Abstract
Objective
Due to inconsistent findings in limited previous cohort studies, the aim of this study was to estimate the obesity effect on periodontitis progression in Thai adults.
Materials and methods
This 10-year retrospective cohort study comprised 2216 employees of the Electric Generation Authority of Thailand (EGAT). Their demographic, medical, and periodontal status was collected. Subjects with periodontitis progression were defined as having ≥ 2 teeth with progression. Additional proximal clinical attachment loss ≥ 3 mm or tooth loss with severe periodontitis at baseline were used to identify disease progression at the tooth level. Central obesity was classified using the waist-hip ratio. Multi-level Poisson regression was used to determine the effect of obesity on periodontitis progression by adjusting for age, sex, education, income, smoking, alcohol drinking, exercise, diabetes mellitus, and hypertension.
Results
The cumulative incidence of periodontitis progression during the 10-year period was 59.6 cases per 100 persons (95% CI: 57.5, 61.6). The univariate analysis indicated that obese subjects had 15% higher risk of progression than that of healthy subjects. However, when confounders were analyzed simultaneously, the effect of obesity was not significant with a risk ratio of 0.98 (95% CI: 0.88, 1.08).
Conclusions
Despite the higher incidence of disease progression in the obese, obesity is not an independent risk factor for periodontitis progression.
Clinical relevance
Obesity and periodontitis progression share many common risk factors. Using the obesity as a preliminary screening for periodontitis progression may be an alternative prevention protocol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has a negative impact on an individual’s health. It is defined as excessive fat accumulation that impairs overall health [1]. The prevalence of being overweight or obese has increased at every sociodemographic index level [2]. Obesity is identified as a risk factor for several non-communicable diseases (NCDs), including cardiovascular disease (CVD), diabetes mellitus (DM), chronic kidney disease, cancers, and musculoskeletal disorders [3,4,5].
Obesity is also suspected as an independent risk factor of periodontitis. Periodontitis is described as the multi-factorial disease [6]. Individuals experience disease initiation and disease progression based on their specific risk factors. Obesity causes low-grade systemic inflammation. The adipocytes in obese individuals increase in size and number. Elevated inflammatory adipokines levels, including tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6), and leptin have been reported [7]. Inflammatory accumulation promotes oral dysbiosis, leading to periodontal destruction [8]. In addition, obesity is also proposed as a risk factor for periodontitis progression by the indirect effect through DM [9, 10]. Elevated TNF-α due to obesity inhibits the insulin signaling pathway, resulting in insulin resistance. Then, immune cell malfunction and the accumulation of advanced glycation end-products (AGEs) from DM stimulate periodontal destruction.
Previous longitudinal studies have explored this risk association; however, the findings were limited and inconclusive. Jimenez et al. [11] investigated the consequences of obesity in a large-scale community-based setting and found that obesity had a significant effect on periodontitis incidence. Gorman et al. [12] demonstrated a non-significant effect of being overweight; however, the risk was significantly 52% higher in the obesity group. In contrast, Saxlin et al. [13] investigated a cohort of non-DM and non-smoking individuals for 4 years. They found that the risk of having periodontitis progression in overweight and obese people was comparable with normal weight subjects. The heterogeneity of these results was affected by variations in target populations, measurement, and classification criteria of periodontitis progression and obesity. Thus, more information from longitudinal studies in other ethnic groups using comprehensive and appropriate criteria for periodontitis progression and obesity are still needed. Therefore, the aim of this cohort study was to compare the incidence of periodontitis progression between normal and obese subjects in Thai adults. Moreover, the magnitude of the independent effect of obesity on periodontitis progression was investigated.
Materials and methods
Setting
This study was conducted as a cohort study that conformed with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for reporting observational studies. The secondary data was accessed and utilized from the Electric Generation Authority of Thailand (EGAT) cohort. This is an ongoing workers cohort in Thailand [14] that primarily aims to examine the NCDs risk factors. EGAT employees were randomly selected and enrolled from urban and rural area for undergoing the health survey every 5 years.
Our 10-year-cohort study used the 2003 (EGAT 2/2) survey as a baseline. The 2008 (EGAT 2/3) and 2013 (EGAT 2/4) surveys were used as the follow-up visit at 5 and 10 years, respectively. The subjects who registered for the survey in 2003 and had at least 1 follow-up visit either in 2008 or 2013 or both were included in our analysis.
Oral examination
Dental examinations were performed by periodontists from the Department of Periodontology, Faculty of Dentistry, Chulalongkorn University in mobile dental units. The examinations comprised the number of remaining teeth, periodontal examinations, and evaluation of treatment needs. Detailed measurements and calibration were performed as reported elsewhere [15]. The periodontal examinations included probing depth (PD), and gingival recession (RE), which were carried out on all fully erupted teeth, except third molars and retained roots. The PD and RE were measured using a PCP-UNC15 probe at six sites per tooth. The clinical attachment level (CAL), representing the distance from the cemento-enamel junction to the tip of the periodontal probe, was the sum of the PD and RE values.
Outcome variable
The primary outcome was periodontitis progression at the subject level. The event was counted if a subject had at least 2 teeth with periodontitis progression. Periodontitis progression at the tooth level was defined as having additional proximal CAL loss ≥ 3 mm [16]. If tooth loss had occurred, that tooth was identified as having disease progression when it had severe periodontitis (CAL ≥ 5 mm) at the previous visit.
Anthropometry
The subjects’ weight, height, and waist and hip circumference were measured by trained personnel from Ramathibodi Hospital. Height was measured in centimeters and weight was measured in kilograms when dressed in normal clothing without shoes. The hip and waist circumferences (WC) were measured in centimeters using a conventional measuring tape. The waist-hip ratio (WHR) was calculated by dividing the WC by the hip circumference. Based on these results, the subjects were categorized into 2 groups, normal and obesity, with a cut-off point of 0.9 for males and 0.85 for females.
Statistical analysis
The data are described using mean (standard deviation) and frequency (percentage) for continuous and categorical data, respectively. Multi-level Poisson regression analysis was performed by assigning the periodontitis progression (stable/progressed) as the binary outcome and obesity (normal/obese) as the independent variable. Other covariables known to affect periodontitis progression were included: age (continuous), sex (male/female), education (≤ high school/vocational or diploma/ ≥ Bachelor’s degree), income (< 20,000/20,000–49,999/ ≥ 50,000 baht/month), exercise (none/1–2 times/week/ ≥ 3 times/week), smoking (never/quit/current smokers), alcohol drinking (never/occasional/frequent drinkers), hypertension (systolic blood pressure 140 mmHg or diastolic blood pressure ≥ 90 mmHg or took blood pressure lowering drugs) [17], and DM (fasting blood sugar ≥ 126 mg/dl or took any type of anti-diabetic medication). When possible, the independent and covariables were treated as time-varying covariables. The risk ratio (RR) and their 95% confidence intervals were estimated. The sensitivity analysis included comparing the obesity effect among different obesity measurements. Moreover, the risk was also compared across the alternative definitions of periodontitis progression. The analysis from the primary outcome was defined as Model A. Model B was analyzed using similar criteria as for Model A, except for ignoring tooth loss as a progression component. Model C applied the 2018 AAP/EFP periodontal diseases classification [18] to define periodontitis progression. Periodontitis was defined as having a loss of proximal CAL in two non-adjacent teeth, or a loss of CAL at the buccal or oral ≥ 3 mm with PD > 3 mm. Only disease stages were considered in the analysis. Disease severity at each survey was determined by the interproximal CAL at sites with the greatest attachment loss: (a) stage I (mild periodontitis) CAL 1–2 mm, (b) stage II (moderate periodontitis) CAL 3–4 mm, and (c) stages III–IV (severe periodontitis) CAL ≥ 5 mm. Stage I or II patients were re-classified as stages III–IV if the maximum PD was ≥ 6 mm [19, 20]. Subjects with an increased stage were assumed as having progression. In addition, subgroup analysis based on the baseline severity of periodontitis [21], and smoking status was performed. All statistical analysis was performed using STATA 14.2 software. A p value < 0.05 was considered statistically significant.
Results
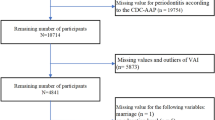
Of the 2686 total subjects in EGAT 2/2, only 2334 participated in EGAT 2/3 or EGAT 2/4. Sixty-eight subjects were initially excluded because of a missing baseline periodontal status. Fifty subjects were further excluded due to missing their periodontal status at follow-up; thus, disease progression could not be determined. The remaining 2216 subjects were included in this cohort study (Fig. S1).
The subjects’ baseline characteristics are described in Table 1. The mean age was 47.3 ± 4.6 years with 72% males. More than 75% of the subjects were middle to high socio-economic status (SES). Half of them were non-drinkers, and 55% never smokers. The prevalence of DM and hypertension at baseline were 6% and 26%, respectively. The proportion of subjects with central obesity defined by WHR was 50%. In addition, the periodontitis prevalence at baseline according to the CDC/AAP definition was 53% for moderate and 29% for severe periodontitis.
During the 10-year follow-up period, 1320 subjects developed the interested outcome, i.e., experiencing periodontitis progression (≥ 2 teeth with progression). The cumulative incidence of disease progression was 59.6 cases per 100 persons per 10 years (95% CI: 57.5, 61.6). Of the total subjects, 31.9% had more than 4 teeth with disease progression, while only 23.1% remained stable in all remaining teeth.
The longitudinal data at the subject level was analyzed using multi-level Poisson regression. The univariate analysis results indicated that age, sex, education, income, DM, hypertension, smoking, and drinking alcohol were associated with periodontitis progression; however, exercise was not (Table S1). Obesity significantly increased the risk of disease progression with a crude RR of 1.15 (95% CI: 1.04, 1.27).
The multivariate model was constructed and presented in Table 2. The magnitude of the risk effect was adjusted by the significant factors from the forward method of co-variable selection, and all known risk factors of disease progression, including age, sex, education, DM, and smoking. The results demonstrated that sex, education, smoking, and DM remained significantly associated with periodontitis progression. However, the effect of obesity was not significant with an adjusted RR of 0.98 (95% CI: 0.88, 1.08) (Table 2).
Sensitivity analysis using various obesity criteria and various definitions of periodontitis progression was performed (Table 3). Model A revealed the results of the main proposed primary outcome with several obesity parameters, including categorical and continuous data. The results demonstrated consistent negative results from all obesity measures. Model B was analyzed using similar disease progression criteria as for Model A, except ignoring tooth loss as a component of progression. The multi-level Poisson regression did not find an independent effect of obesity with any definition of obesity. In Model C, the periodontitis severity was classified by the stages system of the 2018 AAP/EFP periodontal diseases classification. An increase in stage was set as the outcome. According to these criteria, the subjects had periodontitis at baseline with a prevalence of stage I, stage II, and stages III–IV of 1%, 32%, and 67%, respectively. Approximately 70% of the total observations in the multi-level analysis were excluded because they had the maximum thresholds of disease severity, which could not assume progression based on an increased stage. Of 829 total subjects, 557 subjects (67.2%) were identified as having periodontitis progression. Similarly, the effect of obesity on increased severity of periodontitis was not significant.
The results of the subgroup analysis based on severity of periodontitis and smoking status indicated that obesity was not significantly associated with disease progression in any population subtype (Table 4). In the periodontitis diagnosis subgroups, a low level of education and current smoking were significant in subjects with moderate and severe periodontitis. These risk effects were slightly higher in moderate compared with severe periodontitis, and sex was also related with the progression in moderate periodontitis subjects. In addition, sex and education level were considered as independent risk factors among never smokers, while education level was the only factor that affected the outcome in current smokers.
Discussion
This 10-year retrospective cohort study investigated the effect of obesity on periodontitis progression. We found that the risk of disease progression was 15% higher among obese subjects. However, when other confounders were taken into account, there was no significant causal relationship between obesity and periodontitis progression.
In this study, the definition of periodontitis progression according to the 5th European workshop on periodontology was adopted and modified [16]. In case of tooth loss, each tooth was assumed as having progression if it had severe periodontitis at baseline. Our results demonstrated that ~ 60% of the subjects had periodontitis progression. Compared with other studies, the incidence of progression varied affected by study setting, subject characteristics, follow-up period, baseline severity, definition of disease progression, periodontal treatment, and maintenance protocols. For example, Ogawa et al. [22] investigated periodontitis progression in community-based elderly people. They found that 75% of elderly subjects exhibited additional CAL loss ≥ 3 mm at least 1 site over 2 years. In contrast, Lindhe and Nyman reported that only 20% of patients had CAL loss ≥ 3 mm during a 14-year follow-up in a hospital-based setting among periodontitis patients with comprehensive periodontal treatment and underwent regular periodontal maintenance [23].
The causative effect of obesity on periodontitis progression was inconsistent in previous cohort studies. A significant risk effect was found in our univariate analysis; however, it was not found after adjusting for the effect of confounders. Our findings were consistent in the sensitivity and subgroup analysis and also agreed with Saxlin et al. [13] who studied a non-DM and non-smoking cohort. Their analysis revealed a non-significant effect of being overweight or obese on the number of new teeth with periodontal pockets. In contrast, Jimenez et al. [11] estimated the obesity effect in a large-scale population using the Cox proportional hazard model and found a significant effect of being overweight and obese on the periodontitis incidence with an adjusted HR of 1.09 (95% CI: 1.02, 1.18) and 1.30 (95% CI: 1.17, 1.45). However, the low amount of new periodontitis cases (8% from the 20-year follow-up) and low validity of self-reported periodontitis were addressed. Gorman et al. [12] reported the non-significant effect of being overweight (BMI 25.0–29.9 kg/m2); however, the risk was significantly 52% higher in the obesity group (BMI ≥ 30 kg/m2). The WC and waist-to-height ratio (WHtR) were selected as an alternative obesity index, and the results were different among the different criteria. It should be emphasized that the obesity index and their cut-off points were crucial in determining this association. Based on this, comparing the results across the studies and their interpretation should be carefully considered.
Many indices for body anthropometry have been recommended for categorizing obesity, including BMI, WC, WHR, and WHtR. Advantages and limitations of each index have been widely discussed [24,25,26,27,28,29,30,31,32,33]. BMI is most commonly used; however, how well it explains the distribution of body fat is questioned. Weight from muscle mass in the upper limbs, lower limbs, and chest could be sources of misclassification, particularly in masculine person [34].
WC is a globally used as a parameter to quantify central obesity. This measurement directly represents central obesity or visceral adiposity. Brambilla et al. [24] indicated that WC was a better single predictor of visceral adipose tissue compared with BMI. To improve the discrimination performance, height or hip circumference has been used with the WC to calculate their ratios. During the last few decades, WHR and WHtR have become firmly established in medical research. They also have been accepted and used in the universal obesity criteria [35].
Many studies have been performed to identify the best obesity parameter that explains its health burden. The WHtR was indicated as the better indicator compared with BMI and WC for metabolic syndrome prediction [36]. In addition, Lee et al. [37] investigated the association between obesity and the surrogate markers of CVD. Their results demonstrated that BMI, WC, and WHR were positively correlated, and WHR was the best predictor for subclinical atherosclerosis in postmenopausal women. These results suggested that WHtR and WHR may be more suitable than BMI for representing the consequence caused by systemic inflammation. Based on a similar hypothesis with other NCDs, systemic inflammation from adipocyte increases the risk of periodontitis destruction; thus, the WHR was selected as our primary obesity index to reflect central obesity.
Obesity was not significantly independently associated with the periodontitis progression. However, obesity and periodontitis share many common risk factors, e.g., low SES, smoking, and DM. Obesity is a characteristic that can be easily noticed that may obscure other periodontitis risk factors. We found that ~ 70% of the obese subjects had low SES, DM, or smoking habits concomitantly. Therefore, obesity could be used as a screening tool to prevent periodontitis progression. Oral health care and oral health promotion in individuals with obesity should be emphasized by healthcare providers.
The strength of this study was that the causal association of obesity on periodontitis progression was evaluated using an appropriately designed cohort study. Our study was conducted with a large Thai population. The 10-year follow-up period was sufficient for the onset of disease progression. The periodontal examination was performed using the optimum protocol, full-mouth examination with six sites per tooth, by calibrated experienced periodontists. A well-planned collection of the comprehensive medical data including a health questionnaire, physical examination, and laboratory results was performed. Moreover, an advanced statistical analysis, the mixed effect model with the time-varying co-variables pattern, was used to estimate the causal relationship. The variance within- and between-subjects were taken into account.
Our study also has some limitations. First, this study was conducted in a specific group of Thai people; most of whom had a moderate to high SES. Thus, the generalizability of our results might be limited. Secondly, with the retrospective cohort study design, some factors that were potentially associated with periodontitis progression, such as oral hygiene level and history of periodontal treatment, were not collected. Oral hygiene is significantly associated with periodontitis [38], and periodontal treatment resolves or prevent periodontitis progression. The coefficient and significance level of each predictor might be influenced by not analyzing oral hygiene level and periodontal treatment. Third, the gap of 5 years between follow-up periods might be too long. During that period of time, some variables could change. Finally, to categorize disease progression at the subject level, tooth loss was considered as an important variable. However, the reasons for tooth extraction were not collected. A tooth extracted due to dental caries or fracture could bias the interested outcome. In our study, the baseline severity at the tooth level and sensitivity analysis with other definitions of periodontitis progression were used to minimize over-estimation based on this limitation.
Conclusion
Within the limitations of the present study, the progression of periodontitis was common in adults. Approximately 60% of Thai adults experienced disease progression within 10 years. An association between obesity and periodontitis progression was found. Obese subjects had a higher proportion of periodontitis progression compared with normal subjects. However, the effect of obesity on periodontitis progression was not significant when other confounders were simultaneously analyzed. Interestingly, obesity and periodontitis progression share many common risk factors. Using obesity as a preliminary screening for periodontitis progression may be an alternative prevention protocol.
References
Haslam DW, James WP (2005) Obesity Lancet 366:1197–1209. https://doi.org/10.1016/s0140-6736(05)67483-1
Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A et al (2017) Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 377:13–27. https://doi.org/10.1056/NEJMoa1614362
Kotsis V, Jordan J, Micic D, Finer N, Leitner DR, Toplak H et al (2018) Obesity and cardiovascular risk: a call for action from the European society of hypertension working group of obesity, diabetes and the high-risk patient and European association for the study of obesity: part A: mechanisms of obesity induced hypertension, diabetes and dyslipidemia and practice guidelines for treatment. J Hypertens 36:1427–1440. https://doi.org/10.1097/hjh.0000000000001730
Khader Y, Batieha A, Jaddou H, El-Khateeb M, Ajlouni K (2019) The performance of anthropometric measures to predict diabetes mellitus and hypertension among adults in Jordan. BMC Public Health 19:1416. https://doi.org/10.1186/s12889-019-7801-2
Hopkins BD, Goncalves MD, Cantley LC (2016) Obesity and cancer mechanisms: cancer metabolism. J Clin Oncol 34:4277–4283. https://doi.org/10.1200/jco.2016.67.9712
Kornman KS (2008) Mapping the pathogenesis of periodontitis: a new look. J Periodontol 79:1560–1568. https://doi.org/10.1902/jop.2008.080213
Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW (2004) Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 145:2273–2282. https://doi.org/10.1210/en.2003-1336
Hajishengallis G, Lamont RJ (2012) Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol 27:409–419. https://doi.org/10.1111/j.2041-1014.2012.00663.x
Martinez-Herrera M, Silvestre-Rangil J, Silvestre FJ (2017) Association between obesity and periodontal disease. A systematic review of epidemiological studies and controlled clinical trials. Med Oral Patol Oral Cir Bucal 22:e708–e715. https://doi.org/10.4317/medoral.21786
Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y (2005) A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol 76:2075–2084
Jimenez M, Hu FB, Marino M, Li Y, Joshipura KJ (2012) Prospective associations between measures of adiposity and periodontal disease. Obesity 20:1718–1725. https://doi.org/10.1038/oby.2011.291
Gorman A, Kaye EK, Apovian C, Fung TT, Nunn M, Garcia RI (2012) Overweight and obesity predict time to periodontal disease progression in men. J Clin Periodontol 39:107–114. https://doi.org/10.1111/j.1600-051X.2011.01824.x
Saxlin T, Ylostalo P, Suominen-Taipale L, Aromaa A, Knuuttila M (2010) Overweight and obesity weakly predict the development of periodontal infection. J Clin Periodontol 37:1059–1067. https://doi.org/10.1111/j.1600-051X.2010.01633.x
Vathesatogkit P, Woodward M, Tanomsup S, Ratanachaiwong W, Vanavanan S, Yamwong S et al (2012) Cohort profile: the electricity generating authority of Thailand study. Int J Epidemiol 41:359–365. https://doi.org/10.1093/ije/dyq218
Lertpimonchai A, Rattanasiri S, Tamsailom S, Champaiboon C, Ingsathit A, Kitiyakara C et al (2019) Periodontitis as the risk factor of chronic kidney disease: mediation analysis. J Clin Periodontol 46:631–639. https://doi.org/10.1111/jcpe.13114
Tonetti MS, Claffey N (2005) Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. Group C consensus report of the 5th European Workshop in Periodontology. J Clin Periodontol 32(Suppl 6):210–3. https://doi.org/10.1111/j.1600-051X.2005.00822.x
Chalmers J, MacMahon S, Mancia G, Whitworth J, Beilin L, Hansson L et al (1999) 1999 World Health Organization-International Society of Hypertension Guidelines for the management of hypertension. Guidelines sub-committee of the World Health Organization. Clin Exp Hypertens 21:1009–1060. https://doi.org/10.3109/10641969909061028
Tonetti MS, Greenwell H, Kornman KS (2018) Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Clin Periodontol 45(Suppl 20):S149-s161. https://doi.org/10.1111/jcpe.12945
Ortigara GB, Mário Ferreira TG, Tatsch KF, Romito GA, Ardenghi TM, Sfreddo CS et al (2021) The 2018 EFP/AAP periodontitis case classification demonstrates high agreement with the 2012 CDC/AAP criteria. J Clin Periodontol. https://doi.org/10.1111/jcpe.13462
Germen M, Baser U, Lacin CC, Fıratlı E, İşsever H, Yalcin F (2021) Periodontitis prevalence, severity, and risk factors: a comparison of the AAP/CDC case definition and the EFP/AAP classification. Int J Environ Res Public Health 18(7):3459. https://doi.org/10.3390/ijerph18073459
Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ (2012) Update of the case definitions for population-based surveillance of periodontitis. J Periodontol 83:1449–1454. https://doi.org/10.1902/jop.2012.110664
Ogawa H, Yoshihara A, Hirotomi T, Ando Y, Miyazaki H (2002) Risk factors for periodontal disease progression among elderly people. J Clin Periodontol 29:592–597. https://doi.org/10.1034/j.1600-051X.2002.290702.x
Lindhe J, Nyman S (1984) Long-term maintenance of patients treated for advanced periodontal disease. J Clin Periodontol 11:504–514. https://doi.org/10.1111/j.1600-051x.1984.tb00902.x
Brambilla P, Bedogni G, Moreno LA, Goran MI, Gutin B, Fox KR et al (2006) Crossvalidation of anthropometry against magnetic resonance imaging for the assessment of visceral and subcutaneous adipose tissue in children. Int J Obes 30:23–30. https://doi.org/10.1038/sj.ijo.0803163
Chen CC, Wang WS, Chang HY, Liu JS, Chen YJ (2009) Heterogeneity of body mass index, waist circumference, and waist-to-hip ratio in predicting obesity-related metabolic disorders for Taiwanese aged 35–64 y. Clin Nutr 28:543–548. https://doi.org/10.1016/j.clnu.2009.04.017
Chen YM, Ho SC, Lam SS, Chan SS (2006) Validity of body mass index and waist circumference in the classification of obesity as compared to percent body fat in Chinese middle-aged women. Int J Obes 30:918–925. https://doi.org/10.1038/sj.ijo.0803220
Cheng CH, Ho CC, Yang CF, Huang YC, Lai CH, Liaw YP (2010) Waist-to-hip ratio is a better anthropometric index than body mass index for predicting the risk of type 2 diabetes in Taiwanese population. Nutr Res 30:585–593. https://doi.org/10.1016/j.nutres.2010.08.007
Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J (2010) Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk–a review of the literature. Eur J Clin Nutr 64:16–22. https://doi.org/10.1038/ejcn.2009.68
Neovius M, Linné Y, Rossner S (2005) BMI, waist-circumference and waist-hip-ratio as diagnostic tests for fatness in adolescents. Int J Obes 29:163–169. https://doi.org/10.1038/sj.ijo.0802867
Oka R, Miura K, Sakurai M, Nakamura K, Yagi K, Miyamoto S et al (2009) Comparison of waist circumference with body mass index for predicting abdominal adipose tissue. Diabetes Res Clin Pract 83:100–105. https://doi.org/10.1016/j.diabres.2008.10.001
Parikh RM, Joshi SR, Menon PS, Shah NS (2007) Index of central obesity - a novel parameter. Med Hypotheses 68:1272–1275. https://doi.org/10.1016/j.mehy.2006.10.038
Yamborisut U, Kijboonchoo K, Wimonpeerapattana W, Srichan W, Thasanasuwan W (2008) Study on different sites of waist circumference and its relationship to weight-for-height index in Thai adolescents. J Med Assoc Thai 91:1276–1284
Yang F, Lv JH, Lei SF, Chen XD, Liu MY, Jian WX et al (2006) Receiver-operating characteristic analyses of body mass index, waist circumference and waist-to-hip ratio for obesity: screening in young adults in central south of China. Clin Nutr 25:1030–1039. https://doi.org/10.1016/j.clnu.2006.04.009
Rothman KJ (2008) BMI-related errors in the measurement of obesity. Int J Obes 32(Suppl 3):S56–S59. https://doi.org/10.1038/ijo.2008.87
World Health Organization (2011) Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8–11 December 2008. WHO Document Production Services, Geneva, Switzerland
Yang H, Xin Z, Feng JP, Yang JK (2017) Waist-to-height ratio is better than body mass index and waist circumference as a screening criterion for metabolic syndrome in Han Chinese adults. Medicine 96:e8192. https://doi.org/10.1097/md.0000000000008192
Lee HJ, Hwang SY, Hong HC, Ryu JY, Seo JA, Kim SG et al (2015) Waist-to-hip ratio is better at predicting subclinical atherosclerosis than body mass index and waist circumference in postmenopausal women. Maturitas 80:323–328. https://doi.org/10.1016/j.maturitas.2014.12.015
Lertpimonchai A, Rattanasiri S, Arj-Ong Vallibhakara S, Attia J, Thakkinstian A (2017) The association between oral hygiene and periodontitis: a systematic review and meta-analysis. Int Dent J 67:332–343. https://doi.org/10.1111/idj.12317
Acknowledgements
We thank Assoc.Prof. Suphot Tamsailom and Assist.Prof. Kanoknadda Tavedhikul for their advice in study design and data interpretation, Dr. Kevin Tompkins for language editing, and Assoc.Prof. Kitti Torrungruang for periodontal data management in EGAT2/2. We also thank Nisakron Thongmung for organizing the survey and managing the medical data.
Funding
This research was financially supported by Chulalongkorn University.
Author information
Authors and Affiliations
Contributions
Apinun Charupinijkul, Sirikarn Arunyanak and Attawood Lertpimonchai made substantial contributions to the conception and design of the study. Prin Vathesatogkit, Sirikarn Arunyanak, Attawood Lertpimonchai, and Lalitsara Thienpramuk performed the field survey and data collection. Apinun Charupinijkul, Sirikarn Arunyanak, Sasivimol Rattanasiri, and Attawood Lertpimonchai performed data analysis and data interpretation. Apinun Charupinijkul, Sirikarn Arunyanak, and Attawood Lertpimonchai drafted the article and critical revision critically. All authors have given final approval of the version to be published.
Corresponding author
Ethics declarations
Ethics approval
The study protocol was approved by The Ethics Committee of the Faculty of Dentistry, Chulalongkorn University (HREC-DCU 2020–019).
Informed consent
Informed consent was obtained from every subject in the study.
Conflict of interest
The authors declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Charupinijkul, A., Arunyanak, S., Rattanasiri, S. et al. The effect of obesity on periodontitis progression: the 10-year retrospective cohort study. Clin Oral Invest 26, 535–542 (2022). https://doi.org/10.1007/s00784-021-04031-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-04031-2