Abstract
Novel 16S rRNA introns were detected in four new strains within the family Thermoproteaceae. Pyrobaculum oguniense TE7T and Thermoproteus sp. IC-062 housed introns of 32 and 665–668 bp after positions 1205 and 1213 (Escherichia coli numbering system), respectively. Caldivirga maquilingensis IC-167T had two introns of 37 and 140 bp after positions 901 and 908, respectively. Vulcanisaeta distributa IC-065 had a 691-bp intron after position 1391. All the introns larger than 650 bp encoded the LAGLI-DADG type proteins. The intron-encoded proteins of P. oguniense TE7T and Thermoproteus sp. IC-062 are cognate with the proteins encoded by introns inserted at the same position in other Pyrobaculum/Thermoproteus strains and phylotypes. The intron-encoded protein of V. distributa IC-065 is partially related to that of a Pyrobaculum phylotype. A large-scale deletion in the second intron of Caldivirga maquilingensis IC-167T is suspected. Based on these newly found introns and hitherto known 16S rRNA introns, the evolutionary movements of the 16S rRNA introns and the encoded LAGLI-DADG type proteins are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Within the domain Archaea, introns are detected in the genes of tRNAs, rRNAs, and proteins homologous to the eukaryotic centromere-binding factor 5 (Lykke-Andersen et al. 1997; Watanabe et al. 2002). The tRNA introns are distributed widely over the phyla Euryarchaeota and Crenarchaeota, while the introns in the protein-coding genes are found in the genome-sequenced Crenarchaeota strains (Watanabe et al. 2002). Meanwhile, the rRNA introns are so far confined to the 16S rRNA and 23S rRNA genes of the two crenarchaeotic orders Thermoproteales and Desulfurococcales (Burggraf et al. 1993; Dalgaard and Garrett 1992; Itoh et al. 1998; Kjems and Garrett 1985, 1991; Nomura et al. 1998). Furthermore, the rRNA introns occur sporadically even in these two crenarchaeotic orders.
The archaeal rRNA introns are composed of core structures and terminal loops (Lykke-Andersen and Garrett 1994). The former comprise bulge-helix-bulge structures at which the intron is spliced by RNA endonuclease. The lengths of the terminal loops are variable, ranging from several bases to more than 600 bases. The large intronic terminal loops usually carry open reading frames (ORFs) containing the LAGLI-DADG-like motif, which is one of the four conserved amino acid sequence motifs of homing endonucleases (reviewed by Belfort and Roberts 1997; Chevalier and Stoddard 2001), although some of the frames seem to have undergone frame-shift mutations (Itoh et al. 1998; Takai and Horikoshi 1999). Until now, several intron-encoded proteins have been shown to cleave the intronless alleles at the vicinities of the respective intron-insertion sites (Dalgaard et al. 1993; Lykke-Andersen and Garrett 1994; Morinaga et al. 2000). Hyperthermophilic archaea usually possess single 16S rRNA-23S rRNA operons; therefore, the presence of the "homing" endonuclease sequence in the archaeal rRNA introns may confer an infectious nature on the rRNA introns per se. Actually, the 23S rRNA intron of Desulfurococcus mobilis has been shown to invade the intronless allele of Sulfolobus acidocaldarius (Aagaard et al. 1995). Conversely, the rRNA introns can be lost, possibly by recombination with the cDNA copy of the spliced mRNA (Belfort and Perlman 1995). The possibility of losing the ancestral rRNA introns among Thermoproteus strains has been suggested (Itoh et al. 1998). Thus, gain and loss of the rRNA introns are probably common events among these hyperthermophilic crenarchaeotes. Nevertheless, a paucity of the identified rRNA introns has hampered our understanding of the evolution and population dynamics of rRNA introns and the corresponding intron-encoded proteins in the natural environment.
Recently, with an increase of 16S rRNA sequences reported, new 16S rRNA introns have been found in several culturable strains and phylotypes of the family Thermoproteaceae (Itoh et al. 1999, 2002; Sako et al. 2001; Takai and Horikoshi 1999). In this paper, we compare several newly identified 16S rRNA introns and the hitherto known 16S rRNA introns and discuss the possible evolutional movements of the 16S rRNA introns and the encoded LAGLI-DADG proteins.
Materials and methods
Strains
The 16S rRNA introns newly detected in four strains of the family Thermoproteaceae were sequenced in this study. Thermoproteus sp. IC-062 was isolated from hot spring water collected at Sounzan-Onsen, Kanagawa, Japan, where another 16S rRNA intron-containing Thermoproteus sp. IC-061 was isolated (Itoh et al. 1998). Pyrobaculum oguniense TE7T (JCM 10595T) was isolated from hot spring effluent at Oguni-cho, Kumamoto, Japan (Sako et al. 2001). Strain IC-065 (JCM 11215), a strain of the new species Vulcanisaeta distributa designated within the novel genus Vulcanisaeta (Itoh et al. 2002) in the family Thermoproteaceae, was isolated from solfataric soil at Ohwakudani, Kanagawa, Japan. Caldivirga maquilingensis IC-167T (JCM 10307T) was an isolate from hot spring water at Mt. Maquiling, Laguna, the Philippines (Itoh et al. 1999).
Detection of 16S rRNA introns, sequencing, and phylogenetic analysis
Genomic DNA was isolated and purified by the method of Lauerer et al. (1986) or Tamaoka (1994). The 16S rRNA gene was amplified by PCR with the PCR primers described by Itoh et al. (1998) (for Thermoproteus sp. IC-062), Itoh et al. (1999) (for C. maquilingensis IC-167T), Itoh et al. (2002) (for V. distributa IC-065), and Sako et al. (2001) (for P. oguniense TE7T). Sequences of the 16S rRNA genes and cDNAs of the 16S rRNA transcripts were determined as described previously (Itoh et al. 1998; Sako et al. 2001). The phylogenetic analysis was performed with the Clustal X program (Thompson et al. 1997) and the dot matrix analyses were performed with GeneWorks (IntelliGenetics Inc.). The phylogenetic tree was reconstructed by the neighbor-joining method of Saitou and Nei (1987) and was estimated by bootstrap sampling (Felsenstein 1985).
Results and discussion
Phylogenetic relationships of the family Thermoproteaceae
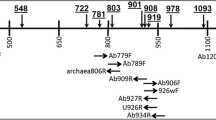
A phylogenetic tree of the family Thermoproteaceae derived from the 16S rRNA exon sequences is shown in Fig. 1. Strain IC-062 was identified as Thermoproteus sp. by cell shape (rod-shaped), growth temperature (up to 95 °C), DNA base composition (56.5 mol%G+C), and a 16S rRNA exon sequence identical to that of strain IC-061.
A phylogenetic tree of the family Thermoproteaceae derived from the 16S rRNA exon sequences. Strains and phylotypes having the 16S rRNA introns are shown in bold letters. The numbers indicate bootstrap values of 1,000 trials (only values greater than 800 are shown). The bar represents 0.01 substitutions per nucleotide position. The sequences cited are as follows: Caldivirga maquilingensis IC-167T, AB013926; Pyrobaculum aerophilum im2T, L07510; Pyrobaculum arsenaticum PZ6T, AJ277124; Pyrobaculum islandicum geo3T, L07511; Pyrobaculum oguniense TE7T, AB029339; Pyrobaculum organotrophum JCM 9190T, AB063647; Thermocladium modestius IC-125T, AB005296; Thermofilum pendens Hvv3T, X14835; Thermoproteus neutrophilus JCM 9278T, AB009616; Thermoproteus tenax, M35966; Thermoproteus sp. IC-033, AB009616; Thermoproteus sp. IC-061, AB009617; Thermoproteus sp. IC-062, AB081846; Vulcanisaeta distributa IC-017T, AB063630; Vulcanisaeta distributa IC-065, AB063639; Vulcanisaeta souniana IC-059T, AB063646; pBA2, AF176346; pHGPA1, AB027539; pHGPA13, AB027540
Members of the genus Pyrobaculum, together with Thermoproteus neutrophilus JCM 9278T and the phylotypes pHGPA1, pHGPA13, and pBA2, formed a coherent clade with more than 98.1% sequence similarities to each other and were closely related to the Thermoproteus strains (≥96.5% sequence similarities). Strains of the genera Vulcanisaeta and Caldivirga were positioned in separate lineages.
Detection of 16S rRNA introns and its core structure
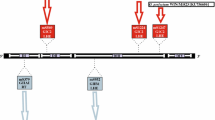
After PCR amplification of the 16S rRNA genes from genomic DNAs, agarose gel electrophoresis of the reaction mixtures revealed that the amplified DNAs of Pyrobaculum oguniense TE7T, Thermoproteus sp. IC-062, Vulcanisaeta distributa IC-065, and Caldivirga maquilingense IC-167T were larger than the normal 16S rRNA genes of Thermoproteaceae strains. Sequence analysis showed that these amplified 16S rRNA genes contained intervening sequences as shown in Table 1. All the inserted sequences possessed the putative intron core structures that exist in all archaeal rRNA introns so far discovered (Lykke-Andersen and Garrett 1994; Itoh et al. 1998; Nomura et al. 1998; Takai and Horikoshi 1999). Moreover, for P. oguniense TE7T, V. distributa IC-065, and C. maquilingensis IC-167T (IC-062 was not examined), the inserted sequences were absent in the cDNAs of the corresponding 16S rRNA transcripts. Thus, the intervening sequences were identified as introns.
Introns found in the genera Thermoproteus and Pyrobaculum
P. oguniense TE7T and Thermoproteus sp. IC-062 had two 16S rRNA introns, after positions 1205 and 1213, that have been detected in several other strains and phylotypes of the genera Thermoproteus and Pyrobaculum, as shown in Table 1. The introns after position 1205 range from 32 to 34 bases in length, and the sequences of introns 061-IV, 062-IV, Pog-IV, pHGPA1-b, and pHGPA13-c are identical or almost identical (only one base difference exists in pHGPA1-b) (see Table 1 for nomenclature of the introns).
The introns after position 1213 range from 662 to 688 bases in length, and the whole intron sequences can be aligned. Among these large introns, 061-V, 062-V, and Tne-V possess ORFs containing the two LAGLI-DADG motifs and occupying almost the whole region of the terminal insert. Homologous ORFs that are apparently shortened by putative occurrence of insertion and deletion in the nucleotide sequences are found in the remaining introns (033-V, Pog-V, pHGPA1-c, and pHGPA13-d). By comparing the nucleotide sequences altogether, reconstruction of the encoded proteins in the four introns (i.e., 033-V, Pog-V, pHGPA1-c, and pHGPA13-d) is theoretically possible. As shown in Fig. 2, the evolutionary relationship based on 190 amino acid positions of the proteins (including the reconstructed proteins) encoded by the 16S rRNA introns inserted after position 1213 agreed well with the phylogenetic tree based on the 16S rRNA exons, with the exception of the Tne-V-encoded protein. The Tne-V-encoded protein is distantly related to the remaining proteins. These findings may indicate that the common ancestor of the genera Pyrobaculum and Thermoproteus had the LAGLI-DADG ORFs in the introns after position 1213 and that the Tne-encoded protein had substituted the original protein. This interpretation, however, needs to be confirmed by identifying the cognate proteins from more new isolates, particularly strains living in the vicinity of Iceland where T. neutrophilus was isolated.
A phylogenetic tree of the LAGLI-DADG proteins encoded by the 16S rRNA introns inserted after position 1213 (in Escherichia coli numbering system). Reconstructed amino acid sequences were used for the putative encoded proteins of 033-V, Pog-V, pHGPA1-c, and pHGPA13-d (see text). The bar represents 0.02 substitutions per amino acid position. See Table 1 for nomenclature of the introns
Among the members having introns after position 1213, Thermoproteus spp. IC-033 and IC-061 as well as pHGPA1 possess another 16S rRNA intron after position 781 (i.e., 033-II, 061-II, and pHGPA1-a, respectively), which encodes another LAGLI-DADG protein (the ORF of 033-II seems to have been derived from frame-shift mutations; Itoh et al. 1998) . Interestingly, the evolutionary distances of the two encoded proteins of Thermoproteus sp. IC-061 and pHGPA 1 are quite similar: the amino acid sequence similarities between introns 061-II and pHGPA1-a and between introns 061-V and pHGPA1-c were 31% and 28%, respectively. These introns might have evolved at similar evolutionary rates in the 16S rRNA genes.
Unlike strain IC-061, strain IC-062 lacks the intron after position 781 in the 16S rDNA. Both strains were isolated from the same sampling site and have identical 16S rRNA exon sequences. Moreover, a close relative of these strains, Thermoproteus sp. IC-033, also possesses an intron after position 781 (Itoh et al. 1998). These facts may suggest that strain IC-062 had lost the intron after position 781.
Introns found in C. maquilingensis IC-167T
In the 16S rDNA of C. maquilingensis IC-167T, two introns, Cma-I and Cma-II, exist in close proximity (Fig. 3). The insertion sites of the two introns appear to be the same as those of pHGPA13-b1 and pHGPA13-b2, respectively. Moreover, the Cma-II intron has the same insertion site as the 16S rRNA intron of Aeropyrum pernix K1T (ApeIα) (Nomura et al. 1998). The bulge-helix-bulge structures and the long stems, particularly the regions adjacent to the bulge-helix-bulge structures, of the two introns of C. maquilingensis IC-167T show high similarities with the counterpart introns of pHGPA13. However, the long stem of Cma-II differs markedly from that of ApeIα. The putative terminal loop of Cma-II (98 bp) is apparently shorter than that of pHGPA13-b2 (571 bp) and ApeIα (653 bp). Within the putative terminal insert of Cma-II, CT-rich stretches resembling an archaeal transcriptional terminator were detected. Such CT-rich sequences are often found near the stop codons of the archaeal rRNA intron ORFs (Itoh et al. 1998). Furthermore, DNA–DNA dot matrix analysis revealed a certain degree of similarity between the terminal insert of Cma-II and the downstream region of pHGPA13-b2 encoding the LAGLI-DADG protein (e.g., the stretch from the 37th to 78th nucleotides of Cma-II and that from the 534th to 575th nucleotides of pHGPA13-b share 69% identity without gaps). This fact implies that the terminal loop sequence of Cma-II may be a remnant of the nucleotide sequence encoding a protein that shared a common trait with the pHGPA13-b2-encoded protein. No significant homology was found between the terminal inserts of Cma-II (or pHGPA13-b2) and ApeIα. At the moment, it is not clear whether these proteins originated from the same ancestral protein.
Intron found in V. distributa IC-065
The 16S rRNA intron (Vdi) found in V. distributa strain IC-065 intervenes at a hitherto unknown intron-insertion position. The intron has a typical core consisting of a bulge-helix-bulge structure and a long stable stem, as shown in Fig. 3. The long terminal insert contains a single ORF, corresponding to 203 amino acid residues, which spans almost the entire insert. The G+C content of the insert is 35.09 mol%, which is significantly lower than the 16S rRNA gene exon. The putative encoded protein had two LAGLI-DADG-like stretches (LMATGVALEG and VLRWAFTLEG). Moreover, AT-rich and CT-rich sequences are found 23–30 bp upstream of the putative start codon of the ORF and around the stop codon of the ORF, respectively. The features described above are consistent with most of the archaeal rRNA introns containing large ORFs. However, protein–protein dot matrix analysis showed no significant similarities between the Vdi-encoded protein and other LAGLI-DADG proteins, except for the protein sequence encoded by 061-II and pHGPA1-a downstream of the second LAGLI-DADG motifs (e.g., the 149th to 172nd amino acid stretch of the Vdi-encoded protein and the 166th to 189th amino acid stretch of the 061-II- and pHGPA1-a-encoded proteins are strictly or strongly conserved, according to the definition of Thompson et al. 1994, in 50% of the residues). This fact may suggest that the Vdi-encoded LAGLI-DADG protein (or those encoded by 061-II and pHGPA1-a) is chimeric in origin.
In the present study, comparison of the 16S rRNA introns and the intron-encoded LAGLI-DADG proteins of strains within the family Thermoproteaceae permits a glimpse of the evolutionary movements of these introns. The introns acquired in the rRNA genes could be coevolved with the rRNA exons, or they could be eliminated from the genes or undergo mutations. Some introns could propagate in another host organism. Thus, the evolutionary or population dynamics of the rRNA introns in the natural environment should be estimated statistically with sizable examples. Direct detection and sequencing of the rRNA intron-containing genes from geographically different geothermal environments, as suggested by Takai and Horikoshi (1999), could be the next strategy for this purpose.
References
Aagaard C, Dalgaard JZ, Garrett RA (1995) Intercellular mobility and homing of an archaeal rDNA intron confers a selective advantage over intron- cells of Sulfolobus acidocaldarius. Proc Natl Acad Sci USA 92:12285–12289
Belfort M, Perlman PS (1995) Mechanisms of intron mobility. J Biol Chem 270:30237–30240
Belfort M, Roberts RJ (1997) Homing endonucleases: keeping the house in order. Nucleic Acid Res 25:3379–3388
Burggraf S, Larsen N, Woese CR, Stetter KO (1993) An intron within the 16S ribosomal RNA gene of the archaeon Pyrobaculum aerophilum. Proc Natl Acad Sci USA 90:2457–2550
Chevalier BS, Stoddard BL (2001) Homing endonucleases: structure and functional insight into the catalysts of intron/intein mobility. Nucleic Acids Res 29:3757–3774
Dalgaard JZ, Garrett RA (1992) Protein-coding introns from the 23S rRNA encoding gene form stable circles in the hyperthermophilic archaeon Pyrobaculum organotrophum. Gene 121:103–110
Dalgaard JZ, Garrett RA, Belfort M (1993) A site specific endonuclease encoded by a typical archaeal intron. Proc Natl Acad Sci USA 90:5414–5417
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Itoh T, Suzuki K, Nakase T (1998) Occurrence of introns in the 16S rRNA genes of members of the genus Thermoproteus. Arch Microbiol 170:155–161
Itoh T, Suzuki K, Sanchez PC, Nakase T (1999) Caldivirga maquilingensis gen. nov., sp. nov., a new genus of rod-shaped crenarchaeote isolated from a hot spring in the Philippines. Int J Syst Bacteriol 49:1157–1163
Itoh T, Suzuki K, Nakase T (2002) Vulcanisaeta distributa gen. nov., sp. nov. and Vulcanisaeta souniana sp. nov., hyperthermophilic, rod-shaped crenarchaeotes isolated from hot springs in Japan. Int J Syst Evol Microbiol 52:1097–1104
Kjems J, Garrett RA (1985) An intron in the 23S ribosomal RNA gene of the archaebacterium Desulfurococcus mobilis. Nature 318:675–677
Kjems J, Garrett RA (1991) Ribosomal RNA introns in archaea and evidence for RNA conformational changes associated with splicing. Proc Natl Acad Sci USA 88:439–443
Lauerer G, Kristjansson JK, Langworthy TA, König H, Stetter KO (1986) Methanothermus sociabilis sp. nov., a second species within the Methanothermaceae growing at 97 °C. Syst Appl Microbiol 8:100–105
Lykke-Andersen J, Garrett RA (1994) Structural characteristics of the stable RNA introns of archaeal hyperthermophiles and their splicing junctions. J Mol Biol 243:846–855
Lykke-Andersen J, Aagaard C, Semionenkov M, Garrett RA (1997) Archaeal introns: splicing, intercellular mobility and evolution. Trends Biochem Sci 22:326–331
Morinaga Y, Nomura N, Sako Y, Uchida A (2000) Substrate recognition of homing endonuclease I–Ape I in a hyperthermophilic archaeon (Abstract). Third International Congress on Extremophiles, Hamburg, Germany, p 212
Nomura N, Sako Y, Uchida A (1998) Molecular characterization and postsplicing fate of three introns within the single rRNA operon of the hyperthermophilic archaeon Aeropyrum pernix K1. J Bacteriol 180:3635–3643
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sako Y, Nunoura T, Uchida A (2001) Pyrobaculum oguniense sp. nov., a novel facultative aerobic and hyperthermophilic archaeon growing at up to 97 °C. Int J Syst Evol Microbiol 51: 303–309
Takai K, Horikoshi K (1999) Molecular phylogenetic analysis of archaeal intron-containing genes coding for rRNA obtained from a deep-subsurface geothermal water pool. Appl Environ Microbiol 65:5586–5589
Tamoka J (1994) Determination of DNA base composition. In: Goodfellow M, O'Donnell AG (eds) Chemical methods in prokaryotic systematics. Wiley, Chichester, pp 463–470
Thompson JD, Higgins DG, Gibson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Watanabe Y, Yokobori S, Inaba T, Yamagishi A, Oshima T, Kawarabayashi Y, Kikuchi H, Kita K (2002) Introns in protein-coding genes in Archaea. FEBS Lett 510:27–30
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Horikoshi
Rights and permissions
About this article
Cite this article
Itoh, T., Nomura, N. & Sako, Y. Distribution of 16S rRNA introns among the family Thermoproteaceae and their evolutionary implications. Extremophiles 7, 229–233 (2003). https://doi.org/10.1007/s00792-003-0314-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-003-0314-y