Abstract
Background
The study aimed to establish a link between blood ethylene oxide (EO) levels and periodontitis, given the growing concern about EO’s detrimental health effects.
Materials and methods
The study included 1006 adults from the 2013–2014 National Health and Nutrition Examination Survey (NHANES) dataset. We assessed periodontitis prevalence across groups, used weighted binary logistic regression and restricted cubic spline fitting for HbEO-periodontitis association, and employed Receiver Operating Characteristic (ROC) curves for prediction.
Results
In the periodontitis group, HbEO levels were significantly higher (40.57 vs. 28.87 pmol/g Hb, P < 0.001). The highest HbEO quartile showed increased periodontitis risk (OR = 2.88, 95% CI: 1.31, 6.31, P = 0.01). A “J”-shaped nonlinear HbEO-periodontitis relationship existed (NL-P value = 0.0116), with an inflection point at ln-HbEO = 2.96 (EO = 19.30 pmol/g Hb). Beyond this, ln-HbEO correlated with higher periodontitis risk. A predictive model incorporating sex, age, education, poverty income ratio, alcohol consumption, and HbEO had 69.9% sensitivity and 69.2% specificity. The model achieved an area under the ROC curve of 0.761.
Conclusions
These findings suggest a correlation between HbEO levels and an increased susceptibility to periodontitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ethylene oxide (EO), also known as ethylene epoxide or oxirane, with a molecular weight of 44.05, is highly soluble in water and organic solvents, and can react with numerous compounds [1]. It’s a versatile volatile organic compound widely utilized in various industries like cleaning, pharmaceuticals, and printing and dyeing. EO is a colorless, transparent liquid at low temperatures and a colorless gas with an ether-like odor at room temperature [2].
Additionally, EO is frequently used for sterilizing medical devices, fumigating food products, and manufacturing cosmetics [1, 3,4,5]. Strict regulations require very low residual EO levels in single-use sterile medical devices, especially face masks [5]. However, while EO offers benefits, it also has associated side effects [6, 7]. In daily life, people may encounter EO through various means, such as inhaling polluted air, vehicle emissions, tobacco smoke, or emissions from household products [2, 8,9,10]. Besides external sources, EO can also be internally generated through processes like gut bacterial reactions or body-wide enzymatic reactions [11, 12]. Endogenous EO concentrations range from 0.13 to 6.9 ppb [13]. EO is a metabolite derived from ethylene, and upon inhalation, it is rapidly absorbed into the bloodstream and subsequently distributed throughout the entire body [14, 15]. As a reactive compound, EO strongly interacts with nucleic acids and proteins [16], forming hemoglobin adducts called HbEO (N-(2-hydroxyethyl) valine), which are reliable indicators of EO exposure [17].
Research suggests that EO exposure may be strongly linked to concerns like inflammation, oxidative stress, insulin resistance, tumors, cardiovascular diseases, and abnormal lipid profiles [18,19,20]. Due to its potential harm to laboratory animals, the United States Environmental Protection Agency (USEPA) classifies EO as a carcinogen [15, 19]. Prolonged EO exposure has been associated with increased risks of various cancers, including leukemia and breast cancer, as well as neurological disorders [9, 21,22,23]. Additionally, extended EO exposure may increase the risk of inflammatory organ damage in rodents [24] and directly elevate oxidative stress levels in the body, potentially resulting in negative consequences related to oxidative stress [6, 25,26,27]. Although research has explored the link between EO exposure and various diseases, no prior investigation has examined the relationship between EO exposure and periodontitis.
Periodontitis is a persistent inflammatory disease marked by progressive destruction of the supporting tissues around teeth. It ranks as the second most common oral condition and has risen to become the 11th most prevalent disease globally [28]. Approximately 3.5 billion individuals worldwide are affected by periodontitis, making it a primary cause of tooth loss among adults aged 35 and above [29, 30]. Estimated productivity losses due to periodontitis could amount to a global cost of around $5.4 billion annually [31]. Moreover, periodontitis is recognized as a standalone risk factor for a range of conditions, including cardiovascular diseases, diabetes, and obesity [32]. The development of periodontal disease involves multiple factors, including genetics and the environment [33, 34]. Currently, there is growing concern about the potential detrimental effects of environmental exposures to substances like PM2.5 and e-cigarette vapor on periodontitis [33, 35, 36]. Hence, identifying other potential environmental factors influencing periodontitis development could provide fresh insights into prevention.
Due to the association between EO and inflammation and oxidative stress, which are potential etiological factors of periodontitis, we hypothesize that exposure to EO may be related to the occurrence of periodontitis. Therefore, our research hypothesis is that the level of HbEO may reflect the extent of EO exposure and be associated with the occurrence of periodontitis. We will utilize data from the 2013–2014 National Health and Nutrition Examination Survey (NHANES) to explore the potential relationship between HbEO levels and periodontitis.
Methods
Study participants
NHANES collects participant data through a comprehensive cross-sectional study conducted jointly by the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS). Its purpose is to assess the health and nutritional status of the U.S. civilian non-institutionalized population. This survey covers a wide range of factors, including demographics, socioeconomic status, diet, health indicators, blood chemistry, and various lab test results. The survey is conducted biennially, and except for restricted data, all relevant information is publicly available on the NCHS website (https://www.cdc.gov/nchs/nhanes/index.htm). Ethical approval was obtained, and informed consent was provided by all participants following the protocols approved by the Research Ethics Review Board of the NCHS.
The NHANES survey, which included newly released blood EO test data, spanned four cycles starting in 2013. Unfortunately, the total oral periodontal examination (FMPE) program was introduced in 2009–2010, but stopped after 2013–2014. This led to the absence of periodontitis-related data in NHANES thereafter. Therefore, this study specifically analyzed the data from 2013 to 2014.
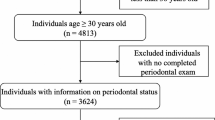
We utilized NHANES data from the 2013-2014 survey, which initially included 10,175 participants. After exclusions for FMPE non-participation (n = 5506), missing EO test (n = 3186), and lacking periodontitis data (n = 332), as well as those with missing data on hypertension (n = 0), hyperlipidemia (n = 0), and diabetes mellitus (DM) (n = 5), we assessed various social habits. These included smoking status (n = 0), alcohol consumption (n = 66), BMI (n = 4), and frequency of flossing and cleaning equipment use (n = 0). Participants with missing socioeconomic status (n = 70), marital status (n = 0), and educational status (n = 0) were also excluded. The final analysis comprised 1006 participants, as illustrated in Fig. 1. Notably, our study holds a broader significance, as it effectively represents a weighted sample from the U.S. population, estimating approximately 12,762,574 individuals.
Flowchart of procedures for participants selection and inclusion. * Missing periodontitis examination data (N=5506): The exclusion of individuals who did not undergo periodontal disease testing refers to participants who were initially not included in the assessment of their periodontal condition. Excluded missing data with periodontitis (N=332): The exclusion of individuals with missing periodontal disease data pertains to participants who agreed to undergo periodontal disease testing but, due to various reasons, did not participate in the actual testing, resulting in missing data for this specific variable
Blood ethylene oxide levels
Participants provided whole blood samples at a mobile examination center, where dedicated laboratory personnel employed a modified Edman reaction method to quantify hemoglobin oxide adducts (HbEO). Previous research has established the exceptional sensitivity of HbEO in detecting EO exposure [17, 37]. All measurements meet NCEH Laboratory Science Division quality control and quality assurance for accuracy and precision of performance standards. Detailed measurements are accessible via the following website: (https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/ETHOX_H.htm).
Evaluation of periodontitis
Following the methodology outlined by Eke et al. in 2012 and adhering to the periodontitis case definition by the U.S. Centers for Disease Control and Prevention and the American Academy of Periodontology (CDC-AAP), NHANES conducted oral periodontal examinations for participants with teeth [38]. At the Mobile Examization Center (MEC), a calibrated dentist conducted a comprehensive oral periodontal examination to ascertain the presence of periodontitis in each subject. Severity categories included mild, moderate, and severe, primarily based on periodontal probing depth (PD) and clinical attachment level (CAL). Detailed criteria can be found in Table S1. For the purposes of this cross-sectional study, the categories of mild, moderate, and severe periodontitis were aggregated for subsequent analysis.
Covariates
NHANES ensures the national representativeness of its data through the utilization of a complex probability sampling design and the application of sample weights. Given that each sample isn’t uniformly chosen, the use of sampling weights significantly facilitates the generation of accurate population estimates. For the household interview, a standardized questionnaire was administered to evaluate demographic characteristics and socioeconomic status, encompassing age, sex, race, BMI, economic status, smoking status, alcohol consumption, flossing and cleaning equipment utilization, marital status, and education level. Furthermore, an assessment of the three prevalent diseases (hypertension, hyperlipidemia, and DM) was conducted in conjunction with relevant laboratory test data.
BMI was calculated by dividing a participant’s weight (kg) by the square of their height (m2). The classification was based on BMI as follows: BMI < 25 (underweight/normal), 25–30 (overweight), and > 30 (obese) [39]. Race categories included non-Hispanic white, non-Hispanic black, Hispanic, and other race (multiracial). Socioeconomic status relied on Family Poverty-to-Income Ratios (PIR) as follows: PIR < 1.3 (low-income), 1.3–3.5 (moderate-income), and > 3.5 (high-income). Smoking status had two categories: no and yes, based on the definition of a smoker (someone who smoked at least 100 cigarettes in their lifetime) [40]. Alcohol consumption was determined by consuming a minimum of 12 alcoholic beverages annually [18]. Moreover, the categorization of flossing and cleaning equipment usage as 0–1, 2–4, and ≥ 5 days a week [41]. Educational level was segmented into three categories: Less than high school, High school, and More than high school. Marital status was classified as Never married, Widowed/Divorced/Separated, and Married/Living with partner. Additionally, the presence of hypertension, hyperlipidemia, and DM was determined as yes or no, based on questionnaire responses and laboratory data, with detailed criteria outlined in Table S2.
Statistical analysis
Due to the presence of probability sampling, we incorporated specialized sample weights (WTSA2YR) in the subsequent analysis. For categorical variables reported as number (%), a chi-square test was used to compare descriptive characteristics of the study population stratified by periodontitis.
HbEO values were divided into quartiles (Q1-Q4), using the lowest quartile (Q1) as the reference group, to further assess the association between HbEO levels and periodontitis through multivariate logistic regression models. Model 1 remained unadjusted for covariates, while Model 2 was adjusted for age, sex, and race. Model 3, building upon Model 2, included additional adjustments for BMI, PIR, smoking status, alcohol consumption, marital status, education level, flossing and cleaning equipment use, DM, hyperlipidemia, and hypertension. Chan et al. emphasized that a variance inflation factor (VIF) exceeding 10 is indicative of significant collinearity issues [42]. In our study, no such severe collinearity was observed among variables including the diagnosis of HbEO, age, sex, race, BMI, PIR, educational level, marital status, smoking status, alcohol consumption, flossing and cleaning equipment use, as well as the presence of hypertension, hyperlipidemia, and DM (all variables had a VIF < 2).
We used Receiver Operating Characteristic (ROC) curve to demonstrate the predictive capabilities of various periodontitis prediction models and to evaluate the role of HbEO in these models. To ascertain whether significant disparities exist in predictive performance among the prediction models, we employed Z-tests to compare the area under the ROC curve (AUC) of each model.
Due to the non-normal distribution of HbEO values, we applied a natural logarithm transformation to achieve normality. To investigate dose-response patterns between HbEO levels and periodontitis prevalence, we examined observations at the 5th, 35th, 65th, and 95th percentiles of the HbEO distribution using restricted cubic spline (RCS) plots with four nodes. Additionally, we conducted stratified analyses to explore interactions among participants’ sex, age, race, BMI, and the three common diseases (hypertension, hyperlipidemia, and DM). Statistical significance was defined as P < 0.05, and the analysis was conducted using R Studio (version 4.3.1) and nhanesR (version 0.9.4.3).
Results
Descriptive characteristics
In this study, a total of 1006 participants from the 2013–2014 NHANES dataset were included. Among them, based on the presence of periodontitis, there were 509 males (50.60%) and 497 females (49.40%). The prevalence of periodontitis differed significantly between male and female groups (P = 0.015). Among individuals with periodontitis, the prevalence was notably higher in the age ≥ 65 years (48.28%) compared to those aged 30–44 (27.74%), 45–54 (34.32%), and 55–64 (38.48%). The prevalence of periodontitis was significantly higher among non-Hispanic black (47.54%) compared to non-Hispanic white (33.01%), Hispanic (40.38%), and other race (34.73%), as illustrated in Table 1.
When HbEO levels were divided into quartiles (Q1-Q4), an ascending trend in the number of periodontitis cases was observed. Remarkably, the prevalence of periodontitis reached 58.99% when HbEO was in the highest quartile (Q4). Regarding BMI categorization, the proportions of underweight/normal, overweight, and obese participants were 26.74%, 32.11%, and 41.15%, respectively. However, the prevalence of periodontitis did not show significant differences among BMI groups (P > 0.05). Furthermore, participants from middle-income households (PIR = 1.3–3.5) (57.68%), those with education levels below high school (67.28%), and former alcohol consumers (52.82%) exhibited notably higher prevalence of periodontitis. Although the prevalence of periodontitis was relatively low (30.05%) for flossing 2 to 4 times per week, there was no significant difference in the prevalence of periodontitis between the three groups for flossing and cleaning equipment use. In this study, the prevalence of periodontitis in individuals with hyperlipidemia, hypertension, and diabetes was found to be 35.9%, 45.20%, and 49.2%, respectively. (Table 1)
Binary logistic regression analysis
We established three models to explore the potential relationship between HbEO levels and periodontitis. In the unadjusted model, we did not account for other covariate factors. The results revealed a significant harmful effect of HbEO at quartile 4 (3.75, 95% CI: 2.01–6.97; P < 0.001). In adjusted model 1, we controlled for participants’ age, sex, and race, and the association remained evident (4.62, 95% CI: 1.79–11.91; P = 0.01). In adjusted model 2, further adjustments were made for BMI, PIR, education level, marital status, smoking status, alcohol consumption, hypertension, hyperlipidemia, DM, and flossing and cleaning equipment use. Consistent with the previous models, the relationship remained significant (2.88, 95% CI: 1.31–6.31; P = 0.01) (Table 2).
Receiver operating characteristic curve
The maximum model encompasses the subsequent variables: sex, age, BMI, race, PIR, marital status, education level, smoking status, alcohol consumption, hyperlipidemia, DM, hypertension, flossing and cleaning equipment use and HbEO. Although this model exhibits the largest AUC (0.771), it incorporates an excessive number of variables, thereby influencing its clinical utility. While considering the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC), we endeavored to identify a balanced point between goodness of fit and the count of parameters to construct candidate model [43, 44]. When evaluating models containing only five or fewer variables, all predictive models demonstrate a notable reduction in predictive performance in comparison to the comprehensive maximum model. However, when six variables are included in the prediction model, the candidate model demonstrates predictive performance akin to that of the comprehensive model. The candidate model comprises sex, age, education level, PIR, alcohol consumption, and HbEO. The AUC of the candidate model (0.761) closely mirrors that of the maximum model (0.771), with a non-significant difference observed in the Z-test for AUC comparison between the two models (Z = 1.581, P = 0.114), as illustrated in Fig. 2. Detailed information about the candidate model can be found in Supplementary Information Table S3. Upon removing HbEO from the candidate model, the resulting predictive model’s AUC notably decreases in comparison to the candidate model (0.743 vs. 0.761, Z = 2.470, P = 0.014). This finding highlights the beneficial influence of HbEO in improving the diagnostic accuracy of the periodontitis prediction model.
Three predictive models, including the maximum, candidate, and candidate after excluding HbEO, were evaluated using receiver operating characteristic (ROC) curves. The Z-test for the area under the ROC curve (AUC) showed no significant difference in predictive performance between the maximum and candidate models for periodontitis (Z = 1.581, P = 0.114). However, there was a significant difference in predictive performance between the candidate and candidate after excluding HbEO models (Z = 2.470, P = 0.014)
Nonlinear relationships exploration
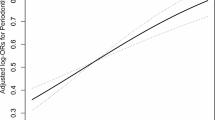
We further investigated potential nonlinear associations. In this regard, we employed RCS regression models to account for potential nonlinearity in the relationship between natural logarithm-transformed EO (ln-HbEO) levels and periodontitis risk. The results revealed a “J”-shaped nonlinear relationship between ln-HbEO and periodontitis (NL-P value = 0.0116) after adjustment for all confounders. The inflection point occurred at an ln-HbEO value of 2.96 (EO = 19.30 pmol/g Hb). Beyond this point, as ln-HbEO levels increased, the risk of periodontitis also increased (Fig. 3).
The full-adjusted relationship between ln-transformed HbEO and periodontitis using Restricted Cubic Spline. The solid line represents the fitted nonlinear curve. The area adjacent to the solid line represents the 95% confidence interval. Abbreviations HbEO, hemoglobin adduct of ethylene oxide; CI, confidence interval
Subgroup analyses
After conducting subgroup analyses based on sex, age, race, BMI, DM, hypertension, and hyperlipidemia, we did not find any significant interactions among the subgroups (P > 0.05). The impact of HbEO on periodontitis remained consistent across these subgroups, as depicted in Fig. 4. Notably, the relationship between HbEO and periodontitis was particularly pronounced in male, those aged 30–44 and 55 years and older, Non-Hispanic White and other race, underweight/normal, and those with hyperlipidemia participants. In subgroup analysis, whether among smokers, individuals with a BMI < 25, or patients with hyperlipidemia, the correlation between HbEO and periodontitis remains significant after stratification.
The odds ratio of periodontitis in participants exposed to probiotics compared to those not exposed was examined, performing subgroup analysis. All analyses were adjusted for age, sex, race, body mass index, poverty income ratio, education, marital status, smoke, alcohol, hyperlipidemia, hypertension, diabetes mellitus, and prebiotics, but not for the specific stratification variables of interest
Discussion
Our study aimed to investigate the potential link between EO exposure and periodontitis. We considered several covariates, including age, sex, race, BMI, marital status, PIR, education level, alcohol consumption, smoking status, hyperlipidemia, hypertension, DM, flossing and cleaning equipment use, and HbEO levels. From what we understand, this is the first study that verifies a potential significant positive association between EO exposure and periodontitis. We utilized a nationally representative sample from the US population in 2013–2014, resulting in a final cohort of 1006 participants after assessing inclusion and exclusion criteria.
The results of the study showed that median HbEO levels were significantly higher in periodontitis patients than in the non-periodontitis group (40.57 pmol/g Hb vs. 28.87 pmol/g Hb, P < 0.0001). Notably, even after adjusting for all confounders, the odds ratio (OR) associated with periodontitis in people taking probiotics was 2.88 (95%CI 1.31–6.31, P = 0.01) for high EO exposure (Q4) compared with low EO exposure (Q1). What’s more, a non-linear association of the “J” type was observed between HbEO and the prevalence of periodontitis (NL-P value = 0.0116). This shows that the body may have some tolerance to EO exposure (19.30-55.99 pmol/g Hb), and after this point (EO = 19.3 pmol/g Hb), the prevalence of periodontitis increases with EO exposure.
In our study participants, we observed a higher prevalence of periodontitis in men compared to women. The lowest incidence of periodontitis was found among the non-Hispanic white population. Additionally, there was a notable increase in periodontitis prevalence with increasing age. However, we did not detect a significant difference in periodontitis prevalence among individuals categorized as underweight/normal weight, overweight, and obese. We acknowledge the discrepancy between our findings and previous reports regarding the association between obesity and periodontitis [45]. However, there are also studies indicating that the prevalence of periodontitis does not increase with increasing BMI, which is consistent with our findings [46,47,48]. We speculate that the complexity of the relationship between obesity and periodontitis may explain this inconsistency. Additionally, differences in study populations, designs, and definitions of periodontitis may contribute to the variation in results. Periodontitis was less prevalent among individuals from higher-income households (PIR > 3.5), those with higher education levels, non-smokers, and those without diabetes or hypertension. These findings align with prior NHANES-based research, underscoring the importance of considering these covariates when investigating EO exposure [43, 49,50,51].
Tüornqvist et al. established that HbEO accurately represents cumulative EO exposure in the four months leading up to measurement [52]. In our study, the prevalence of periodontitis in the smoking group was notably high at 47.20%, indicating significant harm associated with smoking in relation to periodontitis. It’s important to note that research by Jain has highlighted smoking as one of the most prevalent means by which the general population is exposed to EO [9]. After stratifying our data by smoking habits, we similarly observed a closer association between EO exposure and periodontitis within the smoking group (1.329, 95% CI 1.044–1.693, P = 0.024 vs. 1.632, 95% CI 0.822–3.239, P = 0.148). Hence, cumulative EO exposure appears to be a significant environmental pollution factor in the development of periodontitis. In line with our hypothesis, scholar Yu has also proposed that smokers, who are also EO-exposed individuals, may adopt unhealthy dietary habits due to insulin resistance, resulting in abnormal fat breakdown and a decrease in BMI [7]. Similarly, our study confirmed this association, showing a significant positive correlation (1.644, 95%CI 1.118–2.418, P = 0.015) between EO exposure and the prevalence of periodontitis in individuals with underweight/normal (BMI < 25). Research by Zhu and colleagues has indicated a potential link between HbEO and abnormal blood lipid levels [25]. Moreover, lipid abnormalities can contribute to periodontal damage either directly through involvement in systemic inflammatory pathways or indirectly through factors like glycosylated hemoglobin (HbA1c) and obesity [53]. In our stratified analysis of hyperlipidemia, we observed that within the hyperlipidemia group, an increase in EO exposure corresponded to an increased prevalence of periodontitis, and this association was statistically significant. Our subgroup analyses further support the hypothesis that EO’s impact on lipid profiles, inflammation, and metabolism plays a role in periodontitis development.
EO’s hydrolytic metabolism generates ethylene glycol, used in antifreeze and related products [20, 54]. Ethylene glycol can bind with glutathione (GSH), leading to S-carboxymethyl GSH and S-carboxymethyl homocysteine formation. This disrupts sulfhydryl (-SH) group production, reducing GSH levels, and causing oxidative stress damage [51], which can contribute to periodontitis [55].
Both EO and its metabolites have potential hazards, stimulating mucous membranes, cells, and the central nervous system [56, 57]. Growing evidence indicates that EO exposure may play a role in conditions like asthma, chronic obstructive pulmonary disease, cardiovascular diseases, and lipid abnormalities by activating inflammation [25, 27, 58, 59]. Thus, we propose that EO exposure, even before contributing to systemic chronic inflammatory disease [60,61,62], may have affected oral health, although it has received less attention.
The American Academy of Periodontology (AAP) underscores the significance of conducting risk assessments in the evaluation of periodontal health [63]. As predictive models related to periodontitis continue to emerge, it becomes increasingly crucial to further our understanding of early risk factors for periodontal disease, such as age, gender, BMI, obesity, smoking, diabetes, and oral hygiene practices, and their impact on oral health and quality of life for individuals. Our study results indicate that EO exposure positively influences the diagnostic performance of periodontitis prediction models. Nevertheless, it’s important to acknowledge that there is a modest enhancement of 1.8% (AUC: 0.743 vs. 0.761) when taking EO exposure into account compared to a prediction model that excludes it.
This study has limitations. Firstly, it used a cross-sectional design, requiring further prospective research to confirm the causal link between these variables. Secondly, like other epidemiological studies, unmeasured other confounding factors such as sleep patterns, eating habits and neuropsychiatric status may impact the results. Future research should consider including a broader range of potential influencing factors to enhance the understanding of the findings.
Conclusion
Our study found a link between blood EO levels and periodontitis prevalence. Particularly, when EO exposure exceeds 19.30 pmol/g Hb, periodontitis prevalence consistently rises. This discovery is noteworthy, highlighting the need for attention to this issue and providing fresh insights into addressing oral health challenges arising from environmental pollution. However, our study’s design requires further validation through prospective longitudinal cohort studies.
Data availability
The NHANES dataset is publicly available online, accessible at cdc.gov/nchs/nhanes/index.htm.
References
Gimeno P et al (2018) Identification and quantification of ethylene oxide in sterilized medical devices using multiple headspace GC/MS measurement. J Pharm Biomed Anal 158:119–127
Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for ethylene oxide (draft for public comment). Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.]. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=734&tid=133
Kowalska A, Manning L (2022) Food Safety Governance and Guardianship: the role of the private Sector in addressing the EU Ethylene Oxide Incident. Foods 11(2)
Zekri N et al (2023) Physicochemical characterization and antioxidant properties of essential oils of M. pulegium (L.), M. suaveolens (Ehrh.) And M. Spicata (L.) from Moroccan Middle-Atlas. Foods 12(4)
Dias FN et al (2009) Sterilization of medical devices by ethylene oxide, determination of the dissipation of residues, and use of Green Fluorescent Protein as an indicator of process control. J Biomed Mater Res B Appl Biomater 91(2):626–630
Jones RR et al (2023) Ethylene oxide emissions and incident breast cancer and non-hodgkin lymphoma in a US cohort. J Natl Cancer Inst 115(4):405–412
Yu XY, Song P, Zou MH (2018) Obesity Paradox and Smoking Gun: a mystery of statistical Confounding? Circ Res 122(12):1642–1644
Rubinstein ML et al (2018) Adolescent exposure to toxic volatile Organic Chemicals from E-Cigarettes. Pediatrics 141(4)
Jain RB (2020) Associations between observed concentrations of ethylene oxide in whole blood and smoking, exposure to environmental tobacco smoke, and cancers including breast cancer: data for US children, adolescents, and adults. Environ Sci Pollut Res Int 27(17):20912–20919
Bono R et al (1999) Formation of N-(2-hydroxyethyl)valine due to exposure to ethylene oxide via tobacco smoke: a risk factor for onset of cancer. Environ Res 81(1):62–71
Kirman CR et al (2021) Ethylene oxide review: characterization of total exposure via endogenous and exogenous pathways and their implications to risk assessment and risk management. J Toxicol Environ Health B Crit Rev 24(1):1–29
Törnqvist M et al (1989) Unsaturated lipids and intestinal bacteria as sources of endogenous production of ethene and ethylene oxide. Carcinogenesis 10(1):39–41
Sheehan PJ et al (2021) Ethylene Oxide exposure in U.S. populations residing Near sterilization and other Industrial facilities: Context based on endogenous and total Equivalent Concentration exposures. Int J Environ Res Public Health 18(2)
Thier R, Bolt HM (2000) Carcinogenicity and genotoxicity of ethylene oxide: new aspects and recent advances. Crit Rev Toxicol 30(5):595–608
Jinot J et al (2018) Carcinogenicity of ethylene oxide: key findings and scientific issues. Toxicol Mech Methods 28(5):386–396
Ghosh M, Godderis L (2016) Genotoxicity of ethylene oxide: A review of micronucleus assay results in human population. Mutat Res Rev Mutat Res 770(Pt A):84–91
Ogawa M et al (2006) Hemoglobin adducts as a marker of exposure to chemical substances, especially PRTR class I designated chemical substances. J Occup Health 48(5):314–328
Cheang I et al (2022) Inverse association between blood ethylene oxide levels and obesity in the general population: NHANES 2013–2016. Front Endocrinol (Lausanne) 13:926971
Lynch DW et al (1984) Carcinogenic and toxicologic effects of inhaled ethylene oxide and propylene oxide in F344 rats. Toxicol Appl Pharmacol 76(1):69–84
Lundin A, Panas I, Ahlberg E (2007) A mechanistic investigation of ethylene oxide hydrolysis to ethanediol. J Phys Chem A 111(37):9087–9092
Steenland K et al (1991) Mortality among workers exposed to ethylene oxide. N Engl J Med 324(20):1402–1407
Han L, Wang Q (2023) Association between hemoglobin adducts of ethylene oxide levels and the risk of short sleep duration in the general population: an analysis based on the National Health and Nutrition Examination Survey. Environ Sci Pollut Res Int 30(31):76761–76768
Estrin WJ et al (1987) Evidence of neurologic dysfunction related to long-term ethylene oxide exposure. Arch Neurol 44(12):1283–1286
Csanády GA et al (2000) A physiological toxicokinetic model for exogenous and endogenous ethylene and ethylene oxide in rat, mouse, and human: formation of 2-hydroxyethyl adducts with hemoglobin and DNA. Toxicol Appl Pharmacol 165(1):1–26
Zhu X et al (2022) Blood ethylene oxide, systemic inflammation, and serum lipid profiles: results from NHANES 2013–2016. Chemosphere 299:134336
Wu N., et al (2022) Association between blood ethylene oxide levels and the prevalence of hypertension. Environ Sci Pollut Res Int 29(51):76937–76943.
Huang Q et al (2023) Association between ethylene oxide exposure and prevalence of COPD: evidence from NHANES 2013–2016. Sci Total Environ 885:163871
Kassebaum NJ et al (2017) Global, Regional, and National Prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: a systematic analysis for the Global Burden of Diseases, injuries, and risk factors. J Dent Res 96(4):380–387
Akinkugbe AA et al (2016) Systematic Review and Meta-analysis of the Association between Exposure to Environmental Tobacco Smoke and Periodontitis endpoints among nonsmokers. Nicotine Tob Res 18(11):2047–2056
(2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392(10159):1789–1858
Ren ZH et al (2020) Global and regional burdens of oral cancer from 1990 to 2017: results from the global burden of disease study. Cancer Commun (Lond) 40(2–3):81–92
Beukers NG et al (2017) Periodontitis is an independent risk indicator for atherosclerotic cardiovascular diseases among 60 174 participants in a large dental school in the Netherlands. J Epidemiol Community Health 71(1):37–42
Huang K et al (2023) The modification of meteorological factors on the relationship between air pollution and periodontal diseases: an exploration based on different interaction strategies. Environ Geochem Health
Schaefer AS et al (2013) Validation of reported genetic risk factors for periodontitis in a large-scale replication study. J Clin Periodontol 40(6):563–572
Han DH et al Mercury exposure and Periodontitis among a Korean Population: the Shiw ha-Banwol Environmental Health Study. J Periodontol 80(12):1928–1936
Cátala-Valentín AR et al (2022) E-Cigarette aerosols promote oral S. Aureus colonization by delaying an Immune Response and Bacterial Clearing. Cells 11(5)
Yang M et al (2018) High-throughput, simultaneous quantitation of hemoglobin adducts of acrylamide, glycidamide, and ethylene oxide using UHPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 1086:197–205
Eke PI et al (2012) Update of the case definitions for population-based surveillance of periodontitis. J Periodontol 83(12):1449–1454
Curry SJ et al (2018) Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US Preventive Services Task Force Recommendation Statement. JAMA 320(11):1163–1171
SSY AL et al (2019) Association between time since quitting smoking and periodontitis in former smokers in the National Health and Nutrition Examination Surveys (NHANES) 2009 to 2012. J Periodontol 90(1):16–25
Cepeda MS et al Association of flossing/inter-dental cleaning and periodontitis in adu lts. J Clin Periodontol 44(9):866–871
Chan MY et al (2014) Relationship between body mass index and fracture risk is mediated by bone mineral density. J Bone Min Res 29(11):2327–2335
Montero E et al (2019) Development and validation of a predictive model for periodontitis using NHANES 2011–2012 data. J Clin Periodontol 46(4):420–429
Zaorsky NG et al (2022) Pan-cancer analysis of prognostic metastatic phenotypes. Int J Cancer 150(1):132–141
Çetin MB et al (2022) The relationship between body mass index and stage/grade of periodontitis: a retrospective study. Clin Oral Investig 26(2):1937–1945
Eke PI et al (2018) Periodontitis in US adults: National Health and Nutrition Examination Survey 2009–2014. J Am Dent Assoc 149(7):576–588e6
Saxlin T et al (2010) Overweight and obesity weakly predict the development of periodontal infection. J Clin Periodontol 37(12):1059–1067
Khan S et al (2020) Obesity and periodontitis in Australian adults: a population-based cross-sectional study. Int Dent J 70(1):53–61
Li XY et al (2022) The association of healthy eating index with periodontitis in NHANES 2013–2014. Front Nutr 9:968073
Gay IC, Tran DT, Paquette DW (2018) Alcohol intake and periodontitis in adults aged ≥ 30 years: NHANES 2009–2012. J Periodontol 89(6):625–634
Yan Y et al (2022) Periodontitis is Associated with Heart failure: a Population-based study (NHANES III). Front Physiol 13:854606
Törnqvist M et al (2002) Protein adducts: quantitative and qualitative aspects of their formation, analysis and applications. J Chromatogr B Analyt Technol Biomed Life Sci 778(1–2):279–308
Bitencourt FV et al (2023) The role of Dyslipidemia in Periodontitis. Nutrients 15(2)
Moayed M, Mahdavian L (2017) Recycling Monoethylene Glycol (MEG) from the recirculating Waste of an Ethylene Oxide Unit. Open Chem 15(1):167–174
Almerich-Silla JM et al (2015) Oxidative stress parameters in Saliva and its Association with Periodontal Disease and types of Bacteria. Dis Markers 2015:653537
Chang H et al (2023) In vivo toxicity evaluation of tumor targeted glycol chitosan nanoparticles in healthy mice: repeated high-dose of glycol chitosan nanoparticles potentially induce cardiotoxicity. J Nanobiotechnol 21(1):82
da Cunha Mendes GC, da Silva Brandão TR, Miranda Silva CL (2008) Ethylene oxide potential toxicity. Expert Rev Med Devices 5(3):323–328
Zeng G et al (2021) Association between blood ethylene oxide levels and the risk of cardiovascular diseases in the general population. Environ Sci Pollut Res Int 28(45):64921–64928
Li Z et al (2023) The association between ethylene oxide exposure and asthma risk: a population-based study. Environ Sci Pollut Res Int 30(9):24154–24167
Taboza ZA et al (2018) Periodontitis, edentulism and glycemic control in patients with type 2 diabetes: a cross-sectional study. BMJ Open Diabetes Res Care 6(1):e000453
Priyamvara A et al (2020) Periodontal inflammation and the risk of Cardiovascular Disease. Curr Atheroscler Rep 22(7):28
Lertpimonchai A et al (2019) Periodontitis as the risk factor of chronic kidney disease: mediation analysis. J Clin Periodontol 46(6):631–639
(2008) American Academy of Periodontology statement on risk assessment. J Periodontol 79(2):202
Acknowledgements
The authors extend their gratitude to Zhang Jing from Shanghai Tongren Hospital for his substantial contributions to the development of the nhanesR package and webpage. Additionally, the authors express their appreciation to the NHANES databases for granting access to the data.
Funding
This work was funded by the Science and Technology Planning Project of Sichuan Province (2021YJ0170), the Project of Chengdu Science and Technology Bureau (2019-YF05-00498-SN), National Natural Science Foundation of China (82370884), Sichuan Province science and technology plan project (2021YFS0101), National Natural Science Foundation of China (82104069), and Sichuan Science and Technology Program (2022089).
Author information
Authors and Affiliations
Contributions
XY drafted the manuscript and conducted statistical analysis. TYJ, RZY, and Li Linke conducted statistical analysis and contributed to the writing of the methods section. LYJ and XJ were responsible for guiding and reviewing this article. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All the data used in our study were obtained from the National Health and Nutrition Examination Survey (NHANES). NHANES is a nationally representative cross-sectional study conducted under the direction of the National Center for Health Statistics (NCHS) to assess the health and nutrition status of the non-institutionalized population of the United States using a complex, multistage, and probabilistic sampling design. All of the surveys were authorized by the NCHS Ethics Review Board before being conducted, and all participants signed informed consent forms. More information is available at http://www.cdc.gov/nchs/nhanes/.
Consent for publication
Not applicable.
Competing of interests
None of the authors have any potential conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xue, Y., Tang, Y., Ren, Z. et al. Association between blood ethylene oxide levels and the prevalence of periodontitis: evidence from NHANES 2013–2014. Clin Oral Invest 28, 293 (2024). https://doi.org/10.1007/s00784-024-05690-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05690-7