Abstract
Objective
Clinical trials and inconclusive meta-analyses have investigated the effects of omega-3 supplements in children with Attention-Deficit Hyperactivity Disorder (ADHD). We performed a randomised placebo-controlled trial to evaluate the efficacy of omega-3 fatty acids.
Methods
Children aged 6–15 years with established diagnosis of ADHD were randomised 1:1 to receive either supplements containing docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) or a placebo for 3 months. Psychotropic or omega-3-containing treatments were not authorised during the study. The primary outcome was the change in the Attention-Deficit Hyperactivity Disorder Rating Scale version 4 (ADHD-RS-IV). Other outcomes included safety, lexical level (Alouette test), attention (Test of Attentional Performance for Children—KiTAP), anxiety (48-item Conners Parent Rating Scale-Revised—CPRS-R), and depression (Children’s Depression Inventory—CDI).
Results
Between 2009 and 2011, 162 children were included in five French child psychiatry centres. The mean age was 9.90 (SD 2.62) years and 78.4% were boys. The inclusion ADHD-RS-IV at was 37.31 (SD 8.40). The total ADHD-RS-IV score reduction was greater in the placebo group than in the DHA–EPA group: −19 (−26, −12) % and −9.7 (−16.6, −2.9) %, respectively, p = 0.039. The other components of the Conners score had a similar variation but the differences between groups were not significant. Two patients in the DHA–EPA group and none in the placebo group experienced a severe adverse event (hospitalisation for worsening ADHD symptoms).
Conclusion
This study did not show any beneficial effect of omega-3 supplement in children with mild ADHD symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Attention-deficit hyperactivity disorder (ADHD) is a neurobehavioral disorder characterised by age-inappropriate levels of inattention, hyperactivity, and impulsivity. According to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) [1, 2] there are three categorical subtypes of ADHD: a predominantly inattentive subtype, a predominantly hyperactive–impulsive subtype, and a combined inattentive and hyperactive–impulsive subtype. ADHD is a frequent disorder, with an estimated prevalence of 5% and a sex ratio of approximate 4:1 [2,3,4,5].
The reference treatments for ADHD are stimulant medications, such as methylphenidate (MPH) [6]. This drug is licensed for ADHD in children over 6 years of age, and has very strict prescription rules. It should only be initiated by an appropriately qualified healthcare professional with expertise in ADHD, and this should be based on a comprehensive assessment and diagnosis [7, 8]. Prescription should be limited to 28 days, and can be renewed if necessary. There are many side effects (mostly decreased appetite, and sleep problems [9, 10]), contraindications, (e.g. hyperthyroidism or thyrotoxicosis, severe depression, anorexia nervosa, history of suicide or suicidal thoughts, psychosis, and schizophrenia), warnings, and precautions for use (e.g. cardiovascular disorders, psychiatric disorders, growth, seizures) [11]. For these reasons, alternative treatments are greatly needed. Several authors found that clinical signs of essential fatty acid deficiency were systematically associated with ADHD [12]. Furthermore, children and adults with ADHD have been shown to have significantly lower plasma and blood concentrations of polyunsaturated fatty acids (PUFA) [13, 14]. Parletta et al. reported that children with ADHD had low levels of EPA, DHA and AA, as well as a high ratio of n − 6/n − 3 PUFAs, and that these correlated significantly with symptoms [15]. These findings support the therapeutic hypothesis that PUFA supplementation could reduce the attention and behaviour problems associated with ADHD.
Several studies and clinical trials have been performed to assess the effect of PUFA supplementation in ADHD [16, 17]. In a meta-analysis published by the Cochrane Collaboration in 2012 that included 13 trials and 1011 participants, most trials had a small sample size and some had a high attrition bias [18]. The authors concluded that, overall, there was little evidence that PUFA supplementation was beneficial and further high-quality research needed to be performed. Other meta-analyses, based mainly on the same studies, concluded that PUFA supplementation produced small but significant reductions in ADHD symptoms although the clinical significance of these effects remains to be determined [19], or that PUFA were modestly effective in the treatment of ADHD [20]. Our objective was, therefore, to perform a sufficiently powered randomised placebo-controlled trial to investigate the efficacy of an omega-3 supplement to improve ADHD symptoms in children with diagnosed ADHD.
Methods
This was a randomised (1:1), double-blind, placebo-controlled clinical trial. Randomization was performed according to a pre-established blocked randomization list, stratified by centre. This list was generated by the study statistician. To ensure concealment, all participating centres called the coordinating centre for group allocation (centralised randomization). This allowed a direct verification of eligibility criteria before inclusion. Patients, investigators, and the coordination centre were blinded to group allocation.
The study population included children and adolescents aged 6–15 years referred for hyperactivity symptoms to five reference centres for learning disabilities in France. ADHD diagnosis was performed by child psychiatrists specialised in ADHD according to DSM-IV-TR criteria. Briefly, children had to have at least six hyperactivity–impulsivity symptoms for six months or more, and/or at least one of six inattention symptoms for six months or more; certain symptoms had to be present before the age of 7 years, and there was a functional impairment in two or more environment (school, home), with a clinically significant alteration in the social, school, or family functioning. Symptoms had not to be part of another psychiatric disorder [1, 2].
Exclusion criteria were: known intolerance to omega-3 fatty acids, intake of fatty acid/fish oil dietary supplements for more than 1 week during the 3 months preceding inclusion, or MPH or other ADHD drug during the month preceding inclusion. Children who required MPH treatment were also excluded to ensure equipoise.
Intervention
The studied dietary supplement consisted of soft capsules containing fish oil rich in vitamin A, D, and E. The daily dosage was based on available data on recommended dietary intakes and doses used in previous studies: for children aged 6–8 years, EPA (eicosapentaenoic acid) 336 mg and DHA (docosahexaenoic acid) 84 mg; for children aged 9–11 years, EPA 504 mg and DHA 126 mg, and for children aged 12–15 years EPA: 672 mg and DHA 168 mg [18]; capsules also contained 100 µg vitamin A, 1.25 µg vitamin D, and 3.5 mg vitamin E. Treatment duration was 3 months, during which other hyperactivity treatments and other omega-3 supplements or psychotropic drugs were not allowed. The placebo capsules were indistinguishable from active capsules; this was assessed using panel testing. They were composed of olive oil, the same amount of vitamin A, D, and E, with traces of marine lipid concentrate: EPA (18%), DHA (12%), totalling 4.83 mg, to give the capsules a similar taste and smell. Strawberry flavour was added to improve compliance, which was assessed by pill count. Participants, care providers, those assessing outcomes, study coordinators and monitors were blinded to the administered treatment. Compliance was the number of days with supplement/placebo intake divided by the number of days the supplement/placebo should have been taken. Good compliance was defined as compliance ≥70% during the study overall, and during the last month of treatment.
Outcomes
The primary outcome was the parent-rated 18-item Attention-Deficit Hyperactivity Disorder Rating Scale (ADHD-RS) version IV [21, 22], measured at inclusion and 3 months. ADHD-RS-IV was scored at each follow-up visit, i.e. monthly. ADHD-RS-IV is an 18-item scale that rates symptoms of ADHD as outlined in the DSM-IV-TR. Each item is scored on a 4-point scale, ranging from 0 (no symptoms) to 3 (severe symptoms) and yielding a total score (range 0–54) and to two sub-scores (inattention and hyperactivity/impulsivity) [22]. Secondary outcomes included change of scores between baseline and 3 months derived from the following scales: (i) the long form of the revised Conners Parent Rating Scale (CPRS-R:L) [23] that includes 48 items divided into 7 dimensions (total score and sub-scores by dimension); the “L’Alouette” test, a standardised test for reading in French [24] which results in a lexical age; (ii) the battery of Attentional Performance Tests for Children (KiTAP for 6–10 years and TAP for 11–15 years), with a focus on three tests: Distractibility (6–10 years only), Flexibility, and Go/NoGo (the main items considered for evaluation of improvement after 3 months was the number of false responses and the Go/NoGo test) [25]; and (iii) The Children’s Depression Inventory (CDI) [26], a 27-item scale that is self-rated, symptom oriented, and results in a depression score.
Statistics
The sample size was calculated to demonstrate a greater 3-month reduction of the total ADHD-RS-IV in the group of children receiving DHA–EPA than in the placebo group; i.e. −5 in the placebo group vs. −10 in the DHA–EPA group (absolute reduction), with a common standard deviation of 10 (expected effect size of 0.5 SD), alpha equal to 5%, and 90% power, based on previous studies in ADHD with atomoxetine [27, 28]. The necessary sample size was 160 children, 80 per group.
The primary analysis planned in the protocol was a linear regression of the relative reduction (%) of the ADHD-RS-IV total score between baseline and 3 months (dependant variable). The independent variables were the treatment group and the covariates age and sex. A centre effect was also investigated (main effect and interaction after grouping the small centres). The significance threshold was 0.05. The statistical plan of analysis was amended to include the four measures at baseline, 1, 2, and 3 months in a longitudinal analysis of repeated measurements. The dependent variable was the total ADHD-RS-IV score. This analysis was performed using a mixed linear model (with the nlmixed procedure of SAS) for censored data (Tobit model to take into account the bounded distribution of the score). The independent variables were the time of evaluation (months), the type of treatment (DHAEPA or placebo), and its interaction with the time of evaluation, which is considered as the intervention effect. Age and sex were tested as covariates. The longitudinal analysis was also performed for each sub-score (inattention and hyperactivity/impulsivity).
When answers to less than half of the items of any component of the score were missing, answers were imputed from the answers to the other items of the same component [29]. In the ITT analysis of the primary outcome the last completed questionnaire was used to replace a missing questionnaire at 3 months. There were missing ADHD-RS values at 3 months only for 2 patients and also at 2 months for 3 patients; the LOCF replacement method was used for all of them. A sensitivity analysis was performed without replacing any data. A per protocol (PP) analysis was also performed in the same way as the primary analysis but restricted to patients without major protocol deviation (exclusion criteria, ADHD score missing at 3 months, compliance <70%, or received MPH).
Regression analyses were performed on CPRS-R:L and CDI scores using a mixed linear model for censored data, the independent variables being the time of evaluation (3 months versus baseline), the treatment group and its interaction with the time of evaluation. A similar analysis was used for the L’Alouette test with adjustment for sex and age. Univariate tests were used for the change in KITAP at three months (selected tests only, see outcomes section), depending on the nature of the variable (t test or Wilcoxon test for quantitative variables).
Results
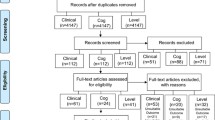
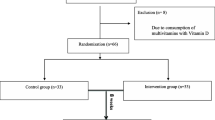
From January 2009 to December 2010, 162 children were included. Follow-up ended in March 2011 once all the planned visits were completed. Five patients had at least one missing ADHD-RS-IV questionnaire and their primary outcome could not be calculated for the per protocol analysis (3 prematurely withdrew, and 2 were lost to follow-up, 3 in the EDA–DHA group, and 2 in the placebo group); they were maintained in the intention to treat analysis. One patient in the EDA–DHA group was excluded because of an exclusion criterion. Four other children received MPH during the study (two in each group); they were maintained in the ITT analysis, but not in the PP analysis. Four had a compliance <70% and were excluded from the PP analysis (3 in the DHA–EPA group, 1 in the placebo group). The flow diagram of patients is shown Fig. 1. The compliance was good overall (n = 153, 97.5% were good compliers), and was not different between groups; n = 75 (96.2%) in the omega-3 group and n = 78 (98.7%) in the placebo group had good compliance. There was no significant difference in baseline characteristics between groups. Just over three-quarters (78.4%) of participants were boys, the mean age at inclusion was 9 years (SD 2.6), and the mean total ADHD-RS-IV was 37.3 (SD 8.4), i.e. with mild to moderate ADHD symptoms (Table 1).
The ADHD-RS-IV score decreased between inclusion and 3-month follow-up in both groups. The mean (95% CI) relative change of the ADHD-RS-IV between inclusion and 3 months was −19 (−26, −12) % in the placebo group and −9.7 (−16.6, −2.9) % in the DHA–EPA group. The decrease was significantly higher in the placebo group (p = 0.039, primary analysis, Table 2) with an observed effect size of +0.33 SD for the relative reduction. A sensitivity analysis with exclusion of patients with missing ADHD-RS-IV data did not modify the direction of the difference (p = 0.023). The PP analysis involved 148 children, (71 DHA–EPA and 77 placebo); children with missing follow-up ADHD score at 3 months, presenting an exclusion criterion, with compliance <70%, or who received MPH were excluded (Fig. 1). The method was the same as the primary analysis, and found similar results for the primary outcome (p = 0.084). For the longitudinal analysis of the ADHD-RS-IV score, the estimated values of the slopes in the placebo and DHA–EPA groups were −2.4 and −1.2 points per month, respectively. This score reduction over time was smaller in the DHA–EPA group compared to the placebo group; with a difference of 1.2 (0.2, 2.3) point per month (p = 0.026) and no difference at baseline (intercept: p = 0.38). The addition of covariates in the longitudinal analysis (age, sex, and centre) did not change the slope estimations. This was done after grouping small centres and it did not have any effect on the results. The same trends where found for the longitudinal analyses of the two sub-scores (inattention and hyperactivity/impulsivity). The difference of slope was 0.6 (−0.003, 1.2) point per month for inattention (p = 0.051) and 0.6 (0.05, 1.1) point per month for hyperactivity/impulsivity (p = 0.034). The change difference between groups at three month was 3.9 (0.5, 7.2) for ADHD total score, 1.8 (−0.1, 3.7) for inattention sub-score and 2.1 (0.3, 3.8) for hyperactivity/impulsivity sub-score (Table 2). The effect size was, respectively, 0.36, 0.30, and 0.36 in favour of placebo.
Secondary analyses performed on the CPRS-R and CDI scores did not find any significant difference between treatment groups. The reading age (L’Alouette test) between inclusion and month 3 increased by a mean 3 months, and there was no significant difference between groups (p = 0.28).
Evaluation using the KiTAP tests at inclusion found a higher number of false responses (Go/NoGo test: 4.8 vs. 6.3, p = 0.016), and a longer reaction time (mean difference 140 ms, p = 0.02) in the placebo group. These were the only significant differences found among the KiTAP components at inclusion. The 3-month evaluation found a longer reaction time in the placebo group (mean difference 101 ms, p = 0.02). This was the only significant difference found among the KiTAP components at 3 months.
Considering the absolute changes between inclusion and 3 months for the items considered, i.e. the items of the Go/NoGo test and the number of false responses for flexibility and distractibility tests, no statistically significant difference was found.
Safety analysis
Eleven (14.9%) children in the DHA–EPA group experienced 13 adverse events, 2 of which were judged to be related to the study treatment: hip pain, fatigue, headache, fever and cough (n = 2), dermatitis, allergic reaction (n = 2), abdominal pain, diarrhoea (n = 3), and depression. Eight children (10.7%) in the placebo group experienced 10 adverse events: fatigue, influenza (n = 2), abdominal pain, dermatitis, swollen eyes, vomiting, and diarrhoea (n = 3). Two patients in the DHA–EPA group experienced a severe adverse event (hospitalisation for worsening ADHD symptoms).
Discussion
This randomised double-blind trial found an improvement from baseline ADHD-RS-IV score in favour of the placebo group compared with omega-3 supplementation. The size of the benefit (+9 [0.3, 18.3] %), was statistically significant (p = 0.039), and clinically relevant [30] with 3.9 [0.5,7.2] points difference. This benefit, however, was not found statistically significant in the PP analysis but was confirmed in secondary analyses on the absolute change (ADHD total score and hyperactivity/impulsivity sub-score were in favour of placebo, and inattention sub-score was not statistically significant but the between-group difference was in the same direction). As there is no biological explanation for a greater effect in the placebo group compared with the active group, the result could be incidental.
The trial reported herein has the second largest sample size of all clinical trials published so far in the field, and was able to demonstrate an effect size of 0.3 in favour of the placebo which is a smaller effect than for which the study was calibrated; therefore a lack of power cannot explain the results [18, 31]. The results also seem robust because the analysis was based on the ITT principle involving 93% of included patients. This compares favourably to the majority of studies included in published meta-analyses [18,19,20, 31] that had a high proportion of loss to follow-up (18% on average [18]). Furthermore, participant compliance to treatment was good.
One limit of the study is that we did not measure blood levels of n − 3 unsaturated acids. We were, therefore, unable to verify whether the treatment was effective when there is a deficit in such fatty acids, as has been reported elsewhere [16]. Additionally, the dose used for 9- to 11-year-old children (EPA 504 mg and DHA 126 mg) is close to those previously shown to induce significant increases in plasma PUFA in children in the same age range: 345 mg DHA [26], 480 mg DHA, 40 mg AA, 96 mg GLA, and 24 mg α-tocopheryl acetate [32], and 500 mg EPA, 2.7 mg DHA, 10 mg Vitamin E [33]. The results are therefore unlikely to be due to an insufficient dose. The 3-month duration is similar to the duration of treatment and follow-up in most studies included in meta-analyses, which varies from 4 weeks (1 study) to 16 weeks (3 studies). Even though Hawkey and Nigg (2014) found no moderating effects of the duration of supplementation [31], we cannot exclude that longer treatment durations might have shown some benefit.
The results confirm those published by a previous meta-analyses reported by Gillies et al. (2012) [18] and who found no statistically significant differences in teacher ratings of overall ADHD symptoms (four trials, 324 participants; SMD 0.05, 95% CI [−0.18; 0.27]); inattention (three trials, 260 participants; SMD 0.26, 95% CI [−0.22; 0.74]) or hyperactivity/impulsivity (three trials, 259 participants; SMD 0.10, 95% CI [−0.16; 0.35]). In a more recent meta-analysis Hawkey and Nigg (2014) [31] combined “the best available parent and teacher report of inattention/hyperactivity”, stating: “despite finding a statistically reliable effect, the small effect size leads us to agree…that there is not enough evidence to recommend omega-3 fatty acids…as an alternative to existing empirically supported pharmacological and behavioural treatments”. Nonetheless, they concluded that “There is sufficient evidence to consider omega-3 fatty acids as a possible supplement to established therapies”. It must be emphasised that this review included reports of studies outside the scope of ADHD, [32,33,34,35,36]. We pooled data reported by Gillies et al. [18] and Hawkey and Nigg [31] for ADHD children, and calculated the standardised mean difference updated with data of the present study; there was no overall effect of DHA–EPA on ADHD-RS-IV: 0.01, 95% CI [−0.16; 0.17], p = 0.92 (data not shown).
Other meta-analyses concluded that PUFA supplementation produced small but significant reductions in ADHD symptoms, although the clinical significance of these effects remains to be determined [19], or that PUFA were modestly effective in the treatment of ADHD [20]. These meta-analyses analysed mainly the same studies. Bos and Oranje also published a review, and concluded that there is only limited evidence to support that omega-3 PUFA supplementation is beneficial in brain disorders, such as Alzheimer’s disease ADHD, major depressive disorder, and schizophrenia [37]. Furthermore, Lange et al. conclude that the benefits of PUFAs are much smaller than the effect sizes observed for traditional pharmacological treatments of ADHD. The effectiveness of PUFA supplements in reducing medication dosage needs to be confirmed [17].
The overall lack of efficacy of DHA–EPA is due to either an absence of a pharmacological effect or by the selection of children with moderate ADHD with little possibility of improvement. In the present study, the baseline mean total ADHD-RS-IV score was 37.3, which corresponds to “mild to moderate” ADHD [30]. It is possible that this population might not benefit from DHA–EPA supplementation. In this regard, it is of note that children requiring MPH were excluded since this study was placebo-controlled and children had to be able to receive either omega-3 or placebo for the study duration.
Burgess et al. criticised that previous studies did not select subjects on the basis of essential fatty acid status or frequency of symptoms of essential fatty acid deficiency [14]. The present study did not include either baseline fatty acid status, dietary intake (including fish consumption), or an assessment of change in plasma/RBC n − 3 LC-PUFA during PUFA exposure. Randomization might have avoided systematic differences between groups; however, this is a limitation of the present investigation. Yet it is of note that European adolescents do not have a sufficient dietary intake of PUFA as reported in the multicentre European study Helena (Healthy Lifestyle in Europe by Nutrition in Adolescence) which described the dietary fatty acids intake of a representative sample of an urban European adolescent population (including French adolescents), using 24 h recalls. Their mean total fat intake was 33.3 (SD 1.2) % of total energy intake. In most adolescents, the PUFA intake was too low, [38]. In this context, a supplementation might be useful, and a benefit in ADHD patients possible.
There is accumulating evidence that DHA–EPA supplementation does not improve symptoms of children with ADHD, and one may wonder whether it is worth continuing to perform clinical trials in this field. However, it remains to be elucidated whether certain children benefit from DHA–EPA supplementation, and, if this is the case, how these may be identified. Two large trials NCT02248948 with an expected sample size of 220 and NCT00819429 with 438 patients are on-going. Their results will add evidence on the efficacy of omega-3 rich dietary product for alleviating ADHD symptoms and may close the debate.
Conclusions
This study did not demonstrate the efficacy of supplementing children with omega-3 rich dietary products for alleviating ADHD symptoms in children with mild ADHD symptoms.
The study was approved by the regional ethics committee (Comité de Protection des Personnes Sud Est II), and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The study was registered in a clinical trials registry before recruitment (#NCT00770627). Both parents had to sign the informed consent form, and each participant ascent to participate, either orally or in writing.
Acknowledgements: The study was sponsored by the URGO laboratories. The sponsor had a role in the study design. The study was conducted, analysed and the article was written independently from the funding entity.
Coordinating centre: Lyon Clinical Investigation Center, Tassadit Bouaziz Clinical Research associate, Investigating centres: Child and Adolescent psychiatry, Neurological Hospital, Lyon, France; Emeline Peyric, Ingrid Lamoury, and Mathilde Vernet, Psychologists, Cecile Lassalle, Anne Henry, Alexandra Berthier, Marie Maud Geoffray, Daniel Gérard, Iris Vulliez-Degraix, Bertille Chiffolot, Associate Investigators. Child psychiatry, Mother Child and Women Hospital, Lyon, France: Laurence Lion-Francois, and Christelle Rougeot, Investigators. Pediatric Neurology Amiens: Laurent Querné PhD. Child and Adolescent psychiatry, Robert Debré Hospital, Paris, France: Diane Purper-Ouakil, Sara Bahadori, Baudoin Forgeot d’Arc, and Muriel Asch Investigators. Psychological Medicine, Saint Eloi Hospital, Montpellier, France: Anne Gramond, Pierre Raysse, Florence Pupier, Investigators.
Alves Fernandes Rodrigo; Penaud Lucile: EUDIPHARM Master students, Institut de Pharmacie Industrielle de Lyon, Université Claude Bernard Lyon I. Professor René Ecochard (statistics). Professor Hemamali Perera, Natalie (Sinn) Parletta, PhD, Bruno Harlé, Jean-Marc Sapori (Lyon, Centre Antipoisons, 24H/7d Emergency unblinding service).
We thank Philip Robinson (DRCI, Hospices Civils de Lyon) for help in manuscript preparation.
References
APA (2010) Diagnostic and statistical manual of mental disorders, 4th edn, text revision (DSM-IV-TR). American Psychiatric Association. 943 pp
American Psychiatric Association (1996) DSM4 V, Manuel diagnostique et statistique des troubles mentaux. Traduction française, Paris, Masson, 1056 pp
Cantwell DP (1996) Attention deficit disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry 35(8):978–987
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA (2007) The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 164(6):942–948
Faraone SV, Sergeant J, Gillberg C, Biederman J (2003) The worldwide prevalence of ADHD: is it an American condition? World Psychiatry 2(2):104–113
Schachter HM, Pham B, King J, Langford S, Moher D (2001) How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. CAMJ 165(11):1475–1488
Attention deficit hyperactivity disorder: diagnosis and management. NICE guidelines [CG72] Published date: September 2008 Last updated: February 2016
Storebø OJ, Krogh HB, Ramstad E, Moreira-Maia CR, Holmskov M, Skoog M, Nilausen TD, Magnusson FL, Zwi M, Gillies D, Rosendal S, Groth C, Rasmussen KB, Gauci D, Kirubakaran R, Forsbøl B, Simonsen E, Gluud C (2015) Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ 25(351):h5203
Kidwell KM, Van Dyk TR, Lundahl A, Nelson TD (2015) Stimulant medications and sleep for youth with ADHD: a meta-analysis. Pediatrics 136(6):1144–1153
Burgess JR, Stevens L, Zhang W, Peck L (2000) Long-chain polyunsaturated fatty acids in children with attention-deficit hyperactivity disorder. Am J Clin Nutr 71:327–330
Mitchell EA, Aman MG, Turbott SH, Manku M (1987) Clinical characteristics and serum essential fatty acid levels in hyperactive children. Clin Pediatr (Phila) 26(8):406–411
Burgess JR, Stevens L, Zhang W, Peck L (2000) Long-chain polyunsaturated fatty acids in children with attention-deficit hyperactivity disorder. Am J Clin Nutr 71(1 Suppl):327S–330S
Parletta N, Niyonsenga T, Duff J (2016) Omega-3 and omega-6 polyunsaturated fatty acid levels and correlations with symptoms in children with attention deficit hyperactivity disorder, autistic spectrum disorder and typically developing controls. PLoS ONE 11(5):e0156432
Richardson AJ, Puri BK (2000) The potential role of fatty acids in attention-deficit/hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids 63(1–2):79–87
Lange KW, Hauser J, Lange KM, Makulska-Gertruda E, Nakamura Y, Reissmann A, Sakaue Y, Takeuchi Y (2017) The role of nutritional supplements in the treatment of ADHD: what the evidence says. Curr Psychiatry Rep 19(2):8
Gillies D, Sinn JKh, Lad SS, Leach MJ, Ross MJ (2012) Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev, 7: p. CD007986
Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, Stevenson J, Danckaerts M, van der Oord S, Döpfner M, Dittmann RW, Simonoff E, Zuddas A, Banaschewski T, Buitelaar J, Coghill D, Hollis C, Konofal E, Lecendreux M, Wong IC, Sergeant J, European ADHD Guidelines Group (2013) Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry 170(3):275–289
Bloch MH, Qawasmi A (2011) Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 50(10):991–1000
DuPaul GJ, Power TJ, Anastopoulos AD (1998) ADHD Rating Scale IV: checklists, norms, and clinical interpretations. Guilford Press, New York (NY)
Mercier C, Roche S, Gaillard S et al (2016) Partial validation of a French version of the ADHD-rating scale IV on a French population of children with ADHD and epilepsy. Factorial structure, reliability, and responsiveness. Epilepsy Behav 58:1–6
Conners CK (1969) A teacher rating scale for use in drug studies with children. Am J Psychiatry 126(6):884–888
Lefavrais P. Test de l’Alouette, Éditions du Centre de Psychologie Appliquée. 2ème édition ed. 1967, Paris
Finch AJ Jr, Saylor CF, Edwards GL (1985) Children’s depression inventory: sex and grade norms for normal children. J Consult Clin Psychol 53(3):424–425
Michelson D, Allen AJ, Busner J et al (2002) Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 159(11):1896–1901
Michelson D, Faries D, Wernicke J et al (2001) Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 108(5):E83
Staquet Maurice J, Hays Ron D (1998) Peter M. Methods and Practice, Fayers. Quality of Life Assessment in Clinical Trials
Goodman D, Faraone SV, Adler LA et al (2010) Interpreting ADHD rating scale scores: linking ADHD rating scale scores and CGI levels in two randomized controlled trials of Lisdexamfetamine Dimesylate in ADHD. Primary Psychiatry 17(3):44–52
Hawkey E, Nigg JT (2014) Omega-3 fatty acid and ADHD: blood level analysis and meta-analytic extension of supplementation trials. Clin Psychol Rev 34(6):496–505
Stevens L, Zhang W, Peck L et al (2003) EFA supplementation in children with inattention, hyperactivity, and other disruptive behaviors. Lipids 38(10):1007–1021
Gustafsson PA, Birberg-Thornberg U, Duchén K et al (2010) EPA supplementation improves teacher-rated behaviour and oppositional symptoms in children with ADHD. Acta Paediatr 99(10):1540–1549
Itomura M, Hamazaki K, Sawazaki S et al (2005) The effect of fish oil on physical aggression in schoolchildren—a randomized, double-blind, placebo-controlled trial. J Nutr Biochem 16(3):163–171
Kirby A, Woodward A, Jackson S, Wang Y, Crawford MA (2010) A double-blind, placebo-controlled study investigating the effects of omega-3 supplementation in children aged 8–10 years from a mainstream school population. Res Dev Disabil 31(3):718–730
Richardson AJ, Burton JR, Sewell RP, Spreckelsen TF, Montgomery P (2012) Docosahexaenoic acid for reading, cognition and behavior in children aged 7–9 years: a randomized, controlled trial (the DOLAB Study). PLoS One 7(9):e43909. doi:10.1371/journal.pone.0043909. Epub 2012 Sep 6
Bos DJ, van Montfort SJ, Oranje B, Durston S, Smeets PA (2016) Effects of omega-3 polyunsaturated fatty acids on human brain morphology and function: what is the evidence? Eur Neuropsychopharmacol 26(3):546–561. doi:10.1016/j.euroneuro.2015.12.031. Epub 2015 Dec 21. Review
Vyncke KE, Libuda L, De Vriendt T, Moreno LA, Van Winckel M, Manios Y, Gottrand F, Molnar D, Vanaelst B, Sjöström M, González-Gross M, Censi L, Widhalm K, Michels N, Gilbert CC, Xatzis C, Cuenca García M, de Heredia FP, De Henauw S, Huybrechts I; HELENA consortium (2012) Dietary fatty acid intake, its food sources and determinants in European adolescents: the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Br J Nutr 108(12):2261–273. doi:10.1017/S000711451200030X. Epub 2012 Feb 28
Koletzko B, Uauy R, Palou A, Kok F, Hornstra G, Eilander A, Moretti D, Osendarp S, Zock P, Innis S (2010) Dietary intake of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in children—a workshop report. Br J Nutr 103(6):923–928
Voigt RG, Llorente AM, Jensen CL, Fraley JK, Berretta MC, Heird WC (2001) A randomized, double-blind, placebo-controlled trial of docosahexaenoic acid supplementation in children with attention-deficit/hyperactivity disorder. J Pediatr 139(2):189–196
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare they have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Cornu, C., Mercier, C., Ginhoux, T. et al. A double-blind placebo-controlled randomised trial of omega-3 supplementation in children with moderate ADHD symptoms. Eur Child Adolesc Psychiatry 27, 377–384 (2018). https://doi.org/10.1007/s00787-017-1058-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-017-1058-z