Under osmotic stress conditions trehalose together with glycerol is significant for the survival and growth by yeast cells acting as membrane protector, as a supplementary compatible solute or as a reserve carbohydrate that may be mobilized during stress (Hounsa et al. 1998). Both carbohydrates function as carbon and energy reserves, which could be used by cells also under conditions of starvation, respiratory adaptation and in vegetative cells during the stationary phase of growth. In general, activation of the proteins involved in regulation of glycogen metabolism and trehalose synthesis is a part of the general stress response mechanism of cells (Parrou et al. 1997; Hohmann 1997).
Dependent on the peculiarities of the strains, the accumulation level of intracellular storage compounds was different; R. creatinivora strain PB12 had the highest total content of trehalose and glycogen in glucose-amended complex medium. The change of the carbon source from glucose to phenol resulted in a remarkable increase of the intracellular content of glycogen for the two R. creatinivora strains PB7 and PB12, but the accumulation of trehalose by these two strains was decreased. Only, C. terreus strain PB4 showed increased trehalose accumulation compared with the first stage of incubation in medium with glucose.
The production of EPS is a characteristic feature of many adherent biofilms. The EPS are believed to assist not only in the attachment process but also to protect the underlying cells from fluctuation in the surrounding environment, including desiccation, penetration of antibiotics, biocides, poisoning components (Nichols et al. 1989) and heavy metals (Allison 1993). Immobilization of microorganisms and its related stimulation of EPS production is necessary for protection from being damaged and to maintain continuous cell growth and phenol degradation under conditions of toxic high phenol concentration (Chung et al. 1998).
The change of the carbon source influenced EPS synthesis by the studied strains to a different extent. In spite of decreased EPS production by all three strains during the second stage of cultivation R. creatinivora strain PB12 presented higher ability to maintain EPS production compared with other strains (only 40% decrease compared with first stage of cultivation). This coincides with the higher total content of intracellular storage compounds during the first stage of incubation by this strain and could be also connected with the remarkable decrease of the intracellular trehalose level by about 70%. According to our data, the intracellular content of trehalose was inversely connected with EPS synthesis. The amount of EPS depends greatly on the availability of carbon substrates (both inside and outside the cell) and on the balance between carbon and other limiting nutrients (Sutherland 2001). Trehalose as endogenous disaccharide accumulated in yeast cells during stationary phase of growth on fermentable carbon substrates was considered as a potential carbon source for fungi under various stressing and/or oligotrophic conditions (Thevelein 1984). Based on the data obtained in our study we assume that the intracellular trehalose pool could be used for maintenance of EPS synthesis during incubation of cells in MSM with phenol, but it depends also on the peculiarities of each strain.
The ability of the studied cold-adapted yeast strains to accumulate high concentrations of intracellular storage compounds and synthesize EPS could allow them to overcome negative environmental conditions and to form biofilm. Under normal osmotic conditions in MSM with 200 mg phenol l−1 better biofilm formation was observed for all strains during immobilization on zeolite. The C. terreus strain PB4 was more active in biofilm formation on both carriers than the other strains, but this strain was not the best phenol degrader, possibly because of the formation of a rich exopolysaccharide layer, which decreased the diffusion of phenol to the cell surface. The two R. creatinivora strains, PB7 and PB12, showed a better phenol degrading activity than C. terreus strain PB4. They also were able to form biofilm in phenol-containing medium under high osmotic conditions. The ability of both R. creatinivora strains, especially strain PB12, to accumulate high amounts of trehalose under favourable cultivation conditions is the base for subsequent EPS synthesis and biofilm formation in presence of phenol under oligotrophic and high osmotic growth conditions. The active accumulation of storage compounds could possibly enable characteristic properties of members of R. creatinivora, that is, to produce high amounts of biomass, to degrade a broad spectrum of monoaromatic compounds and to grow in the presence of high concentrations of pollutants under oligotrophic growth conditions (Bergauer et al. 2005). The data obtained in this study indicate cold-adapted yeast strains as promising candidates for the biological cleaning of phenol-contaminated soil, groundwater and wastewater in climates, where low temperatures can otherwise limit microbial degradation.
References
Aleksieva Z, Ivanova D, Godjevargova T, Atanasov B (2002) Degradation of some phenol derivates by Trichosporon cutaneum. Process Biochem 37:1215–1219
Allison DG (1993) Biofilm-associated exopolysaccharides. Microbiol Eur 1:16–19
Bastos AE, Moon DH, Rossi A, Trevors JT, Tsai SM (2000) Salt-tolerant phenol-degrading microorganisms isolated from Amazonian soil samples. Arch Microbiol 174:346–352
Bergauer P, Fonteyne PA, Nolard N, Schinner F, Margesin R (2005) Biodegradation of phenol and phenol-related compounds by psychrophilic and cold-tolerant alpine yeasts. Chemosphere 59:909–918
Chang YH, Li CT, Chang MC (1998) Batch phenol degradation by Candida tropicalis and its fusant Biotechnol Bioeng 60:391–395
Coplin DL, Cook D (1990) Molecular genetics of extracellular polysaccharides biosynthesis in vascular phytopathogenic bacteria. Mol Plant Microbe Interact 3:271–279
Chung TS, Loh KC, Goh SK (1998) Development of cellulose acetate membrane for bacteria immobilization to remove phenol. J Appl Polym Sci 68:1677–1688
Ehrhardt HM, Rehm HJ (1985) Phenol degradation by microorganisms adsorbed on activated carbon. Appl Microbiol Biotechnol 21:32–36
Francois J, Neves MJ, Hers HG (1991) The control of trehalose biosynthesis in Saccharomyces cerevisiae: evidence for a catabolic inactivation and repression of trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase. Yeast 7:575–587
Garcia MJ, Rios G, Ali R, Belles JM, Serrano R (1997) Comparative physiology of salt tolerance in Candida tropicalis and Saccharomyces cerevisiae. Microbiology 143:1125–1131
Gerhardt P (ed) (1981) Manual of methods for general bacteriology. American Society for Microbiology, WA, pp173–174
Grinberg AE, Clesceri LS, Eaton AD (eds) (1992) Phenols. In: Standard methods for the examination of water and wastewater, 18th edn. WA, pp5-30–5-33
Herbert D, Phipps PJ, Strange RE (1971) Chemical analysis of microbial cells. In: Norris JR, Ribbons DW (eds) Methods in microbiology, vol 5B. Academic Press, NY, pp210–344
Hohmann S (1997) Shaping up: the response of yeast to osmotic stress. In: Hohmann S, Mager WH (eds) Yeast stress responses. RG Landes Company, Austin TX, pp 101–145
Hounsa CG, Brandt EV, Thevelein J, Hohmann S, Prior BA (1998) Role of trehalose in survival of Saccharomyces cerevisiae under osmotic stress. Microbiology 144(Pt 3):671–680
Juares-Ramirez C, Ruiz-Ordaz N, Cristiani-Urbina E, Galindez-Mayer J (2001) Degradation kinetics of phenol by immobilized cells of Candida tropicalis in a fluidised bed reactor. World J Microbiol Biotechnol 17:697–705
Keweloh H, Heipieper HJ, Rehm HJ (1989) Protection of bacteria against toxicity by immobilization in calcium alginate. Appl Microbiol Biotechnol 31:383–389
Kurtz A, Crow SA Jr (1997) Transformation of chlororesorcinol by the hydrocarbonoclastic yeasts Candida maltosa, Candida tropicalis and Trichosporon iovide. Curr Microbiol 35:165–168
Margesin R, Gander S, Zacke G, Gounot AM, Schinner F (2003) Hydrocarbon degradation and enzyme activities of cold-adapted bacteria and yeasts. Extremophiles 7:451–458
Margesin R, Fonteyne PA, Redl B (2005) Low temperature biodegradation of high amounts of phenol by Rhodococcus spp. and basidiomycetous yeasts. Res Microbiol 156:68–75
Meyer AF, Lipson DA, Martin AP, Schadt CW, Schmidt SK (2004) Molecular and metabolic characterization of cold-tolerant alpine soil Pseudomonas sensu stricto. Appl Environ Microbiol 70:483–489
Nichols WW, Evans MJ, Slack MPE, Walmsley HL (1989) The penetration of antibiotics into aggregates of mucoid and non-mucoid Pseudomonas aeruginosa. J Gen Microbiol 135:1291–1303
Panek A (1963) Function of trehalose in baker’s yeast (Saccharomyces cerevisiae). Arch Biochem Biophys 100:422–425
Pardo C, Lapena MA, Gacto M (1991) On the role of trehalose in yeast cells subjected to hyper osmotic shock. Microbiologia 7:42–48
Parrou JL, Teste MA, Francois J (1997) Effect of various types of stress on the metabolism of reserve carbohydrates in Saccharomyces cerevisiae: genetic evidence for a stress-induced recycling of glycogen and trehalose. Microbiology 143:1891–1900
Ruiz-Ordaz N, Hernandez-Manzano E, Ruiz-Lagunez JC, Cristiani-Urbina E, Galindez-Mayer J (1998) Growth kinetic model that describes the inhibitory and lytic effects of phenol on Candida tropicalis yeast. Biotechnol Prog 14:966–969
Sampaio JP (1999) Utilization of low molecular weight aromatic compounds by heterobasidiomycetous yeasts: taxonomic implications. Can J Microbiol 45:491–512
Savenkova L, Gercberga Z, Muter O, Nikolaeva V, Dzene A, Tupureina V (2002) PHB-based films as matrices for pesticides. Process Biochem 37:719–722
Stewart PR (1975) Analytical methods for yeasts. In: Prescott DM (ed) Methods of cell biology, vol 12. Academic Press, NY, pp111–147
Sutherland I (2001) Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147:3–9
Thevelein JM (1984) Regulation of trehalose mobilization in fungi. Microbiol Rev 48:42–59
Trevelyan WE, Harrison JS (1956) Studies on yeast metabolism 7 Yeast carbohydrate fraction Separation from nucleic acid, analysis and behaviour during anaerobic fermentation. Biochem J 63:23–33
Vandevivere P, Kirchman DL (1993) Attachment stimulates exopolysaccharide synthesis by a bacterium. Appl Environ Microbiol 59:3280–3286
van Dijck P, Colavizza D, Smet P, Thevelein JM (1995) Differential importance of trehalose in stress resistance in fermenting and nonfermenting Saccharomyces cerevisiae cells. Appl Environ Microbiol 61:109–115
Wase DAJ, Hough JS (1966) Continuous culture yeast on phenol. J Gen Microbiol 42:13–23
Wiemken A (1990) Trehalose in yeast, stress protectants rather than reserve carbohydrate. Antonie Van Leeuwenhoek 58:209–217
Zaicev GM (1995) Utilization of halogenated benzenes, phenols and benzoates by Rhodococcus opacus GM-14. Appl Environ Microbiol 61:4191–4193
Acknowledgement
This work was funded by fifth framework programme in a frame of EC project QLRT-2000-00163 “Multifunctional permeable barriers carrying well-performing microbial biofilms for treatment of mixed pollutant plumes.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Horikoshi
Rights and permissions
About this article
Cite this article
Krallish, I., Gonta, S., Savenkova, L. et al. Phenol degradation by immobilized cold-adapted yeast strains of Cryptococcus terreus and Rhodotorula creatinivora . Extremophiles 10, 441–449 (2006). https://doi.org/10.1007/s00792-006-0517-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-006-0517-0