Abstract
Objectives
The aim of the present study is to evaluate the possible correlation between sealer penetration into dentinal tubules and sealing ability both in presence and absence of smear layer.
Materials and methods
Fourteen maxillary central incisors were treated with 5.25 % NaOCl +10 % EDTA to remove the smear layer (SL-free group) or 5.25 % NaOCl without EDTA (SL group). Root canals were filled using #25 Thermafil Obturators with Topseal sealer labelled with 0.1 wt% rhodamine B. Sealing ability was measured as fluid filtration rate with a fluid-flow meter using water supplemented with 0.3 % calcein fluorescent dye. Specimens were sectioned, observed under confocal microscope to co-localize the presence of sealer (rhodamine B labelling) into dentinal tubules and gaps (calcein labelling) into the root canal. The depth of sealer penetration into dentinal tubules and the percentage of sealer penetration around the root canal were measured at 3, 5 and 8 mm from the apex.
Results
No significant differences between groups were observed in fluid filtration rate nor in depth of calcein penetration. Sealer penetration depth and percentage into dentinal tubules were not significantly different between groups, except at 8-mm level in absence of smear layer.
Conclusion
Sealer penetration at 3- and 5-mm levels was not influenced by smear layer while it was significantly reduced at 8-mm level. Fluid filtration rate was not correlated either with depth of calcein penetration nor with sealer penetration into dentinal tubules.
Clinical relevance
The sealing ability of Topseal sealer is not affected by presence or absence of smear layer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The main objectives of endodontic therapy are the eradication or reduction in the number of microorganism from the root canal system and the prevention of recontamination and regrowth [1]. The tridimensional root filling could prevent coronal infection or re-infection [2, 3]. However, root canal fillings are affected by gaps that alter their sealing ability [4]. Gaps may be connected and may create a sort of complex spiral network that affects the tridimensional morphology of the root filling [5]. The interface between dentin and filling material is usually the critical area where gaps are higher and influenced by the presence of debris and dentin grooves [6, 7]. For these reasons, there is a considerable interest of the related literature in studying the techniques of root filling and materials. Especially, the ability of endodontic sealers to penetrate into dentinal tubules has been investigated [8–12].
Endodontic sealers are normally used in association with gutta-percha to create a stable bond between dentinal wall and core material; moreover, they fill up potential gaps and irregularities. The sealer penetration into dentinal tubules is considered a reliable outcome of root filling materials, because in theory, it could improve the seal of the root filling [13, 14]. Nevertheless, in literature, there is no agreement on the correlation between the sealer penetration into dentinal tubules and sealing ability [8, 15, 1, 16] and a positive correlation has never been established [17–21].
All endodontic instrumentation techniques produce smear layer [6, 7, 22], and its presence and its thickness may affect the penetration and adhesion of the sealer inside dentinal tubules. Moreover, smear layer and smear plug presence could reduce the sealer fluidity along the root canal and its adaptation and may increase the number of porosities and voids [13, 23, 24]. Therefore, its removal is possible by using EDTA or other chelating agents [25, 26] that open dentinal tubules allowing the sealer penetration inside the tubules. On the other hand, the endodontic literature is not univocal on the advantages that the removal of smear layer would bring to the quality of the seal preventing micro-leakage. The obstruction of dentinal tubules by smear layer and smear plug may restrict bacterial penetration even if it allows fluid filtration [27–29].
The aims of the present study are (1) to investigate the sealing ability of a resin based sealer associated with gutta-percha core-carried technique in presence/absence of smear layer by means of fluid filtration test, (2) to detect the presence of gaps between the root walls and the filling material by means of the addition of a fluorescent dye (calcein) to the fluid of the filtration apparatus, (3) to evaluate the depth and percentage of penetration into dentinal tubules of the sealer labelled with rhodamine B in the presence or absence of smear layer by means of confocal laser scanning microscopy (CLSM) and (4) to investigate the possible correlation between the presence/absence of smear layer and the sealing ability and sealer penetration into dentinal tubules of a resin based sealer.
Materials and methods
Root canal preparation and filling
Fourteen maxillary central incisors with mature apices were selected from a pool of extracted human permanent teeth. Teeth had been extracted for surgical reasons and stored in distilled water at 4 °C. Radiographs were taken in a mesio-distal and bucco-lingual plane to determine canal anatomy, to establish the canal shape (circularity of cross-section) and to determine the presence of any root curvature and lateral canals. Selection criteria of the teeth were similar size of canal at apex, similar circular cross-section of canal and no elliptic section, absence of caries and previous fillings, absence of lateral canals and root curvature and no sclerotic dentine. Roots were inspected with an endodontic optical microscope (OMPI Pico; Carl Zeiss Meditec Inc., Jena, Germany) to detect crack formation or resorption areas. Teeth with root fractures, root caries, evidence of periapical resorptive processes or multiple canals were excluded. The crown of each tooth was removed at the cement enamel junction (CEJ) using a size 701 high-speed fissure bur under water spray to obtain a working length of 13 ± 1 mm. One single operator was responsible for all procedures. The coronal part of each canal was pre-flared using Gates Glidden drills (Dentsply Maillefer, Ballaigues, Switzerland) sizes 1 to 4 inserted in the middle and coronal third. The working length (WL) was measured by insertion of a 21-mm #10 K file (Dentsply Maillefer, Ballaigues, Switzerland) until its tip appeared at the apical foramen under microscopic vision at ×10.
Samples were divided into two experimental groups (n = 5 for group) and two control groups (n = 2 for group) that had a comparable canal width. One control group was used for subsequent confocal laser scanning microscopic analysis and one for fluid filtration test. All groups resulted homogeneous according to canal width, measured on radiographs at 5 mm from the apex, using the analysis of variance (Anova, P > 0.05).
All root canals were shaped by using NiTi WaveOne Primary reciprocating files (size #25 and 8 % taper, Dentsply, Maillefer, Ballaigues, Switzerland) up to 1 mm from the WL, then WL was re-checked with a #10 K file and eventually WaveOne Primary file was used at full WL [30]. All instruments were used in a slow in-and-out pecking motion according to the manufacturer’s instructions mounted on an X Smart plus engine (Dentsply, Maillefer, Ballaigues, Switzerland).
In SL-free group, every time the WaveOne Primary file was pulled out, the canal was irrigated with 1 mL of 5.25 % NaOCl pH 12 (Ogna, Muggiò, Italy), as antibacterial and tissue-dissolving agent for 30 s, followed by 0.5 mL of 10 % EDTA pH 6.8 (Ogna, Muggiò, Italy), as calcium chelating agent to remove the smear layer for 30 s using different syringes supporting a 27-gauge needle (Molteni, Firenze, Italy).
In SL group 2, the same protocol was used but without EDTA.
A final irrigation of 2.0 mL 5.25 % NaOCl left in place for 3 min was performed for each group. After preparation, all canals, except one positive control for subsequent fluid filtration analysis, were dried with paper points (Dentsply Maillefer) and filled by Thermafil Obturators #25 (Dentsply, Tulsa Dental Specialities, Johnson City, TN, USA) (lot. n° 1250047501) with Topseal sealer (Dentsply, Tulsa Dental Specialities, Johnson City, TN, USA) (lot. n° 1207000709).
One additional tooth was subject to protocol of SL-free group and one to protocol of SL group in order to verify by means of ESEM analysis (EVO50 EP, Carl Zeiss NTS GmbH, Oberkochen, Germany) the removal of the smear layer (Fig. 1).
Sealer with rhodamine B preparation
The root canal sealer was mixed manually according to the manufacturer’s directions. Rhodamine B dye (Carlo Erba Reagenti, Arese, Italy) was added to the Topseal sealer in approx. 0.1 wt% in order to mark the sealer and make it visible as red colour under confocal laser scanning microscope (CLSM) observation (Leica TCS SP2 AOBS, Mannheim, Germany).
The sealer was placed in the canal by means of a size 21-mm #15 K file to 1.0 mm short of the WL in a pumping motion for 5 s. After obturation, a portion of coronal Thermafil Obturator was removed using a Thermacut bur (Dentsply, Maillefer) to allow the insertion of the stainless-steel tubing for fluid-flow test. Each sample was exposed to X-ray detection to check the placement of root filling material to the WL, to verify the thickness of the apical filling and to detect the presence of voids inside the filling material. All filled roots were washed with distilled water for 5 s and then lightly dried with gently air blasting. The coronal portion of each root was fixed with cyanoacrylate adhesive (Rocket, Corona, CA, USA) to a Plexiglass block 2.1 × 2.1 × 0.6 cm crossed by a segment of 18-gauge stainless-steel tubing. The stainless-steel tubing was directly connected to the root canal opening. Finally, the external surface of all roots was double coated with nail varnish, except for the canal orifice and the apical foramen (2 mm from apical surface free from varnish) and allowed to dry as previously described by Gandolfi and Prati [31].
As negative control for confocal laser scanning microscopic analysis, two roots were prepared as described earlier and filled with #25 Thermafil Obturator and sealer without rhodamine B dye.
The teeth were stored in simulated body fluid (Hank’s Balanced Salt Solution, HBSS) at 37 °C for 7 days. The fluid flow rate was measured 1 week after root filling.
Fluid filtration and calcein tests
A high-precision digital fluid-flow meter was used for microleakage measurements [31–33]. Each sample was connected to the filtration apparatus with polyethylene tubing (Fisher Scientific, Pittsburg, PA, USA). A water-filled syringe suspended at 70 cm height provided a hydrostatic pressure of 6.895 kPa to the coronal portion of the canal. The deionized water inside the system was supplemented with 0.3 % calcein (Sigma-Aldrich, Saint Louis, MO, USA) to detect the fluid penetration into the root canal by the successive analyses with laser confocal microscope. Calcein was evidenced under confocal microscope as green colour.
A Gilmont microsyringe (Gilmont Instruments, Great Neck, NY, USA) was used to position the air bubble in the hydraulic system [31, 32]. After system stabilization (3 min), the fluid filtration rate of each sample was measured for 3 min (test period) and repeated three times in succession. The micrometric forward movement of a 1.0-mm air bubble inside a microcapillary was measured in micrometres using a microscope connected to a digital camera. Linear measurements were converted to microlitres per minute.
As positive control, one root was prepared as described earlier and filled with #25 Thermafil Obturator without sealer and uncoated with varnish. As negative control, one root was prepared as described earlier and filled with #25 Thermafil Obturator and sealer; moreover, its apex was sealed with a dentin bonding system and entirely covered with nail varnish. Positive and negative control samples were examined at the beginning of the experimental session.
Sectioning and confocal laser scanning microscopic analysis
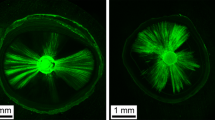
After fluid filtration testing, whole roots were embedded in cold curing methacrylate resin (Technovit 3040, Heraeus Kulzer, Wehrheim, Germany) and serially and transversally sectioned with a saw microtome (Leica SP 1600, Nussloch, Germany) under continuous water irrigation to obtain approximately 24 different root section from CEJ to apex of 200 μm thickness. Every section was examined on a confocal laser scanning microscope (Leica, Mannheim, Germany), using the specific excitation wavelengths for rhodamine B (red) and calcein (green). An Ar laser was used as the light source for calcein excitation at a wavelength of 496 nm and the emission was collected at 515 nm while a HeNe laser was used for rhodamine B excitation at a wavelength of 540 nm and the emission was collected at 590 nm. Each section was observed using a ×10 lens and the images were obtained performing 20 scans of 7 μm step size under both excitation wavelengths in order to co-localize the potential presence of the sealer (rhodamine B) and of a gap (calcein). The images were acquired with LCS Lite 2.61.1537 software (Leica) (Fig. 2).
CLSM images of one single sample without smear layer. a Coronal section showing the green fluorescence of calcein labelling a gap between the filling material and canal wall and some dentinal tubules. b–d section at a more apical level: b the green fluorescence shows calcein labelling a void not filled by sealer; c the red fluorescence shows rhodamine B labelled sealer around the root in the same section. d Merged images
Calcein penetration along the root canal was measured from CEJ considering the number of sections positive to calcein and multiplying this number for the thickness of each section (200 μm) plus that of the saw blade (300 μm), i.e. if the number of positive section is 10, the depth of calcein penetration is 10 × 500 = 5000 μm.
The penetration of the sealer labelled with rhodamine B into dentinal tubules was measured using the straight-line tool of ImageJ software (National Institutes of Health, USA) at 8 standardized points starting from the inner side of canal wall (Fig. 3a) only at 3-, 5- and 8-mm levels from the apex [34]. The percentage of sealer penetration (Fig. 3b) was calculated by measuring the rhodamine B-stained surfaces of the canal wall and dividing these values for the circumference of the root canal itself [8]. To perform the measurements, the segmented-line tool of ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA) was used. Data were recorded and averaged. Representative sections were obtained with additional zoom to confirm the presence of sealer tags (Fig. 4). Table 1 reports an overview of the actions taken during the study of each group.
The straight-line tool of ImageJ software was used on the CLSM image to measure the depth of sealer penetration at eight standardized points starting from the inner side of canal wall (a). The segmented-line tool of ImageJ software was used to measure the circumference of the root canal and the surface (blue lines) where the sealer penetrate into dentinal tubules to calculate the percentage of sealer penetration (b)
Statistical analysis
Assuming a difference in fluid filtration rate between groups of 0.004 μL/min, with a standard deviation of 0.002 μL/min (data obtained in a pilot study) at an a-level of 0.05 for a two-sided test with a power of 0.80, a size of four specimens for each group was required. Mean, standard deviation and range were used to describe the data. Shapiro-Wilk test was performed to verify the fitting of the parameters studied to Gaussian distribution. According to the distribution, Mann-Whitney U test and t test, after controlling for the homoscedasticity of the variances by means of Levene test, were carried out to compare the fluid filtration rate, in the presence or absence of smear layer. Kendall tau correlation coefficient was used to associate fluid filtration rate with depth of calcein penetration, depth and percentage of sealer penetration. α-level was a priori set at 0.05.
Results
Fluid filtration evaluation
After the fluid filtration test, the negative control root exhibited no leakage, while the positive control root showed a high level of leakage. In SL-free group, the average fluid filtration rate was 0.073 ± 0.027 μL/min while in SL-group was 0.065 ± 0.011 μL/min but no significant difference between the groups was observed (P = 0.567).
As for depth of calcein penetration, no significant difference of distribution from Gaussian model was evidenced (Shapiro-Wilk test: P = 0.222). Depth of calcein penetration along the root canal was higher in SL-group than SL-free group (Table 2) even if no significant difference was observed (P = 0.318).
Sealer penetration into dentinal tubules
No rhodamine B fluorescence was observed in the negative control group. The depth of sealer penetration into dentinal tubules increased, not significantly, from 3- to 8-mm level from the apex in both groups (Table 3). Only for 8-mm level from the apex, the mean was significantly higher in absence of smear layer (P = 0.022).
Table 4 presents the percentage of sealer penetration around the root canal at 3-, 5- and 8-mm levels from the apex. The value of the percentage of sealer penetration increases, not significantly, from the 3-mm level to the 8-mm level in both groups. Average values in SL-free group were always higher than SL group at 3- and 5-mm levels, but the difference was not significant; only at 8-mm level, this difference was significant (P = 0.006).
Correlation analysis
No correlation has been found between fluid filtration rate and depth of calcein penetration both in presence or absence of smear layer (Fig. 5).
No significant correlations were observed between fluid filtration rate, percentage of sealer penetration around the root canal and depth of sealer penetration into dentinal tubules both in presence or absence of smear layer.
Discussion
Few studies have provided information about the distribution of endodontic sealer within the dentinal tubules and the potential presence of voids using Thermafil Obturator technique by using confocal laser scanner microscopy [16, 35, 36].
CLSM sample preparation is a non-destructive process and does not require dehydration of the specimens compared to scanning electronic microscopy. Rhodamine B was used with Topseal in order to make the sealer fluorescent. Rhodamine B does not affect the chemical-mechanical behaviour of the sealer considering the small amount used (0.1 wt%) [36]. Moreover, CLSM allows the study of a volume of dentin; in fact, it permits the visualization not only of the sample surface but also in depth within the section [37], and in particular, it allows to observe the dentinal tubules not perpendicular to canal walls [16].
This is the first in vitro study that used two dyes at the same time (rhodamine B and calcein) to evaluate their penetration along the root canal from coronal to apical area. Other researchers used multiple dyes simultaneously to evaluate the morphology of the tooth restoration interface [38–40].
From a methodological point of view, it is important to emphasize that in the present study, the same samples were used to analyse different areas. Rhodamine B and calcein can be analysed separately by using CLSM since they have different excitation and emission wavelength. In this study, rhodamine B was used as a marker for the sealer to determine the penetration of the sealer into dentinal tubules.
Laboratory leakage models have been questioned in recent years [41]. To our knowledge, this is the first study that employs calcein, a fluorescent chelating agent, dissolved into the water of the fluid filtration apparatus. Therefore, this allows to observe where the fluid penetrates and to verify if a correlation exists between fluid filtration rate and penetration depth along the root canal of the calcein-labelled fluid. A noteworthy finding of this study is the appearance of calcein along the canal; the correlation analysis showed that fluid filtration rate and depth of calcein penetration are not correlated. The lack of correlation might be ascribed to the fact that the same amount of fluid might fill, for example, a large gap located in the coronal third or fill small gaps that might connect to create a fine network into the root canal “filled” space; therefore, a high value of fluid filtration does not necessarily mean deep penetration of the fluid itself.
The present study shows that calcein may penetrate in a short time (12 min) large portions of interface and may penetrate into the root canal for 4500 μm in absence of smear layer and 5800 μm in presence of smear layer. Therefore, in these in vitro conditions, 12 min is a sufficient time to create a deep infiltration.
If calcein can reach deep dentin and medium/apical root third, it can be assumed that hydrolytic enzymes and other toxic/bacterial substances may penetrate into the canal and may affect the integrity of sealer/gutta-percha system.
The presence of smear layer can increase the risk for fine porosities and may create alteration at sealer interface [25]. Published researches state that open tubules may create condition to increase the penetration of sealer into the tubules, while the presence of smear layer obstructs the sealer penetration [42, 43].
In this study, sealer penetration into the tubules was similar at 3- and 5-mm levels of the root canal both in presence or absence of smear layer. This result is in agreement with Kuçi et al. [12] who evaluated the dentinal tubules penetration of the sealers AH26 and MTA Fillapex either in the presence or absence of smear layer. Zhou et al. [44] showed that some commercially available sealers, among which AH Plus (Topseal) was present, have similar flow ability, that is they have a pseudoplastic behaviour with an increased flow when shear rate is increased during obturation; this physical property influences the ability to fill voids, gaps or accessory canal and to penetrate into dentinal tubules. The presence of smear layer limits the sealer penetration but not completely blocks its penetration [10]. Even an in vivo study has shown that the sealer penetration into tubules also takes place in presence of a thick smear layer [45]. In the present study, only at 8-mm levels from the apex penetration depth is significantly higher in absence of smear layer. This is probably due to the progressive increase of density of dentinal tubules from apical foramen toward the crown [46]. Moreover, it is likely that at 8-mm level, since the root canal is wider with respect to lower levels, the amount of irrigant is greater compared to 3- and 5-mm levels; therefore, the higher the quantity, the lesser the saturation of the irrigant solution, especially that EDTA might be more effective in the removal of smear layer.
No correlation has been found between sealer penetration into dentinal tubules and sealing ability (fluid filtration rate) both in presence and absence of smear layer using the same core-carrier-based technique.
Unexpectedly, the distribution of the sealer around the root canal, in most samples, was greater in the bucco-lingual direction and there was no penetration in the mesio-distal direction. This phenomenon, described in the past as “butterfly effect” [47–49], is due to the dentinal tubular sclerosis that differs in the mesio-distal and bucco-lingual direction [50, 51]. Root sections with the butterfly effect have a lower density of tubules in mesio-distal direction, corresponding to the wings of the butterfly [49].
Conclusions
According to the experimental conditions and results obtained in this in vitro study, it is possible to conclude that:
-
1.
The sealing ability of Topseal sealer measured using fluid filtration rate and the penetration depth along the root canal of calcein-labelled fluid were not affected by presence or absence of smear layer.
-
2.
No correlation was found between fluid filtration rate and depth of calcein penetration along the root canal.
-
3.
The presence or absence of smear layer does not influence the penetration percentage and depth of the rhodamine B-labelled sealer into dentinal tubules at 3- and 5-mm levels from the apical foramen. Conversely, at 8-mm level, the presence of smear layer significantly reduces the sealer penetration percentage and depth into dentinal tubules.
-
4.
No correlation was found between fluid filtration rate, percentage, and depth of sealer penetration into dentinal tubules both in presence or absence of smear layer.
References
Mamootil K, Messer HH (2007) Penetration of dentinal tubules by endodontic sealer cements in extracted teeth and in vivo. Int Endod J 40:873–881
Torabinejad M, Ung B, Kettering JD (1990) In vitro bacterial penetration of coronally unsealed endodontically treated teeth. J Endod 16:566–569
Trope M, Chow E, Nissan R (1995) In vitro endotoxin penetration of coronally unsealed endodontically treated teeth. Endod Dent Traumatol 11:90–94
Ardizzoni A, Generali L, Righi E, et al. (2014) Differential efficacy of endodontic obturation procedures: an ex vivo study. Odontology 102:223–231
Gandolfi MG, Parrilli AP, Fini M, Prati C, Dummer PM (2013) 3D micro-CT analysis of the interface voids associated with Thermafil root fillings used with AH Plus or a flowable MTA sealer. Int Endod J 46:253–263
Foschi F, Nucci C, Montebugnoli L, et al. (2004) SEM evaluation of canal wall dentine following use of Mtwo and ProTaper NiTi rotary instruments. Int Endod J 37:832–839
Prati C, Foschi F, Nucci C, Montebugnoli L, Marchionni S (2004) Appearance of the root canal walls after preparation with NiTi rotary instruments: a comparative SEM investigation. Clin Oral Invest 8:102–110
Gharib SR, Tordik PA, Imamura GM, Baginski TA, Goodell GG (2007) A confocal laser scanning microscope investigation of the Ephiphany obturation system. J Endod 33:957–961
Balguerie E, van der Sluis L, Vallaeys K, Gurgel-Georgelin M, Diemer F (2011) Sealer penetration and adaptation in the dentinal tubules: a scanning electron microscopic study. J Endod 37:1576–1579
Kara Tuncer A, Tuncer S (2012) Effect of different final irrigation solutions on dentinal tubule penetration depth and percentage of root canal sealer. J Endod 38:860–863
Bolles JA, He J, Svoboda KKH, Schneiderman E, Glickman GN (2013) Comparison of vibringe, endoactivator and needle irrigation on sealer penetration in extracted human teeth. J Endod 39:708–711
Kuçj A, Alaçam T, Yavaş O, Ergul-Ulger Z, Kayaoglu G (2014) Sealer penetration into dentinal tubules in the presence or absence of smear layer: a confocal laser scanning microscopic study. J Endod 40:1627–1631
White RR, Goldman M, Lin PS (1984) The influence of the smear layer upon dentinal tubule penetration by plastic filling materials. J Endod 10:558–562
Çalt S, Serper A (1999) Dentinal tubule penetration of root canal sealers after root canal dressing with calcium hydroxide. J Endod 25:431–433
Patel DV, Sherriff M, Ford TR, et al. (2007) The penetration of RealSeal primer and Tubliseal into root canal dentinal tubules: a confocal microscopic study. Int Endod J 40:67–71
Ordinola-Zapata R, Bramante CM, Bernardineli N, et al. (2009) A preliminary study of the percentage of sealer penetration in roots obturated with the Thermafil and RealSeal-1 obturation techniques in mesial root canals of mandibular molars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:961–968
Şen BH, Pişkin B, Baran N (1996) The effect of tubular penetration of root canal sealers on dye microleakage. Int Endod J 29:23–28
Engel GT, Goodell GG, McClanahan SB (2005) Sealer penetration and apical microleakage in smear-free dentin after a final rinse with either 70 % isopropyl alcohol or Peridex. J Endod 31:620–623
Stevens RW, Strother JM, Mcclanahan SB (2006) Leakage and sealer penetration in smear-free dentin after a final rinse with 95 % ethanol. J Endod 32:785–788
De Deus G, Brandão MC, Leal F, et al. (2012) Lack of correlation between sealer penetration into dentinal tubules and sealability in nonbonded root fillings. Int Endod J 45:642–651
Machado R, Silva Neto UX, Cameiro E, Fariniuk LF, Westphalen VPD, Cunha RS (2014) Lack of correlation between tubular dentine cement penetration, adhesiveness and leakage in roots filled with gutta percha and an endodontic cement based on epoxy amine resin. J Appl Oral Sci 22:22–28
McComb D, Smith DC (1975) A preliminary scanning electron microscopic study of root canals after endodontic procedures. J Endod 1:238–242
Cergneux M, Ciucchi B, Dietschi JM, Holz J (1987) The influence of the smear layer on the sealing ability of canal obturation. Int Endod J 20:228–232
Shahravan A, Haghdoost AA, Adl A, Rahimi H, Shadifar F (2007) Effect of smear layer on sealing ability of canal obturation: a systematic review and meta-analysis. J Endod 33:96–105
Şen BH, Wesselink PR, Türkün M (1995) The smear layer: a phenomenon in root canal therapy. Int Endod J 28:141–148
Çalt S, Serper A (2002) Time-dependent effects of EDTA on dentin structures. J Endod 28:17–19
Saleh IM, Ruyter IE, Haapasalo M, Ørstavik D (2002) The effects of dentine pretreatment on the adhesion of root-canal sealers. Int Endod J 35:859–866
Michelich VJ, Schuster GS, Pashley DH (1980) Bacterial penetration of human dentin in vitro. J Dent Res 59:1398–1403
Torabinejad M, Handysides R, Khademi AA, Bakland LK (2002) Clinical implications of the smear layer in endodontics: a review. Oral Surg Oral Med Oral Phatol Oral Radiol Endod 94:658–666
Generali L, Righi E, Todesca MV, Consolo U (2014) Canal shaping with WaveOne reciprocating files: influence of operator experience on instrument breakage and canal preparation time. Odontology 102:217–222
Gandolfi MG, Prati C (2010) MTA and F-doped MTA cements used as sealers with warm gutta-percha. Long-term study of sealing ability. Int Endod J 43:889–901
Iacono F, Pedullà E, Rapisarda E, Prati C, Gandolfi MG (2014) Long term stability of different obturation systems after 12 months in simulated body fluids. Gen Dent 62:20–23
Camilleri J, Gandolfi MG, Siboni F, Prati C (2011) Dynamic sealing ability of MTA root canal sealer. Int Endod J 44:9–20
Bitter K, Paris S, Martus P, Schartner R, Kielbassa AM (2004) A confocal laser scanning microscope investigation of different dental adhesives bonded to root canal dentine. Int Endod J 37:840–848
Weis MV, Parashos P, Messer HH (2004) Effect of obturation technique on sealer cement thickness and dentinal tubule penetration. Int Endod J 37:653–663
Kok D, Húngaro Duarte MA, Abreu Da Rosa R, Wagner MH, Pereira JR, Só MV (2012) Evaluation of epoxy resin sealer after three root canal filling technique by confocal laser scanning microscopy. Microsc Res Tech 75:1277–1280
D’Alpino PH, Pereira JC, Svizero NR, Rueggeberg FA, Pashley DH (2006) Use of fluorescent compounds in assessing bonded resin-based restorations: a literature review. J Dent 34:623–634
Watson TF (1989) A confocal optical microscope study of the morphology of the tooth/restoration interface using Scotchbond 2 dentin adhesive. J Dent Res 68:1124–1131
Griffiths BM, Watson TF (1995) Resin–dentin interface of scotchbond multi-purpose dentin adhesive. Am J Dent 8:212–216
Hilton TJ (2002) Can modern restorative procedures and materials reliably seal cavities? In vitro investigations. Part 1. Am J Dent 15:198–210
De Deus G (2008) New directions in old leakage models. Int Endod J 41:720–721
Oksan T, Aktener BO, Sen BH, Tezel H (1993) The penetration of root canal sealers into dentinal tubules. A scanning electron microscopic study. Int Endod J 26:301–305
Kokkas AB, Boutsioukis AC, Vassiliadis LP, Stavrianos CK (2004) The influence of the smear layer on dentinal tubule penetration depth by three different root canal sealers: an in vitro study. J Endod 30:100–102
Zhou HM, Shen Y, Zheng W, Li L, Zheng YF, Haapasalo M (2013) Physical properties of 5 root canal sealers. J Endod 39:1281–1286
Vassiliadis LP, Sklavounos SA, Stavrianos CK (1994) Depth of penetration and appearance of Grossman sealer in the dentinal tubules: an in vivo study. J Endod 20:373–376
Mjör IA, Nordhal I (1996) The density and branching of dentinal tubules in human teeth. Arch Oral Biol 41:401–412
Beust TB (1931) Reactions of the dentinal fibril to external irritation. J Am Dent Assoc 18:1060–1073
Vasiliadis I, Darling AI, Levers BGH (1983) The amount and distribution of sclerotic human root dentine. Arch Oral Biol 28:645–649
Russel AA, Chandler NP, Haumann C, Siddiqui AY, Tompkins GR (2013) The butterfly effect: an investigation of sectioned roots. J Endod 39:208–210
Van Huysen G (1960) The microstructure of normal and sclerosed dentin. J Prosthet Dent 10:976–982
Giardino L, Cavani F, Generali L (2016) Sodium hypochlorite solution penetration into human dentine: a histochemical evaluation. Int Endod J. doi:10.1111/iej.12641
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The work was supported by the Department of Surgery, Medicine, Dentistry and Morphological Sciences with Transplant Surgery, Oncology and Regenerative Medicine Relevance, University of Modena and Reggio Emilia, Modena, Italy.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Generali, L., Prati, C., Pirani, C. et al. Double dye technique and fluid filtration test to evaluate early sealing ability of an endodontic sealer. Clin Oral Invest 21, 1267–1276 (2017). https://doi.org/10.1007/s00784-016-1878-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-016-1878-0