Abstract
Thermostable polymers cast as thin, porous coatings or membranes may be useful for concentrating and stabilizing hyperthermophilic microorganisms as biocatalysts. Hydrogel matricies can be unstable above 65°C. Therefore a 55-μm thick, two layer (cell coat + polymer top coat) bimodal, adhesive latex coating of partially coalesced polystyrene particles was investigated at 80°C using Thermotoga maritima as a model hyperthermophile. Coating permeability (pore structure) was critical for maintaining T. maritima viability. The permeability of bimodal coatings generated from 0.8 v/v of a suspension of non-film-forming 800 nm polystyrene particles with high glass transition temperature (Tg= 94°C, 26.9% total solids) blended with 0.2 v/v of a suspension of film-forming 158 nm polyacrylate/styrene particles (Tg≈ −5°C, 40.9% total solids) with 0.3 g sucrose/g latex was measured in a KNO3 diffusion cell. Diffusivity ratio remained above 0.04 (Deff/D) when incubated at 80°C in artificial seawater (ASW) for 5 days. KNO3 permeability was corroborated by cryogenic-SEM images of the pore structure. In contrast, the permeability of a mono-dispersed acrylate/vinyl acetate latex Rovace SF091 (Tg~10°C) rapidly decreased and became impermeable after 2 days incubation in ASW at 80°C. Thermotoga maritima were entrapped in these coatings at a cell density of 49 g cell wet weight/liter of coating volume, 25-fold higher than the density in liquid culture. Viable T. maritima were released from single-layer coatings at 80°C but accurate measurement of the percentage of viable entrapped cells by plate counting was not successful. Metabolic activity could be measured in bilayer coatings by utilization of glucose and maltose, which was identical for latex-entrapped and suspended cells. Starch was hydrolyzed for 200 h by latex-entrapped cells due to the slow diffusion of starch through the polymer top coat compared to only 24 h by suspended T. maritima. The observed reactivity and stability of these coatings was surprising since cryo-SEM images suggested that the smaller low Tg polyacrylate/styrene particles preferentially bound to the T. maritima toga-sheath during coat formation. This model system may be useful for concentrating, entrapment and stabilization of metabolically active hyperthermophiles at 80°C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine hyperthermophiles are being investigated as a source of numerous thermostable enzymes, for biotransformations, and for the production of novel carotenoids (Pantazaki et al. 2002; Hicks and Kelly 1999). However, marine microorganisms capable of growth at extreme temperatures in acidic or alkaline environments may themselves be useful biocatalysts for multi-step oxidations, reductions, for the production of acids, generation of H2, or as a stable form of membrane-associated enzymes. In particular, the ‘toga’ protein sheath of Thermotoga species, which can increase the cell membrane area several-fold, may be a useful extra-cellular platform for membrane-associated biocatalysis. Development of processes that achieve faster reaction rates with lower cooling costs by using hyperthermophiles as whole-cell biocatalysts have so far not been realized due to a lack of methods to concentrate and stabilize their activity. There is a single report of using Pyrococcus furiosus for bioreductions (van den Ban et al. 1998), several reports of using Thermotoga species (Schröder et al. 1994; Van Ooteghem et al. 2002) and Caldicellulosiruptor saccharolyticus for production of H2 (Van Niel et al. 2003), and stabilization of Acetogenium kivui for one year for the formation of acetate from CO2/H2 (Rainina et al. 1994).
Although there are exceptions (Hicks and Kelly 1999; Holst et al. 1997; Adams 1995), many marine hyperthermophiles grow slowly, are sensitive to high shear rates, and only reach a low cell density in culture (108 to 6×109 cells/ml, 4 g/l wet cell weight, <1.5 g/l cell dry weight) (Holst et al. 1997; Sharp and Raven 1997). Heterologous gene expression has been reported in Thermus thermophilus (see for example Park et al. 2004), however most hyperthermophiles are not yet amenable to genetic manipulation. Because of their low culture density and the lack of genetic methods to increase their specific reactivity, hyperthermophiles as biocatalysts have low volumetric productivities unless fed-batch, dialysis or cell-recycle methods are used to concentrate and extend their activity (Holst et al. 1997). Surprisingly, only thermostable poly(vinyl alcohol) cryogels (PVAG) have been evaluated as matrix materials to stabilize non-growing hyperthermophiles as biocatalysts (Rainina et al. 1994). Designing an adhesive matrix that can be cast as a thin coating or membrane with stable porosity at 80°C in high salt concentration to entrap hyperthermophiles without loss of metabolic activity, however, is a challenging composite porous film problem that has only recently begun to be investigated (Gebhard et al. 2004; Jons et al. 1999).
The limitations of soft hydrogel and some cryogel matricies for entrapment of viable microbial cells are well established: extreme mass transfer limitations; cell leakage; low specific reactivity; poor mechanical stability; significant manufacturing costs; and poor thermostability above 65°C (Webb and Devakos 1996; Wijffels 2001). Gel matricies that have been investigated for immobilization of thermophiles include: Caldariella acidophila in crude egg white (De Rosa et al. 1981), Sporotrichum thermophile in 2.5% agar (Singh et al. 1990), Bacillus stearothermophilus in 0.66% Gelrite and 0.66% alginic acid (Worden et al. 1991), and entrapment of Acetogenium kivui in PVAG at 65°C (Ryabokon et al. 1996; Rainina et al. 1994). Recently, immobilized Escherichia coli expressing Sulfolobus trehalosyl-dextrin forming enzymes was reported immobilized in an alginate matrix at 75°C, but the high temperature was used only for cell permeabilization and to inactivate unwanted host proteins; the cells were non-viable (Di Lernia et al. 2002; Schiraldi et al. 2003). No matricies have been reported for entrapment of viable microorganisms at temperatures >65°C. In addition, none of the porous matricies reported are capable of being cast into mechanically stable adhesive coatings or stand-alone membranes.
The above limitations can be overcome by devising porous thermostable latex polymer coatings and films containing ~50% by volume of viable cells (Lyngberg et al. 1999a; 1999b; 2001). The entrapment of viable microorganisms in latex coatings is dependent upon coating microstructure (nano-porosity) following coat drying, storage, and rehydration. The reactivity of these coatings (and when used as stand-alone films or membranes) is determined by their thinness (<100 μm), permeability, volume fraction of viable cells, and cellular-specific activity, which can be increased after film formation by gene induction. Reactive E. coli latex coatings have been stored dry or frozen for months and re-hydrated immediately prior to use with retention of viability, and the capability for mer-lux and lacZ expression under nitrogen starvation conditions (Lyngberg et al. 1999b; Swope and Flickinger 1996).
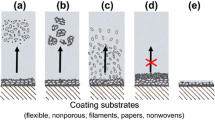
Nano-porous latex coatings cast entirely from low Tg acrylate/vinyl acetate lattices lose permeability due to wet coalescence (Solheid 2003; Lyngberg et al. 2001). In order to block polymer particle wet coalescence at hyperthermophilic temperatures (>60°C) hard polystyrene particles with high glass transition temperature, Tg (melting point >200°C, depending upon polymer molecular weight) could be used. However these latex particles by themselves are non-film forming under conditions compatible with entrapment of living cells. Polystyrene particles can form nano-porous coatings and films when coalesced with softer (low Tg) acrylate film-forming polymer particles (Fig. 1). The particles are blended in ratios such that the smaller film-forming acrylate particles cannot completely fill the void space between the larger particles (Tzitzinou et al. 2000; Ma 2002). The result of this is that the smaller particles are drawn towards the contact points of the larger particles by capillary forces exerted upon them during drying and function as ‘glue’. Bimodal blends can generate larger pores than monodispersed low Tg lattices (see scale bar, Fig. 1). The disadvantage is that the smaller particles migrate during film formation and hence can be distributed non-uniformly.
Formation of a porous latex coating or film. a Monodispersed latex with arrested polymer particle coalescence (Lyngberg et al. 2001). b Bimodal latex blend consisting of large hard (high Tg) polymer particles + small soft (low Tg) polymer particles
Cantwell et al. (1995) first reported entrapment of microorganisms in a blend of hard and soft polymer particles having a Tg in the range of −60°C up to 60°C. However, this study did not use these blends in thin coatings—only flocculates, 1–2 mm diameter spherical aggregates, and 2 mm diameter fibrils. Whole cell-immobilized enzyme activity was reported, but no data was presented on cell viability following entrapment, aggregate permeability, or the stability of the porosity of these polymer structures at >60°C.
Here we report a method to cast 55 μm thick nano-porous patch coatings composed of two layers (cell coat + polymer topcoat) using a polystyrene/polyacrylate latex bimodal blend with stable porosity at 80°C. The polymer particles and the pore space generated as a result of arrested polymer particle coalescence are smaller than the embedded microorganisms creating interconnected nano-pores (Fig. 1). The cell coat contains a high concentration of metabolically active non-growing T. maritima embedded in nano-porous latex; the sealant top coat is nano-porous latex. The embedded T. maritima retain the ability to utilize carbohydrates and hydrolyze starch after coat drying and rehydration in ASW at 80°C.
Materials and methods
Bacterial strains, chemicals, media, and growth conditions
Thermotoga maritima DSM 3109 (obtained from RM Kelly, North Carolina State University, Raleigh, NC, USA) was cultured in ASW-based medium supplemented with 0.1% yeast extract (Difco Laboratories, Detroit, MI, USA), 0.5% tryptone (Difco), 0.1% starch (soluble starch, reagent grade, MCB, Norwood, OH, USA) and 2 ml/l resazurin from a 2 g/l stock (ASW-starch medium) (Kester et al. 1967; Brown et al. 1993, Liebl et al. 1997). ASW was composed of (per liter): NaCl 15.0 g, Na2SO4 2.0 g, MgCl2·6H2O 2.0 g, CaCl2·2H2O 0.50 g, NaHCO3 0.25 g, K2HPO4 0.10 g, KBr 50 mg, H3BO3 20 mg, KI 20 mg, Fe(NH4)2(SO4)2 15 mg, Na2WO4·2H2O 3 mg, and NiCl2·6H2O 2 mg. The medium was filter-sterilized through a 0.2 μm pore size filter. Cells were grown in 50 ml volumes in 125-ml serum bottles with crimped caps, and anaerobic conditions were attained by heating the medium to 98°C for 30–90 min, sparging with nitrogen for 1 min and adding 0.1 ml of a 50 g/l Na2S·9H2O stock solution. Anaerobic bottles were inoculated with 1 ml from an overnight culture and incubated in a hot oil bath at 80°C. Growth of suspended cells was monitored by the increase in OD at 600 nm. Cultures were also plated for determination of viability by colony counting on 1% Gelrite ASW medium in 80 ml Wolfe anaerobic bottles (Bellco, Vinland, NJ, USA) incubated at 80°C in an incubator within an anaerobic chamber (Forma Model 1025) following the method of Sharp and Raven (1997).
Experiments on latex-entrapped and suspended cells were conducted in 125 ml serum bottles containing 20 ml of medium incubated at 80°C. Carbohydrate utilization experiments were conducted in ASW plus maltose (0.25 g/l) and glucose (0.25 g/l) without yeast extract or tryptone. Starch hydrolysis was determined in ASW-starch medium without tryptone and yeast extract. Chemicals were reagent grade and were obtained from Fisher Scientific, Fairlawn, NJ, USA or Sigma Chemical Company, St. Louis, MO, USA.
Determination of latex coating permeability
For determination of coating permeability, 34-mm diameter latex coatings without bacteria were punched with a hole punch (Osborne punches, McMaster Corp, Chicago, IL, USA) from a coating prepared at room temperature in air at approximately 50% relative humidity and delaminated from a 127 μm thick stainless steel shim substrate (Artus Corporation, Englewood NJ, USA) or photographic paper (Ilford multigrade RC MGC.44M, Ilford Imaging, UK). The permeability of these stand-alone films was determined using a half-cell diffusion apparatus with KNO3 as the diffusant (Lyngberg et al. 2001). This method was modified by injecting KNO3 directly into the donor cell using a 3 ml syringe with a 30 cm long 16 gauge needle (Solheid 2003; Charaniya 2004). The data were analyzed by Nightingale’s equation (Cussler 1998) using a pseudo-steady-state assumption and the permeability of latex films expressed as an effective diffusivity (Deff), which was reported as the ratio of Deff/D, a number between 0 and 1. D is the diffusivity of KNO3 in water which is 1.899×10−5 cm2/s at 30°C and infinite dilution (Geankoplis 1984). Average film thickness was determined from five different places on each patch using a digital micrometer with a 0.79 cm2 circular footpad (Model 1D-C112GEB, Mitutyo Corporation, Japan).
Latex entrapment of Thermotoga maritima
Thermotoga maritima was entrapped in either 1.27 cm diameter or 3.81 cm diameter bi-layer coatings of the following two polymer formulations:
-
1.
Rovace SF091 coatings. Rovace SF091 is a mono-dispersed acrylate/vinyl acetate copolymer latex emulsion with a Tg~10°C, 280 nm polymer particle diameter, 50.5% total solids with 15 ppm KathonLX biocide (Rohm and Haas Co, Spring House, PA, USA) adjusted to pH~7. The coating formulation consisted of 1.2 g wet cell paste gently mixed with 0.3 ml of 50% sterile glycerol in water and 0 – 0.7 g sucrose/g dry latex solids from a filter sterilized stock solution of 0.58 g/ml sucrose that was added to increase coating permeability (see below). The top coat (without cells) contained 5% glycerol on a water-free basis. Entrapment in 1.27 cm diameter Rovace SF091 coatings was performed at 4°C as previously described (Lyngberg et al. 2001) with the exception that all drying and coating took place under a nitrogen atmosphere in a disposable glove bag (SPILFYTER, JV Manufacturing, Green Bay, WI, USA). Nitrogen gas was continually purged through the bag at approximately 2,800 l/h to control the relative humidity so that coat drying was at a rate similar to that in a 4°C cold room.
-
2.
Bimodal blend coatings of Rhopaque 1055 and JP1232. Rhopaque 1055 is a 800 nm diameter hollow non-film-forming polystyrene particle with a Tg=94°C, 26.9% total solids, adjusted to pH ~7 containing 250 ppm Proxel biocide (Rohm and Haas Co., Spring House, PA, USA) which cannot coalesce at ambient temperature. JP1232 is a 158 nm diameter polyacrylate/styrene particle with a Tg≈ −5°C, 40.9% total solids, adjusted to pH 7 that does not contain biocide (Rohm and Haas Co., Spring House, PA, USA). For coatings consisting of blends of Rhopaque 1055, JP1232, and T. maritima, 3.81 cm diameter patches were prepared using the same patch coating method but at room temperature and in a nitrogen atmosphere glove bag.
Thermotoga maritima was grown overnight in 20, 125 ml serum bottles containing 50 ml ASW-starch medium. The culture broth was harvested into two cooled 500 ml centrifuge bottles (no head space in each bottle) and centrifuged at 2,800×g at 4°C for 25 min. The cell pellets were washed in 100 ml ASW, pooled into one 500 ml centrifuge bottle, and centrifuged at 2,800×g for 25 min. The total wet-cell pellet yield was 1.9 g/l. The bimodal blend latex coating formulation was prepared by blending 10 ml of pH 7 Rhopaque 1055 with 2.5 ml of pH 7 JP 1232 and the appropriate amount of glycerol and sucrose using a small spatula. This mixture was blended with wet-cell paste in the ratio of 0.3 g wet-cell paste to 1 ml coating liquid. The bended coating liquid was transferred into a plastic anaerobic hood with a nitrogen flow rate of approximately 2,800 l/h. Patches were generated on 100 μm thick polyester sheets (DuPont Melinex 454, Tekra Corp, NJ, USA) using a 77 μm thick adhesive vinyl mask (Con-Tact, Stamford, CT, USA) punched with a hole punch (McMaster, Corporation, Chicago, IL, USA) and applied to the polyester substrate with a rubber roller (Lyngberg et al. 2001). Here the method was modified by using a number 48 Mayer rod (Paul N.Gardner, Pompano Beach, FL, USA) for the cell coat which resulted in a dry cell coat thickness of 55±5 μm. After drying for 60 min at ~28°C, biolayer patches were top coated with the same latex coating liquid without T. maritima, using a number 48 Mayer rod and a 155 μm thick tape spacer. Following top coat drying for 60 min at ~28°C, patches were cut from the sheet and transferred using tweezers to 125 ml serum bottles containing 20 ml of medium. The wet cell coat volume deposited per patch was equivalent to 125 μl of cell coat formulation prior to water evaporation during coat formation. Therefore, suspended cell controls were 125 μl of cell coat liquid inoculated into serum bottles containing 20 ml of ASW medium.
Maltose and glucose analysis
Maltose and glucose were determined using a Dionex DX500 HPLC equipped with a PA1 anion exchange column and an amperometric detector. Samples were diluted 1–10 in distilled and deionized water containing 15 mg/l fucose as internal standard. Elution was isocratic at 0.08 M NaOH. Concentrations of maltose and glucose were determined as the ratio of the peak area to the internal standard peak. Analysis of unknown polysaccharides was evaluated using a Thermo Finnigan LCQ electro spray ESI mass spectrometer (ES-MS).
Cryogenic scanning electron microscopy
High resolution cryogenic scanning electron microscopic images of T. maritima were generated by withdrawing 5 μl from liquid cultures and depositing the liquid on one side of a perforated carbon film on a copper TEM specimen grid (Ted Pella, Redding, CA, USA). Carbon film hole size ranged from 1 μm to 10 μm. Filter paper was used to blot the other side of the carbon film leaving a thin liquid film spanning the holes, and the grids were then plunged into liquid ethane (−182.8°C) cooled by liquid nitrogen. The vitrified samples were mounted on a Gatan 626 cryo-transfer stage (Gatan, Pleasanton, CA, USA) in liquid nitrogen vapor and transferred into a pre-cooled Balzars MED 010 sputter device (Balzars Union, Balzars, Lichenstein) against a counter flow of dry nitrogen gas. Ice was partially sublimed at −108°C and 2×10−9 bar for 10 min. A 2–3 nm thick layer of platinum was sputtered onto the samples differentially at −120°C and the samples were then transferred to a Hitiachi S900 in-the-lens field emission SEM (FESEM). Specimens were imaged at 1 KeV and 0.65×10−11 amperes at −170°C to reduce charging and electron beam damage.
T. maritima entrapped in Rhopaque HP1055 and JP1232 bimodal latex composites or cell free blends of polymer particles were prepared for cryogenic SEM by coating onto 5 mm by 5 mm pieces of silicon wafers at room temperature in air followed by drying between 1 min and 10 min following the methods of Thiagarajan et al. (1999) and Huang et al. (1999). Samples of T. maritima immobilized in Rovace SF091 containing 0.4 v/v sucrose were coated onto silicon wafers or stainless steel shims and dried for 1 min to 30 min. Rehydrated samples were incubated in ASW at room temperature or at 80°C for 10 min or at 30°C in ASW also for 10 min prior to freezing in liquid nitrogen for cryo-SEM imaging.
Results and discussion
Determination of T. maritima viability in SF091 acrylate/vinyl acetate latex coatings
Suspension cultures of T. maritima examined using cryo-SEM prior to mixing with latex had the characteristic toga sheath coating extending beyond the end of each cell or covering pairs of cells (Fig. 2). No cocci-shaped cells characteristic of later stationary phase morphology were observed. The maximum specific growth rate of control cultures grown at 80°C in 50 ml of ASW-starch medium was 0.30 h−1 with a cell yield of 1.9 g wet cell weight/liter.
We have previously developed a method for determining the viability of latex-entrapped microorganisms recovered by agar plating by shaking or mild sonication to release cells from patches (either delaminated from a stainless steel substrate or attached to a polyester substrate) (Solheid 2003; Lyngberg et al. 1999a, b). Viable T. maritima cells were recovered in ASW as colonies on 1% Gelrite in Wolfe anaerobic bottles from the SF091 and 1055/1232 coating mixtures and from non-top-coated rehydrated latex patches. However, this method was not sufficiently reproducible to accurately determine the fraction of viable T. maritima cells that may have been killed by coat drying, and rehydration. Therefore carbohydrate utilization was used as a measure of latex-entrapped T. maritima metabolic activity in comparison to the same volume of cells suspended in the coating mixture prior to latex patch formation.
Coating T. maritima with SF091 latex
Initial latex coating experiments were performed by entrapping T. maritima in SF091 acrylate/vinyl acetate latex polymer patches (containing glycerol and sucrose to generate coating permeability) using the method previously developed for E. coli by Lyngberg et al. (2001). Glucose and maltose utilization were used as an indicator of T. maritima viability. The effect of several different coating conditions that would result in different degrees of polymer particle coalescence and T. maritima entrapment were investigated prior to rehydrating each patch in ASW-starch medium. All patches were dried at 4°C with the following treatments: (1) cell coat only (no top coat), no further treatment; (2) a cell coat and a top coat dried at 4°C; (3) incubation of the cell coat for 10 min at 37°C to partially coalesce the polymer particles; (4) a coating without T. maritima as a control for glucose degradation at 80°C in ASW. Rehydration of SF091 patches that were coated and dried at 4°C but not incubated for 10 min at 37°C to partially coalesce the polymer particles at ambient temperature (30°C) resulted in redispersion of the latex immediately upon contact with medium. However, SF091 patches not incubated at 37°C but immediately transferred into 80°C ASW medium did not redisperse, indicating that contact with 80°C ASW medium rapidly induced polymer particle coalescence and sufficient particle welding to form a stable film. Patches not incubated at 37°C and without SF091 latex topcoats would release T. maritima cells immediately upon hydration with 80°C ASW medium and as such, were used as controls to determine the effect of latex coating and drying conditions on the viability of the entrapped T. maritima. Gelrite plating and glucose consumption experiments indicated that the cells released from non-top-coated patches were viable (data not shown). However, patches with a topcoat of SF091 latex (incubated or not incubated at 37°C) did not show significant glucose consumption when rehydrated directly in 80°C ASW medium indicating that little metabolic activity was present. This suggests that rapid heat-induced coalescence of the low Tg SF091 topcoat occurs on contact with 80°C ASW resulting in formation of a non-permeable coating.
Cryogenic SEM of T. maritima SF091 latex coatings
Cryogenic SEM images of Rovace SF091 latex coatings where the T. maritima cells were shaken out supported the findings from the glucose consumption experiments (Fig. 3). The appearance of the T. maritima cell craters and the surrounding latex matrix incubated at 30°C was similar to images previously made of SF091 coatings of E. coli (Thiagarajan et al. 1999). The cleanliness of the cell crater surface indicated that the T. maritima cells did not adhere to the SF091 acrylate/vinyl acetate polymer particles. SF091 latex particles not exposed to 80°C formed a partially coalesced nano-porous coating and individual particle contours were clearly visible (Fig. 3a). The microstructure of patches where T. maritima cells were shaken out of the coatings (coatings without a topcoat, incubated 10 min at 37°C and then rehydrated at 80°C) showed a significantly higher degree of particle deformation and compaction (Fig. 3b). This result supported the glucose uptake experiments indicating that temperature-induced wet coalescence of SF091 acrylate/vinyl acetate latex was rapid at 80°C.
Cryogenic-FESEM of the top of T. maritima acrylate/vinyl acetate SF091 latex coatings without a top coat layer. a Coating hydrated at 30°C, 10 min. Porosity visible between the partially coalesced latex particles (striped arrows). Large craters left by released T. maritima (white arrows). b T. maritima coating hydrated at 80°C, 10 min. Reduced porosity between polymer particles (white arrows)
Kinetics of wet coalescence of SF091 latex coatings
Because of the carbohydrate utilization and cryo-SEM evidence of rapid wet coalescence, the rate of loss of SF091 coating diffusivity (Deff/D) at 80°C was determined by incubating patches in water and periodically measuring their diffusivity (Fig. 4). The half-life of loss of permeability of SF091 coatings incubated at 80°C was determined to be 45 min. The temperature and time dependence of the decrease in diffusivity of SF091 acrylate/vinyl acetate latex coatings containing 0.4 g sucrose/g latex to arrest polymer particle coalescence has recently been determined in phosphate buffered saline (PBS) from 5°C to 30°C and can be described by an Arhenius relationship with an activation energy of 108 kJ/mol (Solheid, 2003). A half-life of approximately 80 min was predicted from the activation energy determined in PBS, confirming that the rate of wet coalescence is too rapid for monodispersed low Tg lattices such as SF091 to be useful as a coating matrix at 80°C (Fig. 4).
Rate of loss of diffusivity (Deff/D) of acrylate/vinyl acetate coatings containing 0.4 g sucrose/g SF091 latex incubated at 80°C (filled square). Triplicate determinations. Error bars represent ±1 SD. (filled diamond) Estimated by the relationship Deff/D = 0.05 e−kt, k=A e−Ea/Rt, A=3.5×1015 h−1 (Solheid, 2003)
Coating T. maritima in bimodal latex blends
Permeability of polystyrene/polyacrylate bimodal latex blends
The diffusivity of coatings of 1055/1232 polystyrene/polyacrylate bimodal latex blends cast and dried at room temperature without the addition of sucrose was significantly higher than for mono-dispersed SF091 acrylate/vinyl acetate coatings but decreased with increasing volume fraction of 158 nm, low Tg, JP1232 polymer particles (Fig. 5a) Coatings cast with less than 15% (0.15 v/v) JP1232 did not have sufficient strength to be delaminated intact from the stainless steel substrate and mounted in the diffusion apparatus. A 20% 1232/ 80% 1055 bimodal blend (which corresponds to 23% w/w) was chosen for further investigation. Based upon the ratio of polymer particle diameters, at this weight ratio there are a sufficient number of small particles to surround each large particle, assuming hexagonal packing (Tzizinou and Keddie 2000). The addition of sucrose previously reported to increase latex coating permeability (Lyngberg et al. 2001) was evaluated using the 0.2 v/v 1232/0.8 v/v 1055 bimodal blend and found not to dramatically increase diffusivity (Fig. 5b). However, the addition of 0.3 g sucrose/g latex to this blend was used in order to be consistent with previous work on arresting polymer particle coalescence and enhance coating diffusivity (Charaniya 2004).
a The effect of increasing volume fraction of 158 nm soft JP1232 polyacrylate latex on the diffusivity of a hydrated coating prepared from a bimodal blend with 800 nm hard 1055 polystyrene latex particles. Coatings rehydrated for 30 min in phosphate buffered saline, delaminated from photographic paper, and diffusivity measured at 30°C. Triplicate determinations ±1 SD. b The effect of the addition of sucrose on bimodal blend coating diffusivity
The kinetics of loss of diffusivity of the 0.2 (v/v) 1232/0.8 (v/v) 1055 bimodal blend in comparison to SF091 incubated at 80°C in ASW as a function of time was determined (Fig. 6). The diffusivity of the 1232/1055 bimodal latex blend decreased to about half of the initial value after 2 days and then remained constant while the SF091 coating became non-permeable. The initial decrease in diffusivity for the latex bimodal blend indicated wet compaction – that some polymer particle packing rearrangement was taking place at 80°C after the coating was formed and rehydrated. This indicates that the 800 nm 1055 particles were not permanently fixed in place by the 1232 particles acting as ‘glue’ but continued to rearrange following coat rehydration at 80°C into a tighter packing configuration.
Cryogenic SEM evidence of bimodal blend porous microstructure
Images of cryo-fractures of coatings of the 0.2(v/v) 1232/0.8 (v/v) 1055 bimodal blend microstructure of the fracture surface appeared highly porous with the 800-nm particles arranged in a random pattern with some particles arranged in small groups of hexagonal closest packing (HCP) structures (Fig. 7). The small 1232 particles were not visible, indicating that they were so soft that individual particle contours disappeared by deformation and interdiffusion prior to imaging. Compared to the fracture surface, the coating surface appeared similarly open in packing conformation, with a significantly higher volume fraction of 158 nm coalesced particles in the first one or two particle depths from the surface, indicating that during drying some migration of the small particles had occurred. A high magnification image of individual large polystyrene particles (Fig. 7b) revealed that some 800 nm polystyrene particles appeared coated with JP1232 polymer, giving them a rough appearance. Higher magnification images of the contact surfaces between individual 800 nm particles revealed coalesced polymer appearing as ‘glue’ at the contact points between the larger particles (Fig. 7c). Also, it appeared that some rearrangement occurred after initial contact of the larger particles as indicated by the drawn-out soft polymer strings attached between larger particles. The strings were likely formed by large particles contacting each other and then being drawn apart in a later stage of film formation by cohesive forces.
Cryo-FESEM image of 1055 0.8 v/v and JP1232 0.2 v/v bimodal blend latex coating, dried at 30°C. a View of top surface and fracture edge. b View of fracture edge. c View of white frame in (b) showing particle-to-particle contact point and stretching of JP1232 polymer particles (images courtesy of E. Sutanto)
Microscopic evidence of binding of 1232 latex polymer particles to T. maritima
We attempted to obtain images of T. maritima cells immobilized in the 1232/1055 latex blends similar to in SF091 coatings (Fig. 3). However, this proved very difficult because 1232 acrylate/styrene latex particles agglomerated with the bacterial cells when blended with the cell pellet. Phase contrast microscopy before and after mixing of cells with 0.2 (v/v) 1232/1055 revealed that individual cells were clearly visible in the latex-free cell pellet. Similarly, 1055 particles and cells were visible in the latex blend before mixing with 1232 latex. Following mixing of the two polymer emulsions, no individual T. maritima were visible, but the 800 nm 1055 particles could still be seen. In the mixed state, clumps were visible that appeared to be cells bound together with small latex particles. Two cell to latex blend ratios were investigated, 0.3 v/v and 0.5 v/v wet cell paste to latex (total polymer volume), but no cells were clearly visible in any cryo-SEM freeze fracture images of these mixtures, though the contours of individual 800 nm latex particles were distinctly visible. These findings suggest that agglomeration of the low Tg 1232 acrylate/styrene particles onto the T. maritima toga surface occurs upon mixing .
This result is in contrast to the images of Fig. 3 and many previous cryo-SEM images of latex-embedded viable Gram negative microorganisms obtained in our laboratory using low Tg acrylate polymers (see for example Lyngberg et al. 2001; Thiagarajan et al. 1999). The images in Fig. 3 indicate that the surface of acrylate/vinyl acetate SF091 polymer particles do not adhere to the T. maritima toga sheath and viable cells were recovered from non-top-coated SF091 patches. The 1232 acrylate/styrene particles that are more reactive and have fewer surface acid groups than SF091 appear to adhere to the protein toga sheath of T. maritima masking the cells in cryo-SEM images. Surprisingly, coating the toga sheath of T. maritima with this polymer did not reduce the ability of the cells to utilize carbohydrates nor did it block starch from diffusing to the toga as evident by the observed amylase activity (see below).
Glucose and maltose utilization by concentrated and bimodal latex-entrapped T. maritima
Concentrating T. maritima in 55±5 μm thick two-layer latex coatings increased the cell density approximately 25-fold from a suspended cell density of 1.9 g cell wet weight/liter of medium to 49 g cell wet weight/liter of latex coating volume. No carbohydrate utilization or amylase activity could be detected following entrapment of T. maritima at 80°C in SF091 latex bilayer patches because of rapid polymer particle coalescence resulting in very low diffusivity of the matrix both surrounding the cells and in the topcoat.
Evidence for latex-entrapped T. maritima viability and metabolic activity was obtained in 0.2 v/v 1232/0.8 v/v 1055 bimodal blend patch coatings by comparing the glucose and maltose disappearance (utilization) rates of suspended cells and patches of T. maritima (Fig. 8). This experiment was conducted using the same quantity of cells in media but without a nitrogen source (ASW with 0.25 g/l maltose + 0.25 g/l glucose). Figure 8 shows the utilization of glucose and maltose as a function of time for latex-entrapped and suspended T. maritima. The concentration profiles appeared identical for both suspended and latex-entrapped cells, and maltose utilization is more rapid than glucose uptake. This correlates with Chhabra et al. 2003 who reported that the doubling time for growth of T. maritima on monosaccharides is substantially higher than on the corresponding polysaccharides. Initially there was a large decrease in maltose concentration until 50 h after which it remained constant (Fig. 8). The glucose concentration increased slightly during the same time period after which it decreased slowly during the remaining 150 hours. The similarity between the utilization profiles indicates that latex-entrapped T. maritima are viable and capable of carbohydrate transport that does not appear to be diffusion-limited using this 1232/1055 polymer matrix.
Glucose and maltose production as an indicator of T. maritima amylase activity
The α-amylase of T. maritima has been shown to be toga-associated and 85% or more of the total enzyme activity is accessible on the outside of the cell (Liebl et al. 1997). Bimodal latex-blend entrapped T. maritima patches and suspended T. maritima were incubated in ASW medium containing 1 g/l soluble starch and the maltose + glucose concentration monitored as a function of time as an indicator of amylase activity. Figure 9 shows evidence of amylase activity by the kinetics of glucose + maltose accumulation in the medium. Cell-free controls produced no measurable glucose or maltose (data not shown). Suspended T. maritima produced maltose and glucose immediately following inoculation for 24 h after which the maltose and glucose were consumed below detectable levels following 50 h of incubation. Bimodal latex patches of T. maritima showed little evidence of amylase activity during the first 24 h, presumably due to the slow diffusion of starch to the cells through the polymer top coat. However, after 24 h of incubation amylase activity was evident as glucose + maltose were liberated and accumulated in the medium for 200 h. In comparison to Fig. 8, the observed accumulation of monosaccharides in the medium is the excess starch hydrolysis products over carbohydrate assimilation under nitrogen-starvation conditions. Little maltotriose was detected by HPLC analysis which was in agreement with previous findings that the α-amylase of T. maritima was of the endo-type having no α-1-6 glucoside bond activity (Liebl et al. 1997). During the incubation there was also an accumulation of an extra-cellular polysaccharide that could not be identified further by HPLC or ES-MS due to the high salt concentration and the presence of metal ions. It was determined by HPLC not to be composed of fucose, pyruvate, galactose, fructose, mannose, glucoseamine, sucrose, maltose or sorbitol.
Conclusion
Polystyrene bimodal blend coatings may be a useful model system for engineering a matrix to generate porous, adhesive coatings for entrapment of hyperthermophiles at high concentration so that they can be used under non-growth conditions to increase their volumetric biocatalytic productivity. Other approaches for generating thermostable coatings such as core-shell lattices may also be useful (see for example Kalinina and Kumacheva 1999; Dos Santos et al. 2000). The core shell approach should minimize binding of reactive low Tg ‘shell’ polymers to cell surfaces such as in this case, the T. maritima toga protein sheath. With further development, these methods may be useful for casting perfusive biocatalytic films for high temperature membrane bioreactors (Drioli and Giorno 1999; Sanchez Marcano and Tsotsis 2002) and membranes useful to immobilize and stabilize the activity of a high density of viable hyperthermophiles at a phase boundary for high temperature multi-phase biocatalysis (Cabral et al. 2001).
Abbreviations
- ASW:
-
Artificial seawater
- Deff/D:
-
Ratio of the diffusivity of KNO3 in a latex coating relative to its diffusivity, D, in water
- T g :
-
Polymer particle glass transition temperature
- v/v:
-
Volume fraction of bimodal latex emulsions blended
References
Adams MWW (1995) Large-scale growth of hyperthermophiles. In: Robb FT, Place AR, Sowers KR, Schrier HJ, DasSarma S, Fleischmann EM (eds) Archaea: a laboratory manual. Cold Spring Harbor, New York, pp 47–49
Brown SH, Sjrholm C, Kelly RM (1993) Purification and characterization of a highly stable glucose isomerase produced by the extremely thermophilic eubacterium, Thermotoga maritima. Biotechnol Bioeng 41:878–886
Cabral JB, Mota M, Tramper J (2001) Multiphase bioreactor design. Tayor & Francis, London
Cantwell JB, Mills PDA, Jones E, Stewart RF (1995) Immobilized cells. European Patent Office 0288203B1
Charaniya SP (2004) Optimization of the catalytic activity of bilayer latex coatings for bacterial whole-cell oxidation and reduction. MS Thesis, University of Minnesota
Chhabra SR, Shockley KR, Conners SB, Scott KL, Wolfinger RD, Kelly RM (2003) Carbohydrate-induced differential gene expression patterns in the hyperthermophilic bacterium Thermotoga maritima. J Biol Chem 278:7540–7552
Cussler EL (1998) Diffusion: mass transfer in fluid systems, 2nd edn. Cambridge University Press, Cambridge, pp 22–24
De Rosa M, Gambacorta A, Lama L, Nicolaus B (1981) Immobilization of thermophilic microbial cells in crude egg white. Biotechnol Lett 3:183–186
Di Lernia I, Schiraldi C, Generoso M, De Rosa M (2002) Trehalose production at high temperature exploiting an immobilized cell bioreactor. Extremophiles 6:341–347
Dos Santos FD, Fabre P, Drujon X, Meunier G, Leibler L (2000) Films from soft-core/hard-shell hydrophobic latexes: structure and thermomechanical properties. J Polymer Sci B 38:2989–3000
Drioli E, Giorno L (1999) Biocatalytic membrane reactors. Taylor & Francis, London
Geankoplis CJ (1984) Mass transport phenomena. Edward Brothers, Columbus
Gebhard MS, Lesko PM, Brown AB, Young DH (2004) Porous non-friable polymer films. US Patent 6,750,050
Hicks PM, Kelly RM (1999) Thermophilic microorganisms. In: Flickinger MC, Drew SW (eds) Encyclopedia of bioprocess technology: fermentation, biocatalysis, and bioseparation. Wiley Interscience, New York, pp 2536–2552
Holst O, Manelius Å, Krahe M, Märkl H, Raven N, Sharp R (1997) Thermophiles and fermentation technology. Comp Biochem Physiol 118A:415–422
Huang Z, Thiagarajan V, Lyngberg OK, Scriven LE, Flickinger MC (1999) Microstructure evolution in polymer latex coatings for whole-cell biocatalyst applications. J Coll Interface Sci 215:226–243
Jons S, Ries P, McDonald CJ (1999) Porous latex composite membranes: fabrication and properties. J Membr Sci 155:79–99
Kalinina O, Kumacheva E (1999) A “core-shell” approach to producing 3D polymer nanocomposites. Macromolecules 32:4122–4129
Kester DR, Duedall IW, Connors DN, Pyrkowicz RM (1967) Preparation of artificial sea water. Limnol Oceanogr 12:176–178
Liebl W, Stemplinger I, Ruile P (1997) Properties and gene structure of the Thermotoga maritima α-amylase AmyA, a putative lipoprotein of a hyperthermophilic bacterium. J Bacteriol 179:941–948
Lyngberg OK, Thiagaragan V, Stemke DJ, Schottel JL, Scriven LE, Flickinger MC (1999a) A patch coating method for preparing biocatalytic films with E. coli. Biotechnol Bioeng 62:44–55
Lyngberg OK, Stemke DJ, Schottel JL, Flickinger MC (1999b) A simple single use luciferase based mercury biosensor using latex-film immobilized Escherichia coli HB101. J Ind Microbiol Biotechnol 23:668–676
Lyngberg OK, Ng CP, Thiagarajan V, Scriven LE, Flickinger MC (2001) Engineering the microstructure and permeability of thin multilayer latex biocatalytic coatings containing E. coli. Biotechnol Prog 17:1169–1179
Ma Y (2002) High-resolution cryo-scanning electron microscopy of latex film formation. PhD Thesis, University of Minnesota
Pantazaki AA, Pritsa AA, Kyriakidis DA (2002) Biologically relevant enzymes from Thermus thermophilus. Appl Microbiol Biotechnol 58:1–12
Park H-S, Kayser KJ, Kwak J-H, Kilbane JJ (2004) Heterologous gene expression in Thermus thermophilus: β-galactosidase, dibenzothiophene monooxygenase, PNB carboxy esterase, 2-aminobiphenyl-2,3-diol dioxygenase, and chloramphenicol acetyl transferase. J Ind Microbiol Biotechnol 31:189–197
Pörtner R, Märkl H (1996) Immobilization of the extremely thermophilic archaeon Pyrococcus furiosus in macro-porous carriers. In: Wijffels RH, Buitelaar RM, Bucke C, Tramper J (eds) Immobilized cells: basics and applications. Elsevier, Amsterdam, pp 424–430
Rainina EI, Pusheva MA, Ryabokon AM, Bolotina NP, Lozinsky VI, Varfolomeyev SD (1994) Microbial cells immobilized in poly(vinyl alcohol) cryogels: biocatalytic reduction of CO2 by the thermophilic homoacetogenic bacterium Acetogenium kiuvi Biotechnol Appl Biochem 19:321–329
Ryabokon AM, Kevbrina MV, Pusheva MA, Zubov AL, Lozinsky VI, Rainina EI (1996) Ecologically pure cultures of acetate synthesis on diverse gaseous substrates by homoacetogenic bacteria, entrapped in poly(vinyl alcohol) cryogel. In: Wijffels RH, Buitelaar RM, Bucke C, Tramper J (eds) Immobilized cells: basics and applications. Elsevier, Amsterdam, pp 106–111
Sanchez Marcano JG, Tsotsis TT (2002) Catalytic membranes and membrane reactors. Wiley-VCH, Weinheim, Germany
Schiraldi C, Di Lernia I, Giuliano M, Generoso M, D’ Agnostino A, De Rosa M (2003) Evaluation of a high temperature immobilized enzyme reactor for production of non-reducing oligosaccharides. J Ind Microbiol Biotechnol 30:302–307
Schröder C, Selig M, Schönheit P (1994) Glucose fermentation to acetate, CO2, and H2 in the anaerobic hyperthermophilic eubacterium Thermotoga maritima: involvement of the Embden-Mayerhof pathway. Arch Microbiol 161:460–470
Sharp RJ, Raven NDH (1997) Isolation and growth of hyperthermophiles. In: Rhodes PM, Standbury PF (eds) Applied microbial physiology. IRL, Oxford, pp 23–52
Singh A, Goel R, Johri BN (1990) Production of cellulolytic enzymes by immobilized Sporotrichium thermophile. Enz Microbiol Technol 12:464–468
Solheid C (2003) Characterization of bilayer latex coatings containing viable Gluconobacter oxydans for the oxidation of D-sorbitol to L-sorbose. MS Thesis, University of Minnesota
Swope KL, Flickinger MC (1996) Activation and regeneration of whole cell biocatalysts: initial and periodic induction behavior in starved E. coli after immobilization in thin synthetic films. Biotechnol Bioeng 51:360–370
Thiagarajan VS, Huang Z, Scriven LE, Schottel JL, Flickinger MC (1999) Microstructure of a biocatalytic latex coating containing viable Escherichia coli cells. J Coll Interface Sci 215:244–257
Tzitznou A, Keddie JL (2000) Film formation of latex blends with bimodal particle size distributions: consideration of particle deformability and continuity of the dispersed phase. Macromolecules 33:2695–2708
Tzitznou A, Keddie JL, Geurts JM, Peters ACIA, Satguru R (2000) Film formation of latex blends with bimodal particle size distributions: consideration of particle deformability and continuity of the dispersed phase. Macromol 33:2695–2708
Van de Ban E, Willemen H, Wassink H, Haaker H, Laane C (1998) Bioreductions by Pyrococcus furiosus at elevated temperature. In: Ballesteros A, Plou FJ, Iborra JL, Halling PJ (eds) Progress in biotechnology 15: stability and stabilization of biocatalysts. Elsevier, Amsterdam
Van Niel EWJ, Claassen PAM, Stams AJM (2003) Substrate and product inhibition of hydrogen production by the extreme thermophile Caldicellulosiruptor saccharolyticus. Biotechnol Bioeng 81:255–262
Van Ooteghem SA, Beer SK, Yue PC (2002) Hydrogen production by the thermophilic bacterium Thermotoga neapolitana. Appl Biochem Biotechnol 98–100:177–189
Webb C, Devakos GA (1996) Studies in viable cell immobilization. Academic Press, RG Landes Co, Austin
Wijffels RH (ed) (2001) Immobilized cells. Springer, Berlin
Worden RM, Subramanian R, Bly MJ, Winter S, Aronson CL (1991) Growth kinetics of Bacillus stearothermophilus BR219. Appl Biochem Biotechnol 28/29:267–275
Acknowledgements
The authors would like to thank Professor Robert M. Kelly, Tina D. Rinker, and Paula M. Hicks, North Carolina State University, Raleigh, North Carolina, for their assistance and encouragement in beginning this work, Sridevi Nagarajan for developing the anaerobic plate counting method, Erwin Sutanto for cryo-FESEM images of bimodal blend coatings, and Marcello Fidaleo for helpful comments on preparation of the manuscript. Latex polymers were generously supplied by Matthew Gebhard, Rohm and Haas, Co., Spring House, PA, USA. This work was supported by NIH grant T32/GM08347, DSO DARPA contract N66001-020C-8046, Joe Bielitski Program Director, and the University of Minnesota BioTechnology Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Wiegel
Rights and permissions
About this article
Cite this article
Lyngberg, O.K., Solheid, C., Charaniya, S. et al. Permeability and reactivity of Thermotoga maritima in latex bimodal blend coatings at 80°C: a model high temperature biocatalytic coating. Extremophiles 9, 197–207 (2005). https://doi.org/10.1007/s00792-005-0434-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-005-0434-7