Abstract
Objectives
This pilot randomized controlled clinical trial compares the clinical outcome obtained in persistent periodontal pockets during 9-month follow-up of supportive periodontal step 4 treatment performed by either combining subgingival instrumentation with adjunctively used sodium hypochlorite/amino acid gel and crosslinked hyaluronic acid (xHyA) or subgingival instrumentation alone.
Materials and methods
Study protocol is registered under NCT06438354 at Clinicaltrials.gov. Patients seeking further therapy after completed step 2 non-surgical periodontal treatment underwent either repeated subgingival instrumentation with adjunctive application of sodium hypochlorite/amino acid gel and crosslinked hyaluronic acid (group A) or repeated subgingival instrumentation alone (group B). One calibrated investigator performed the treatment sequence in both groups accordingly. Subgingival instrumentation of the residual pockets was carried out under local anaesthesia using hand- and ultrasonic instruments, as well as air polishing in both groups. Patients were instructed to continue oral hygiene without any restriction. At 3-month re-evaluation treatment was repeated accordingly at sites with persistent 5 mm probing depth and BoP + . Clinical attachment level (CAL), pocket probing depth (PPD), gingival recession (GR), and bleeding on probing (BoP) were recorded at baseline (T1), 3- (T2) and 9-month (T3) post-op, with CAL as a primary outcome measure.
Results
In total 52 patients (20 females and 32 males, mean age 58.4 ± 2.4 years) presenting with 1448 sites which required further periodontal treatment were enrolled. Both groups exhibited homogeneity in terms of age, gender, smoking habit, initial number of sites, and BOP. At 9-month evaluation, PD reduction and CAL gain showed significant differences between the test and control group, favouring the adjunctive treatment. GR tended to exhibit more recovery in the test group compared to the control group. Although BOP frequency effectively reduced in both groups, there was no statistically significant difference between the two groups.
Conclusion
Within the limits of the study, the present data indicates that, during subgingival instrumentation of persistent pockets, the adjunctive usage of sodium hypochlorite/amino acid gel and xHyA sufficiently improves the clinical outcomes. The continuous improvement of CAL in association with the GR scores observed in group A, indicates that sites subjected to adjunctive treatment may indicate a tendency for a regenerative response to treatment within the 9-month follow-up period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontitis, a predominantly chronic inflammatory disease caused by dysbiotic dental plaque biofilms, poses a substantial global health burden. According to the Global Burden of Disease Study, periodontitis ranks sixth worldwide in terms of prevalence, with an alarming 743 million reported cases [1]. Given the progressively aging global population and the increased retention of natural teeth, a heightened global burden is anticipated in the future [2]. Additionally, periodontitis demonstrates bidirectional links with systemic conditions such as atherosclerosis and diabetes [3, 4]. Consequently, the prevention, diagnosis, and effective treatment of periodontitis are paramount.
The primary goal of periodontitis therapy is to resolve periodontal inflammation, expressed by the absence of bleeding, and reduce probing depths to less than 4 mm, preferably alongside with minimal gingival recession [5, 6]. While the efficacy of non-surgical periodontal therapy is evident, residual periodontal pockets are regularly observed at re-evaluation performed during supportive periodontal therapy (SPT). Residual periodontal pockets act as reservoirs for pathogenic bacteria, which lead to the persistence in inflammation and correlate with an increased risk for tooth loss [7]. Since persisting inflammation exhibits a severe risk of disease progression, the timely treatment of respective periodontal pockets is a clinical imperative to establish long-term periodontal stability. Residual or persisting pockets are classified as such if sites present with 4 mm PPD and positive BOP or 5 and more mm in depth. According to the EFP treatment guidelines, these sites are supposed to refer to Step 3 therapy, which may encompass different surgical approaches or repeated instrumentation [8]. However, periodontal surgery may be technically challenging for general practitioners and associated with increased patient morbidity, albeit it remains the most effective treatment option according to the clinical literature in deeper sites [9]. Possible reasons for the inferiority of non-surgical re-instrumentation may include challenging removal of calculus due to restricted accessibility of sites with deep probing depth, or involvement of multi-rooted teeth [10, 11]. For this reason, various adjunctive protocols have been proposed to enhance the efficacy of non-surgical periodontal therapy recently. To date, many adjunctive interventions oscillate between antibacterial effect of local antibiotic chemotherapy, anti-plaque chemical agents, or photodynamic therapy [12, 13]. While the use of biologics, such as enamel matrix derivatives, hyaluronic acid, and platelet-rich fibrin for regenerative periodontal surgery has steadily increased, applying these materials to non-surgical therapy is still uncommon [14,15,16]. Intriguingly, the integrative application of a sodium hypochlorite/amino acid and crosslinked hyaluronic acid (xHyA) as a regenerative adjunct in the non-surgical treatment of residual periodontal pockets has demonstrated significant attachment level gain in two retrospective studies [17, 18]. However, adequately controlled data supporting the efficacy of this protocol during supportive periodontal therapy is lacking.
This pilot randomized controlled clinical trial investigated the efficacy of adjunctive sodium hypochlorite, and xHyA in treating residual periodontal pockets during supportive periodontal therapy.
Materials and methods
Study design
The randomized controlled trial protocol received approval by the Ethics Committee at Witten/Herdecke University (No. 64/2022). The participants informed about the freedom to quit the study whenever they want in agreement with the Helsinki declaration of medical research and confirmed that the data may be used for scientific research. Clinicaltrials.gov registered the study with the ID number: NCT06438354 as a pilot RCT. All participants had been diagnosed with residual periodontal pockets either exceeding 5 mm or equalling 4-5 mm in PPD with bleeding on probing (BOP) at the first re-evaluation after step 2 periodontal therapy [19]. Before enrolment, all patients signed a written informed consent form. After signing the consent form, patients were randomly allocated to either test group (group A, subgingival instrumentation plus adjunctive protocol) or control group (group B, subgingival instrumentation only).
The study was conducted according to current standards of clinical research (CONSORT guidelines: http://www.consort-statement.org/) [20] between August 2022 and October 2023 in a private dental office in Munich, Germany.
Inclusion and exclusion criteria
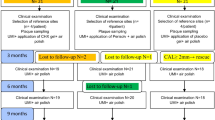
The inclusion criteria encompassed systemically healthy adult individuals who were previously diagnosed with periodontitis according to the clinical practice guideline and who had completed step 1 and 2 therapy initially presenting with untreated periodontitis at stage 3 or 4 (Fig. 1) [8]. In particular, the study focused on persistent periodontal pockets characterized by a probing pocket depth (PPD) of ≥ 5 mm or 4 mm with positive BOP assessed at first re-evaluation following step 2 treatment. The exclusion criteria comprised individuals with unregulated T2DM with an HbA1c scores > 7.5%, other chronic conditions such as rheumatoid arthritis, or pregnancy and lactating. The allocation of sites for therapy per patient was not constrained, and there were no restrictions on the location of residual sites. Both, single- and multi-rooted teeth were considered for the study.
Sample size calculation
At setting the protocol for the study, no numbers for the proposed therapy option were available from literature so far. Conceptualization of a pilot clinical investigation estimated a relevant average difference in CAL of 1 mm between both study groups with a standard deviation of 1 mm. Thus, the number of necessary cases to demonstrate the anticipated effect with a power of at least 80% was estimated with 24 per group. Accounting for possible dropouts during the study period, the number of patients was increased to 27 in each group.
Randomization and blinding
Fifty-two patients were randomized into two treatment groups. A computer-generated randomization table was created. Patients were assigned unique numbers from 1 to 52, and 2 sets of randomized numbers were generated (26 for control group subjects and 26 for test). Allocation concealment was performed using sealed envelopes to be opened before SPT treatment after the probing depth was re-evaluated. The generation of the random sequence allocation and the assignment of participants to interventions were performed by the investigator, who performed the treatment herself (L.B.). Thus, there was no blinding of the investigator possible in study setting.
Treatment protocol
One calibrated investigator (L.B.) performed the subgingival instrumentation under local anesthesia with 4% articaine (Ultracain DS, Sanofi, Germany). The calibration was achieved by measuring the PPD and Recession at the phantom model for diagnosing periodontitis (A-PB Frasaco, Germany) in three separate cycles. The study coordinator (A.F.) controlled the evaluated numbers, calibration fulfilled the requirements since overall agreement between three assessments exceeded 90% level.
Patients enrolled have been re-instructed in oral hygiene measures and treated by the operator (L.B.). The approximal plaque index (API) documented the progress in improving adequate oral hygiene performed by the patients at home [21]. The change in the scores of Approximal Plaque Index (API) compared the level assessed at evaluation after initial therapy with the scores assessed during the study.
The mechanical treatment regimen was identical for sites from both the test and control groups, respectively. In brief, sites underwent subgingival instrumentation by using Gracey curettes (HU-Friedy Group, Chicago, USA), ultrasonic instruments (SONICflex; KaVo, Biberach an der Riß, Germany), and glycine powder air polishing (Airflow; EMS, Vallée de Joux, Switzerland). In the test group (group A), each site intended to treat received subgingivally applied sodium hypochlorite cleaning gel (Perisolv; Regedent AG, Zürich, Switzerland) for 30 s before starting SI. Thereafter, a thorough mechanical debridement of the biofilm followed. The application of sodium hypochlorite gel repeated twice to enhance decontamination effect. An explorer probe (EXS3A6, HU-Friedy Group, Chicago, USA) verified the desired and appropriate instrumentation result. Concomitantly, the sites received 0.2–0.3 ml of xHyA (hyaDENT BG, Regedent AG, Zürich, Switzerland) up to the gingival margin.
Patients received instructions to continue oral hygiene without any restriction after completing the SPT visit regardless treatment group allocation. Subsequently, within the following 7 days, a second application of xHyA (0.2–0.3 ml) was placed subgingivally alongside with the control of oral hygiene. At three months re-evaluation, the treatment was repeated according to patient’s allocation in sites presenting with persistently constant PPD ≥ 5 mm and positive BOP. The final re-evaluation took place 9 months post-treatment (Fig. 1).
Statistical analysis
Descriptive statistics were applied for the metrical variables pocket probing depth (PPD), clinical attachment level (CAL), and recession, including mean, standard deviation, median, minimum, and maximum. For nominal data (BOP), percentages were calculated to summarize the data. The primary parameter was CAL, while PPD, BOP and Rec applied as secondary parameters. To determine univariate differences between groups Analysis of Variance (ANOVA) or Chi-Square-Test in the case of nominal data were calculated. For the analysis of group effects repeated-measures ANOVA models were performed on the outcomes CAL, PPD, GR. In case of variance heterogeneity across time determined by Mauchly's test of sphericity, Greenhouse–Geisser adjusted testing was applied. Treatment group by time interaction effects were additionally quantified using 95% confidence intervals and partial eta square as effect size η2. Values of 0.01–0.06, 0.06–0.14, and more than 0.14 were interpreted as small, medium, or large effects, respectively. Statistical analysis performed with the IBM SPSS 27 software package (IBM Corp.).
Outcomes displayed using estimated marginal means across the time points for both groups. For all analyses, a two-tailed significance level of α = 5% was applied.
Results
The present study enrolled 52 patients with 1448 sites intended to treat after two patients allocated to group A withdrawn participation agreement. The mean age was 58.6 ± 12.4, with 20 patients being females (40%) and 30 males (60%). All patients were normoglycemic, and 15 patients (30%) were smokers. The oral hygiene improved continuously from the baseline level to the final examination 9 months after starting this trial. Figure 2 displays the positive development of oral hygiene performance at mean level for both groups. Both groups exhibited homogeneity in terms of age, gender, initial number of sites (31.38 ± 21.35 vs. 28.63 ± 17.60, respectively; p = 0.622), and BOP frequency at baseline evaluation (Table 1). According to the randomized allocation, 764 sites received the adjunctive protocol (treatment group A), while 684 sites received the subgingival treatment without adjunctives (treatment group B). Summarizing the treated sites by patient allocation, 25 patients underwent treatment by option A and 27 patients by option B. Fifty out of 52 patients completed the study; two dropouts occurred in group B following the 3-month reevaluation. One patient relocated, while the other one had to be excluded due to inadequate compliance. Healing was uneventful in all patients; participants did not report any unexpected adverse events, and the investigator observed neither.
At the outset (T1), baseline mean values for the primary parameter, CAL indicated no significant variance between the two groups (5.85 ± 1.42 mm for group A vs. 6.07 ± 1.59 mm for group B), (p = 0.105). Nevertheless, as the intervention effect unfolded, Group A consistently demonstrated a remarkable enhancement in CAL compared to Group B at both 3- and 9-month visits (T2: 4.44 ± 1.37 mm and T3: 3.76 ± 1.18 mm for group A vs. T2: 4.85 ± 1.66 mm and T3: 4.59 ± 1.70 mm for group B, respectively), with these distinctions holding statistical significance (p = 0.001).
Similarly, the means for PPD showed no statistically significant divergence between Group A and Group B (T1: 4.74 ± 0.99 mm group A vs. 4.69 ± 1.01 mm group B) (p = 0.193, Table 2). As the study progressed, both groups exhibited a notable and significant reduction in PPD (T2: 3.50 ± 1.03 mm in group A and 3.35 ± 1.08 mm in group B; T3: 2.94 ± 0.82 mm and 3.14 ± 1.01 mm, respectively) (Table 2). However, Group A exhibited a more substantial reduction than Group B at both, the 3- and 9-month evaluations and these disparities were statistically significant at the 9-month evaluation (p = 0.001, Table 4).
Gingival Recession (GR) scores were significantly different between Group A and B at baseline. Throughout the 9-month evaluation, Group A displayed insignificant but rather positive changes, while Group B showed little progression in GR depth from Baseline to 3 months, remaining constantly at the same level for a further 6 months. The intergroup difference in mean GR level at both evaluations was statistically significant (p = 0.001) (Fig. 3a-d).
Conversely, the BOP frequency yielded no statistically significant difference between the two groups at any time point, neither at baseline, at 3-months, nor 9-month evaluation, with p-values of 0.796, 0.175, and 0.339, respectively. Details of the clinical parameters and their alterations presented in Table 2. The frequency of pocket closure (PPD ≤ 4 mm / BOP negative) after the 9-month observation period was indifferent in the shallow pockets (4-5 mm). Interestingly, the pocket closure rate decreased in the deeper pockets but was still higher in the treatment group. In the very deep pockets > 7 mm, group A exhibited a pocket closure rate of 94%, while the control group only exhibited 42% pocket closure (Table 3).
The inter-group comparison of the means for all four parameters at any time point of the study displayed by Table 4, showing the respective level of significance and the partial effect size (partial η2).
Discussion
The present study evaluated the clinical effect of the adjunctive combination of sodium hypochlorite/amino acid gel and xHyA for treating residual inflamed periodontal pockets during SPT in a private praxis environment. Clinically, the treatment and the healing were uneventful in all cases. The study results reveal that repeated instrumentation during SPT was an effective option to further improve clinical periodontal parameters in both groups. However, the adjunctive protocol investigated in this study yielded better results than the control group regarding attachment level gain. Intriguingly, the pocket closure rates were significantly higher in test group as compared to the controls when looking at deep residual pockets (Table 3), indicating that the adjunctive protocol was highly beneficial for deep residual sites. Thus, the response in sites designated for surgical intervention due to scores assessed at re-evaluation emphasized the probability of waiving surgery by means of meticulous instrumentation supported by adjunctive use of hypochlorite/amino acids and xHyA.
The three-months evaluation of periodontal conditions after completion of initial periodontal treatment applies to German regulations for treating periodontitis within the health insurance system, but is also backed up by the recent meta-analysis [22]. The data extracted from 29 RCT studies leave no room for the assumption the re-evaluation performed three months after completion of initial subgingival instrumentation may impair ongoing healing. While our group set up the protocol and conducted the clinical part for the step 3 SPT treatment in a private praxis environment, the group from Lithuania used the same adjunctive protocol at step 2 treatment of periodontal pockets [23]. The authors reported significantly better PD reduction over an observation period of 6 months for the test group compared to controls (2.9 ± 0.4 vs 1.8 ± 0.6 mm, p < 0.001, respectively). Similarly, mean CAL gain was statistically higher in the test group compared to the control one (test: 2.6 ± 0.5 vs. control: 1.6 ± 0.6 mm, p < 0.001). The notable pocket closure rate in sites with PPD exceeding 6 mm dropped from total of 298 (8.7%) to 4 (0.1%) in test group (p = 0.003), and from 277 (7.6%) to 35 (1.0%) in the controls. The declining detection rates for five targeted periodontal pathogens in samples obtained from subgingival compartment at three clinical visits accordingly to clinical examinations reflected the abovementioned benefit of the adjunctive protocol applied at step 2 periodontal therapy. The detection frequencies for A.actinomycetemcomitans, P. gingivalis, Pr. Intermedia, T. denticola and T. forsythsia were significantly smaller in the test group vs. controls in 6-month samples [24]. Thus, the counts of the targeted putative periodontal pathogens showed significantly and continuously reduced scores in sites that showed the greatest clinical response in the test group, whereas the controls demonstrated a kind of rebound in colonization by the same microorganisms during the observation period of 6 months. The conformity of clinical and microbiological outcomes underscored the benefit of the combined hypochlorite/ amino acid gel use followed by xHyA application after completed NSPT. Although this study addressed another step in the treatment flow of periodontal pockets, our clinical outcome agreed with the data cited above pointing out the universal benefit proposed approach unfolds in a non-surgical treatment for deep periodontal pockets. The clinical parameters, PPD, CAL, BOP improved significantly from baseline to 6-months reevaluation in a case series which used same adjunctives during step 2 therapy [23].
Similar clinical results we recorded in this study over a period of 9 months post-op. However, the treatment population was recruited from the patients who presented with residual active periodontal pockets and were referred to either step 3 or 4 – the SPT. Intriguingly, the pocket closure rates were substantially higher in the treatment group, while patients with shallow pockets did not seem to benefit from the adjunctive treatment. This observation may partly explain the contradicting results for xHya-supported surgical and non-surgical treatment protocols [25]. A very similar rate in BOP frequency in both groups consistently recorded from baseline to latest re-evaluation may be related to the masking effect which smoking induces on bleeding on probing frequency by duration of the habit [26]. In our study, about 30% of participants in both groups were smokers.
In vitro studies, sodium hypochlorite has proven effective against gram-negative species-dominated biofilms [27]. In addition, coating dentin surfaces with hypochlorite/amino acid also promoted the proliferation of periodontal ligament fibroblasts on these surfaces in vitro [28]. Moreover, subgingivally applied as a singular adjunctive for treating periodontitis, the hypochlorite/amino acid gel has been shown to enhance antimicrobial and therapeutic effects expressed by improved clinical parameters in randomized clinical studies [29, 30]. However, in a randomized clinical trial, the additional use of sodium hypochlorite gel did not affect the outcomes of manual or ultrasonic subgingival instrumentation in a limited group of patients at the SPT stage [31].
Hyaluronic acid, a ubiquitous component in mammalian tissues, exhibits a versatile spectrum of properties. Studies have demonstrated that HA has a bacteriostatic effect, contributing to minimizing bacterial contamination in surgical wounds [32]. Additionally, HA has a fungistatic effect on the growth of Candida albicans [33]. However, using xHyA as an adjunct to mechanical treatment of periodontal pockets failed to unleash a greater effect than subgingival instrumentation alone in a 12-month randomized non-surgical study [14].
Results from animal studies with a surgical approach proved the potential of xHyA to support new attachment formation in a recession canine model, an acute intrabony defect, and an acute furcation Grade 3 model. The microscopic outcome in all these experiments proved new cementum, ligamentum, and bone formation, respectively. Histomorphometric assessment revealed a clinically relevant amount of newly organized tissues in sites treated with xHyA [34,35,36]. HA presence can also promote osteosynthesis by enhancing mesenchymal cell differentiation in critical-sized bone defects and has been shown to stimulate osteoblasts, underscoring a great potential for periodontal regeneration [37, 38].
Accordingly, a randomized controlled trial comparing the efficacy of enamel matrix derivatives and xHya in treating contained three-wall intrabony defects reported equal regenerative outcomes for both biologics [39]. Effective decontamination of the root surface and periodontal pocket is a prerequisite for effective regeneration and represents a major rationale for flap elevation. Same requirement is a particular challenge for achieving regenerative healing by non-surgical approach. The recent animal study performed in chronic infected intrabony pockets by means of non-surgical subgingival instrumentation corroborated at histomorphometrical level the regenerative pattern of tissue formation if the adjunctive hypochlorite/amino acid and xHyA treatment was applied [40].
Naturally, this study exhibits some limitations. Because it is a single-center investigation in a private clinic involving just one investigator, a chance of investigator bias is inherent to this study. Moreover, radiographic validation of the clinical findings is warranted in future studies.
Nonetheless, the study's findings were clinically relevant, advocating for incorporating proposed adjunctive therapy in residual periodontal pocket management. Moreover, the high pocket closure rate of deep sites indicates that the adjunctive use of sodium hypochlorite and xHya may circumvent surgical intervention. Future studies must, therefore employ more independent and blinded investigators to increase the generalizability of findings.
Conclusions
Within the limitations of this mono centre RCT study we consider the proposed adjunctive treatment protocol sufficient in improving clinical conditions in persisting residual periodontal pockets at relevant level during SPT visit. Thus, patients showing off with residual deep sites at reevaluation may carry a sustained benefit from such extended therapy effort.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Witten/Herdecke University, Department of Periodontology.
References
Kassebaum NJ, Smith AGC, Bernabe E, Fleming TD, Reynolds AE, Vos T, Murray CJL, Marcenes W, Collaborators GBDOH (2017) Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J Dent Res 96(4):380–387. https://doi.org/10.1177/0022034517693566
Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J (2017) Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J Clin Periodontol 44(5):456–462. https://doi.org/10.1111/jcpe.12732
Sanz M, Del Castillo AM, Jepsen S, Gonzalez-Juanatey JR, D’Aiuto F, Bouchard P, Chapple I, Dietrich T, Gotsman I, Graziani F, Herrera D, Loos B, Madianos P, Michel JB, Perel P, Pieske B, Shapira L, Shechter M, Tonetti M, Vlachopoulos C, Wimmer G (2020) Periodontitis and Cardiovascular Diseases. Consens Report Glob Heart 15(1):1. https://doi.org/10.5334/gh.400
Isola G, Tartaglia GM, Santonocito S, Polizzi A, Williams RC, Iorio-Siciliano V (2023) Impact of N-terminal pro-B-type natriuretic peptide and related inflammatory biomarkers on periodontal treatment outcomes in patients with periodontitis: An explorative human randomized-controlled clinical trial. J Periodontol 94(12):1414–1424. https://doi.org/10.1002/JPER.23-0063
Nibali L, Sun C, Akcali A, Meng X, Tu YK, Donos N (2017) A retrospective study on periodontal disease progression in private practice. J Clin Periodontol 44(3):290–297. https://doi.org/10.1111/jcpe.12653
Nibali L, Pometti D, Tu YK, Donos N (2011) Clinical and radiographic outcomes following non-surgical therapy of periodontal infrabony defects: a retrospective study. J Clin Periodontol 38(1):50–57. https://doi.org/10.1111/j.1600-051X.2010.01648.x
Matuliene G, Pjetursson BE, Salvi GE, Schmidlin K, Bragger U, Zwahlen M, Lang NP (2008) Influence of residual pockets on progression of periodontitis and tooth loss: results after 11 years of maintenance. J Clin Periodontol 35(8):685–695. https://doi.org/10.1111/j.1600-051X.2008.01245.x
Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, Beglundh T, Sculean A, Tonetti MS, Participants EFPW, Methodological C (2020) Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J Clin Periodontol 47(Suppl 22):4–60. https://doi.org/10.1111/jcpe.13290
Sanz M, Meyle J (2010) Scope, competences, learning outcomes and methods of periodontal education within the undergraduate dental curriculum: a consensus report of the 1st European workshop on periodontal education–position paper 2 and consensus view 2. Eur J Dent Educ 14:25–33
Rateitschak-Pluss EM, Schwarz JP, Guggenheim R, Duggelin M, Rateitschak KH (1992) Non-surgical periodontal treatment: where are the limits? An SEM study. J Clin Periodontol 19(4):240–244. https://doi.org/10.1111/j.1600-051x.1992.tb00460.x
Rabbani GM, Ash MM Jr, Caffesse RG (1981) The effectiveness of subgingival scaling and root planing in calculus removal. J Periodontol 52(3):119–123. https://doi.org/10.1902/jop.1981.52.3.119
Salvi GE, Stahli A, Schmidt JC, Ramseier CA, Sculean A, Walter C (2020) Adjunctive laser or antimicrobial photodynamic therapy to non-surgical mechanical instrumentation in patients with untreated periodontitis: A systematic review and meta-analysis. J Clin Periodontol 47(Suppl 22):176–198. https://doi.org/10.1111/jcpe.13236
Herrera D, Matesanz P, Martin C, Oud V, Feres M, Teughels W (2020) Adjunctive effect of locally delivered antimicrobials in periodontitis therapy: A systematic review and meta-analysis. J Clin Periodontol 47(Suppl 22):239–256. https://doi.org/10.1111/jcpe.13230
Pilloni A, Zeza B, Kuis D, Vrazic D, Domic T, Olszewska-Czyz I, Popova C, Kotsilkov K, Firkova E, Dermendzieva Y, Tasheva A, Orru G, Sculean A, Prpic J (2021) Treatment of residual periodontal pockets using a hyaluronic acid-based gel: A 12 month multicenter randomized triple-blinded clinical trial. Antibiotics (Basel) 10(8). https://doi.org/10.3390/antibiotics10080924
Miron RJ, Fujioka-Kobayashi M, Bishara M, Zhang Y, Hernandez M, Choukroun J (2017) Platelet-rich fibrin and soft tissue wound healing: A systematic review. Tissue Eng Part B Rev 23(1):83–99. https://doi.org/10.1089/ten.TEB.2016.0233
Hagi TT, Laugisch O, Ivanovic A, Sculean A (2014) Regenerative periodontal therapy. Quintessence Int 45(3):185–192. https://doi.org/10.3290/j.qi.a31203
Diehl D, Friedmann A, Liedloff P, Jung RM, Sculean A, Bilhan H (2022) Adjunctive application of hyaluronic acid in combination with a sodium hypochlorite gel for non-surgical treatment of residual pockets reduces the need for periodontal surgery-retrospective analysis of a clinical case series. Materials (Basel) 15(19). https://doi.org/10.3390/ma15196508
Ramanauskaite E, Machiulskiene V, Dvyliene UM, Eliezer M, Sculean A (2023) Clinical evaluation of a novel combination of sodium hypochlorite/amino acid and cross-linked hyaluronic acid adjunctive to non-surgical periodontal treatment: A case series. Oral Health Prev Dent 21(1):279–284. https://doi.org/10.3290/j.ohpd.b4347453
Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, Beglundh T, Sculean A, Tonetti MS, Participants EFPW, Methodological C (2020) Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J Clin Periodontol 47(Suppl 22):4–60. https://doi.org/10.1111/jcpe.13290
Schulz KF, Altman DG, Moher D, Group C (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 152(11):726–732. https://doi.org/10.7326/0003-4819-152-11-201006010-00232
Lange DE, Plagmann HC, Eenboom A, Promesberger A (1977) Clinical methods for the objective evaluation of oral hygiene. Dtsch Zahnarztl Z 32(1):44–47
Paterno Holtzman L, Valente NA, Vittorini Orgeas G, Copes L, Discepoli N, Clementini M (2024) Change in clinical parameters after subgingival instrumentation for the treatment of periodontitis and timing of periodontal re-evaluation: A systematic review and meta-analysis. J Clin Periodontol. https://doi.org/10.1111/jcpe.13985
Ramanauskaite E, Machiulskiene V, Shirakata Y, Dvyliene UM, Nedzelskiene I, Sculean A (2023) Clinical evaluation of sodium hypochlorite/amino acids and cross-linked hyaluronic acid adjunctive to non-surgical periodontal treatment: a randomized controlled clinical trial. Clin Oral Investig 27(11):6645–6656. https://doi.org/10.1007/s00784-023-05271-0
Ramanauskaite E, Machiulskiene Visockiene V, Shirakata Y, Friedmann A, Pereckaite L, Balciunaite A, Dvyliene UM, Vitkauskiene A, Baseviciene N, Sculean A (2024) Microbiological effects of sodium hypochlorite/-amino acids and cross-linked hyaluronic acid adjunctive to non-surgical periodontal treatment. Oral Health Prev Dent 22:171–180. https://doi.org/10.3290/j.ohpd.b5281925
Pilloni A, Zeza B, Kuis D, Vrazic D, Domic T, Olszewska-Czyz I, Popova C, Kotsilkov K, Firkova E, Dermendzieva Y (2021) Treatment of residual periodontal pockets using a hyaluronic acid-based gel: A 12 month multicenter randomized triple-blinded clinical trial. Antibiotics 10(8):924
Al-Bayaty FH, Baharuddin N, Abdulla MA, Ali HM, Arkilla MB, ALBayaty MF (2013) The influence of cigarette smoking on gingival bleeding and serum concentrations of haptoglobin and alpha 1-antitrypsin. Biomed Res Int 2013:684154. https://doi.org/10.1155/2013/684154
Jurczyk K, Nietzsche S, Ender C, Sculean A, Eick S (2016) In-vitro activity of sodium-hypochlorite gel on bacteria associated with periodontitis. Clin Oral Investig 20(8):2165–2173. https://doi.org/10.1007/s00784-016-1711-9
Mueller A, Fujioka-Kobayashi M, Mueller HD, Lussi A, Sculean A, Schmidlin PR, Miron RJ (2017) Effect of hyaluronic acid on morphological changes to dentin surfaces and subsequent effect on periodontal ligament cell survival, attachment, and spreading. Clin Oral Investig 21(4):1013–1019. https://doi.org/10.1007/s00784-016-1856-6
Radulescu V, Boariu MI, Rusu D, Roman A, Surlin P, Voicu A, Didilescu AC, Jentsch H, Siciliano VI, Ramaglia L, Vela O, Kardaras G, Sculean A, Stratul SI (2022) Clinical and microbiological effects of a single application of sodium hypochlorite gel during subgingival re-instrumentation: a triple-blind randomized placebo-controlled clinical trial. Clin Oral Investig 26(11):6639–6652. https://doi.org/10.1007/s00784-022-04618-3
Iorio-Siciliano V, Ramaglia L, Isola G, Blasi A, Salvi GE, Sculean A (2021) Changes in clinical parameters following adjunctive local sodium hypochlorite gel in minimally invasive nonsurgical therapy (MINST) of periodontal pockets: a 6-month randomized controlled clinical trial. Clin Oral Investig 25(9):5331–5340. https://doi.org/10.1007/s00784-021-03841-8
Megally A, Zekeridou A, Cancela J, Giannopoulou C, Mombelli A (2020) Short ultrasonic debridement with adjunctive low-concentrated hypochlorite/amino acid gel during periodontal maintenance: randomized clinical trial of 12 months. Clin Oral Investig 24(1):201–209. https://doi.org/10.1007/s00784-019-02949-2
Pirnazar P, Wolinsky L, Nachnani S, Haake S, Pilloni A, Bernard GW (1999) Bacteriostatic effects of hyaluronic acid. J Periodontol 70(4):370–374. https://doi.org/10.1902/jop.1999.70.4.370
Kang JH, Kim YY, Chang JY, Kho HS (2011) Influences of hyaluronic acid on the anticandidal activities of lysozyme and the peroxidase system. Oral Dis 17(6):577–583. https://doi.org/10.1111/j.1601-0825.2011.01807.x
Shirakata Y, Imafuji T, Nakamura T, Kawakami Y, Shinohara Y, Noguchi K, Pilloni A, Sculean A (2021) Periodontal wound healing/regeneration of two-wall intrabony defects following reconstructive surgery with cross-linked hyaluronic acid-gel with or without a collagen matrix: a preclinical study in dogs. Quintessence Int 0(0):308–316. https://doi.org/10.3290/j.qi.b937003
Shirakata Y, Imafuji T, Nakamura T, Shinohara Y, Iwata M, Setoguchi F, Noguchi K, Sculean A (2022) Cross-linked hyaluronic acid gel with or without a collagen matrix in the treatment of class III furcation defects: A histologic and histomorphometric study in dogs. J Clin Periodontol. https://doi.org/10.1111/jcpe.13694
Shirakata Y, Nakamura T, Kawakami Y, Imafuji T, Shinohara Y, Noguchi K, Sculean A (2021) Healing of buccal gingival recessions following treatment with coronally advanced flap alone or combined with a cross-linked hyaluronic acid gel. An experimental study in dogs. J Clin Periodontol 48(4):570–580. https://doi.org/10.1111/jcpe.13433
de Brito BB, Mendes Brazao MA, de Campos ML, Casati MZ, Sallum EA, Sallum AW (2012) Association of hyaluronic acid with a collagen scaffold may improve bone healing in critical-size bone defects. Clin Oral Implants Res 23(8):938–942. https://doi.org/10.1111/j.1600-0501.2011.02234.x
Sasaki T, Watanabe C (1995) Stimulation of osteoinduction in bone wound healing by high-molecular hyaluronic acid. Bone 16(1):9–15. https://doi.org/10.1016/s8756-3282(94)00001-8
Pilloni A, Rojas MA, Marini L, Russo P, Shirakata Y, Sculean A, Iacono R (2021) Healing of intrabony defects following regenerative surgery by means of single-flap approach in conjunction with either hyaluronic acid or an enamel matrix derivative: a 24-month randomized controlled clinical trial. Clin Oral Investig 25(8):5095–5107. https://doi.org/10.1007/s00784-021-03822-x
Shirakata Y, Nakamura T, Setoguchi F, Imafuji T, Shinohara Y, Matsumura S, Iwata M, Noguchi K, Ramanauskaite E, Sculean A (2024) Histological evaluation of nonsurgical periodontal treatment with and without the use of sodium hypochlorite / amino acids and cross-linked hyaluronic acid gels in dogs. Clin Oral Investig 28(5):281. https://doi.org/10.1007/s00784-024-05674-7
Acknowledgements
The authors are grateful to Regedent AG, Zürich, Switzerland for providing the PI (L.B.) with the study materials.
Funding
Open Access funding enabled and organized by Projekt DEAL. There was no external funding of the study.
Author information
Authors and Affiliations
Contributions
L.B. conducted clinical research, data management, writing the manuscript, A.F. conception of the study protocol, supervision of the study, data collection, writing the manuscript, T.O. data management, statistics, data interpretation, proof reading the manuscript, D.D. writing the manuscript, data interpretation, graphical work.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Benyei, L., Friedmann, A., Ostermann, T. et al. Non-surgical treatment of residual periodontal pockets using sodium hypochlorite/amino acid gel and cross-linked hyaluronic acid—a 9-month pilot randomized controlled clinical trial. Clin Oral Invest 28, 513 (2024). https://doi.org/10.1007/s00784-024-05906-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05906-w