Abstract
Introduction
An ideal pulpotomy agent for primary molars has been sought for many years. Recently, new materials that allow regeneration of residual pulp tissue have been developed. In this study, we compared the preliminary clinical results obtained using Biodentine and mineral trioxide aggregate (MTA) as pulp-dressing agents in pulpotomies of primary molars.
Methods
A randomized clinical study was performed in children aged 4–9 years with at least one primary tooth with decay or caries requiring pulp treatment. A total of 90 primary molars requiring pulpotomy were randomly allocated to the MTA or Biodentine group, and 84 pulpotomies were performed. Clinical and radiographic evaluations were undertaken 6 and 12 months after treatment. All teeth were restored with a reinforced zinc oxide–eugenol base and stainless steel crowns. Statistical analysis using Fisher’s exact test was performed to determine the significant differences between the groups.
Results
A total of four clinical failures were observed; all involved gingival inflammation. The clinical success rate in the MTA Group after 12 months was 92 % (36/39), whereas the Biodentine Group obtained 97 % (38/39) (p = 0.346). All radiographic failures were observed at the 12-month follow-up evaluation. One molar from MTA Group showed internal resorption obtaining a radiographic success rate of 97 % (38/39). Two molars from the Biodentine Group showed radiographic failure (1 internal resorption and 1 periradicular radiolucency) obtaining a radiographic success rate of 95 % (37/39).
Conclusions
Biodentine showed similar clinical results as MTA with comparable success rates when used for pulpotomies of primary molars. However, longer follow-up studies are required to confirm our findings.
Clinical relevance
This article demonstrates the effectiveness of Biodentine as a primary teeth pulpotomy material, performing similar results as MTA at 12-months evaluation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulpotomy remains the most common treatment for pulp cavities exposed by caries in symptom-free primary molars [1]. During pulpotomy, the coronal pulp is amputated, and the remaining vital radicular pulp tissue surface is treated with a pulp-dressing agent [2]. Although Formocresol (FC) has long been considered the gold standard, researchers are questioning its use in this procedure due to its possible mutagenic and toxic effects [3]. Different materials such as calcium hydroxide, ferric sulfate, and glutaraldehyde have been studied to identify an alternative to the use of FC [4]. With the development of materials that are both biocompatible and bioinductive, the emphasis has shifted from preservation to regeneration of residual pulp tissue [5, 6]. Mineral trioxide aggregate (MTA; Dentsply, Tulsa, OK, USA) has gained popularity among pediatric dentists for use in pulpotomy because of its excellent sealing ability, biocompatibility, and ability to stimulate hard tissue formation [7]. Indeed, when applied directly onto the pulp, MTA has been found to induce reparative dentin bridge formation [8]. This effect seems to be partially due to the fact that MTA induces release of bioactive molecules such as transforming growth factor-β1 (TGF-β1) from powdered human dentin and pulp cells [9, 10] and this growth factor has been shown to be involved in odontoblastic differentiation [11]. This is why several authors advocate the use of MTA over FC because of its excellent clinical and radiographic results [12, 13]. A recent meta-analysis comparing MTA to FC in 30 clinical articles from 7 databases reported superior clinical success with MTA (95 %) than FC (success rate 87 %) [14].
The notable biological properties of Portland cement have led to the development of Biodentine (Septodont, St. Maur-des-Fosses, France). This new calcium silicate-based cement is packaged as powder and liquid. According to the manufacturer, the powder consists mainly of tricalcium silicate, calcium carbonate, and zirconium oxide. The aqueous component is made up of water, calcium chloride (to accelerate setting), and a modified polycarboxylate (as a superplasticizer). A single measure of liquid is dispensed into a disposable capsule containing the Biodentine powder and mixed with a mixing device for 30 s [10]. Biodentine was developed to combine the high biocompatibility and bioactivity of calcium silicates with enhanced properties such as rapid setting time (conferred by the calcium chloride) and high strength (conferred by the low water-to-cement ratio, made possible by the water-soluble superplasticizing agent) [15]. An in vitro study has evaluated the use of Biodentine in pulpotomies of primary pig teeth. Hard tissue formation was observed after 90 days in all samples [16]. Another in vitro investigation in human molars demonstrated by tomographic evaluation that Biodentine showed the highest thickness of dentin bridges in comparison with MTA and other materials [17]. These properties make Biodentine a possible choice for use as a pulp-dressing agent for pulpotomies in primary molars.
In this study, we aimed to clinically and radiographically evaluate and compare the performance of MTA and Biodentine as pulp-dressing materials following pulpotomy in human primary molars in a 12-month follow-up.
Materials and methods
Study design and participants
This is a randomized open label clinical trial. This study was conducted between February 2012 and April 2013. The project was evaluated and approved by the Ethics Committee of the Universitat Internacional de Catalunya, Sant Cugat del Valles, Barcelona, Spain (Approval Reference: D-34-LBD-09). The study was designed in accordance with the 2010 Consolidated Standards of Reporting Trials statement, Updated Guidelines for Reporting Parallel Group Randomized Trials [18]. This report is part of a larger study, which has been registered at ClinicalTrials.gov (Identifier: NCT01591278).
The participants were selected from patients attending the Department of Paediatric Dentistry at the Universitat Internacional de Catalunya. The procedures, possible discomfort or risks, and possible benefits were explained to the participants and their parents/guardians. Informed consent was obtained from the parents/guardians before participation.
Patients eligible to participate were healthy individuals aged 4–9 years requiring pulpotomy in one or two primary molars. The criteria for the selection of teeth to be included in the study comprised the following:
-
Carious pulp exposure in symptom-free vital primary molars found during the removal of caries
-
No clinical or radiographic evidence of pulp degeneration (excessive bleeding from the root canal, internal root resorption, or inter-radicular and/or furcal bone destruction)
-
The potential for proper restoration of the tooth with a minimum of three walls present
-
Physiologic resorption of less than one third of the root
The exclusion criteria included the presence of systemic pathology and any history of allergic reaction to local anesthetics or to the constituents of the test pulp-dressing agents.
Sample size calculation
Sample size was performed under the assumption of non-inferiority study. Pulpotomy with MTA have a 95 % of clinical success. Similar results can be achieved with Biodentine (maximum difference of 10 % in clinical success between groups). Accepting an alpha risk of 0.05 and a beta risk of 0.2 in a one-sided test, a total of 78 subjects are necessary (36 in MTA group and 36 in Biodentine group). It has anticipated a drop-out rate of 10 %.
Study procedures and outcomes
A single postgraduate student in pediatric dentistry performed the procedures, and one investigator checked to ensure that the pulp was exposed during preparation and that the teeth were suitable for pulpotomy. The molars were randomly assigned to either the Control (MTA) or Experimental (Biodentine) Groups using a random number table.
The primary molars were anesthetized using 4 % articaine with 1:100,000 epinephrine (Ultracain®; Normon S. A., Madrid, Spain) administered by inferior alveolar nerve block for mandibular primary molars and by buccal infiltration for maxillary primary molars [19] using a maximum of one carpule. Rubber dam isolation was used in all cases. Following the removal of caries and exposure of the vital pulp, access to the pulp chamber was obtained using a #330 high-speed bur with a water spray. The access was refined with round burs in a slow-speed handpiece. Subsequently, the coronal pulp tissue was removed using a sterile slow-speed round bur (#6 or #8) [20, 21]. Complete removal of the pulp tissue down to the canal orifices was ensured by visual inspection and corroborated by a second investigator. Bleeding from the remaining pulp tissue was controlled by the application of slight pressure for 5 min [20, 22] with a sterile cotton pellet moistened with saline solution. If bleeding started again at this point, the tooth was eliminated from the study. The remaining radicular pulp tissue was treated with either MTA or Biodentine, as described below.
Mineral trioxide aggregate (control group)
The radicular tissue was covered with MTA paste obtained by mixing MTA powder with sterile saline in a ratio of 3:1 in accordance with the manufacturer’s instructions.
Biodentine (experimental group)
The radicular tissue was covered with Biodentine obtained by mixing Biodentine powder with a single dose of liquid in accordance with the manufacturer’s instructions.
Restoration
The pulp chambers of the molars in both groups were filled with a polymer-reinforced zinc-oxide–eugenol restorative material (Intermediate Restorative Material [IRM]; Dentsply Caulk, Milford, DE, USA). Periapical radiographs were taken immediately after the procedure to ensure that the dressing agents had been placed correctly. All molars were restored with stainless steel crowns (3M ESPE, St. Paul, MN, USA) cemented with glass ionomer cement (Ketac-Cem; 3M ESPE, St. Paul, MN, USA).
Recall visits
At the 6-months and 1-year recall visits, clinical and radiographic examinations were performed. Teeth were evaluated clinically by a single investigator and scored as a clinical success if the patient had no symptoms of pain, and there was no swelling or gingival inflammation, fistulation, or pathologic mobility. Postoperative radiographs were taken using digital radiography (VistaScan; Durr Dental Medics Iberica S. A., Barbera del Valles, Barcelona). For each patient, the radiographs were evaluated independently by two observers experienced in pedodontics. An HP Compaq LA2205 screen was used for the evaluation of the radiographs [23]. Teeth were scored as a radiographic success if they showed no evidence of internal or external resorption or periradicular radiolucency.
Statistical analysis
The Kappa index was measured to assess inter-observer agreement. A tooth-level analysis was performed. Results are presented as valid % or mean (SD). The comparisons between groups at 6 and 12 months were performed using the Chi-square test (exact Fisher test with observed frequencies <5) for categorical variables. P value <0.05 was considered to be statistically significant. All statistical analyses were performed using a statistical software package (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.).
Results
The final study population comprised 68 children (35 boys, 33 girls) with a mean age (± standard deviation) at the time of treatment of 6.6 (± 1.3) years.
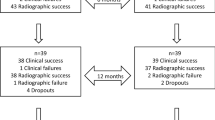
A total of 90 primary molars were initially included in the study, but six were excluded because of uncontrollable bleeding during treatment (a flow chart of molars included in the study is shown in Fig. 1). In total, 84 pulpotomies were performed on teeth randomly assigned to either the MTA or Biodentine Groups. Twenty-five patients had one pulpotomy with Biodentine, 27 patients had one pulpotomy with MTA, and 16 patients had two pulpotomies (one with MTA and one with Biodentine). This makes a total of 41 pulpotomies with Biodentine and 43 pulpotomies with MTA. The types of teeth were as follows: 24 mandibular first primary molars, 18 mandibular second primary molars, 24 maxillary first primary molars, and 18 maxillary second primary molars. After 6 months of follow-up, all molars were clinically and radiographically evaluated without any drop-out; whereas, at the 12-month follow-up evaluation, which had a recall rate of 93 % (78/84), six molars could not be checked as a result of four drop-outs from the MTA Group and two drop-outs from the Biodentine Group (Fig. 1). The Kappa index obtained was 0.8.
Clinical findings
After 6 months of follow-up, three clinical failures had occurred. All involved gingival inflammation (two molars from the MTA Group and one from the Biodentine Group). One molar from the MTA Group showed gingival inflammation at the 12-month follow-up visit. These molars were re-evaluated after educating the patient on oral hygiene, and the gingival inflammation was resolved. No clinical failures were observed in the Biodentine Group at the 12-month follow-up evaluation. Therefore, the clinical success rate in the MTA Group after 12 months was 92 % (36/39), whereas the clinical success rate in the Biodentine Group after 12 months was 97 % (38/39; Table 1) (p = 0.346). No patients showed signs of pain, tooth mobility, fistula, or swelling.
Radiographic findings
No evidence of internal or external resorption or periradicular radiolucency was observed in any molar in either group at the 6-month recall (Fig. 2). All radiographic failures were observed at the 12-month follow-up evaluation. One molar from the MTA Group showed internal resorption; therefore, use of MTA yielded a radiographic success of 97 % (38/39). Use of Biodentine yielded a radiographic success of 95 % (37/39). One molar showed internal resorption and a second exhibited periradicular radiolucency (Table 1; p = 0.635).
Radiographic findings in two molars included in the study. A1 Immediate radiograph of a first mandibular primary molar after pulpotomy with mineral trioxide aggregate. B1 Immediate radiograph of a first mandibular primary molar after pulpotomy with Biodentine. A2 and B2 Radiographic findings for A1 and B1, respectively, at the 12-month follow-up
Discussion
This randomized clinical trial was conducted to evaluate the preliminary effects of the use of Biodentine and MTA as pulp-dressing agents during pulpotomies of primary molars. We observed similar clinical and radiologic success rates in both groups (97 % total success for Biodentine and 96 % for MTA).
With the development of materials that are both biocompatible and bioinductive, the search for alternative pulpotomy agents has shifted from preservation to regeneration of the remaining pulp tissue [6]. One material that has demonstrated potential for pulp regeneration is MTA [24]. In this study, MTA was considered the control treatment, because good results obtained with MTA in previous studies are comparable to those obtained with FC [25–29]. Despite these high rates of success of MTA in the use of pulpotomies, there is still little evidence of its superiority to other materials [4]. We obtained 94 % total success rate for MTA, and this finding is comparable to those obtained in other studies [26, 30].
Both MTA and Biodentine are primarily composed of tricalcium silicate. The biocompatibility of both materials has been established [24, 31, 32]. Indeed, a comparison of pulp response to pulpotomy in dog teeth demonstrated that both MTA and Biodentine induced dentin bridge formation in all investigated samples [33]. MTA has a long setting time of 3–4 h [34], whereas Biodentine sets in just 12 min as a result of the addition of calcium chloride to the mixing liquid [10]. Another difference between these materials is the superior compressive strength of Biodentine compared with MTA and its possible application as an enamel replacement material for up to 6 months [15, 35]. Here, we used IRM as a temporary restorative material for comparison with MTA. However, one major advantage of Biodentine is that it can be placed as a permanent dentin substitute under the crown in a single session.
We experienced a high degree of clinical and radiographic success using both Biodentine and MTA as pulp-dressing agents during pulpotomies of primary molars. This success rate, which was high whatever the tooth type, indicates that both materials seem to be suitable for pulpotomy of all primary molar teeth. Such clinical success may be due to the fact that both materials lead to a non-porous dentin bridge formation as demonstrated in vivo and in clinic [17, 36]. While our clinical protocol cannot bring a scientific explanation to the obtained results, experimental works performed on fibroblast cell cultures and on entire human tooth cultures demonstrated that this dentin regeneration seems to be due to the fact that when these materials are applied to pulp fibroblasts, the later release TGF-β1 and this growth factor recruits pulp stem cells which regenerate the missing dentin in the form of reparative dentin bridge [10, 37]. Also, recent data reported an anti-bacterial activity of both MTA and Biodentine which have been shown to inhibit the growth of oral bacterial strains including Streptococcus mutants and Enterococcus faecalis [38, 39]. This antibacterial property is very significant to the restoration clinical success. Indeed, a retrospective work on factors influencing the pulp response to cavity restorations reported that the presence of bacteria on the cavity walls is the main factor influencing pulp reaction under restorative materials and, consequently, the clinical success [40].
Two of the three radiographic failures reported in our clinical trial involved internal root resorption; this finding was also reported in our previous clinical trial of pulp agents [41]. All molars in the previous study that showed internal root resorption were left for observation if asymptomatic and did not show any signs of clinical failure. In this study, these molars are under observation and remain asymptomatic. Regarding clinical results, all clinical failures in the present study involved gingival swelling, which has long been considered a clinical failure after a primary molar pulpotomy [20, 26, 29, 42, 43]. However, the molars suffering from gingival swelling observed in this study were resolved by basic oral hygiene instructions, as was done in our previous clinical trial on pulpotomies [34]. Although no clear evidence has demonstrated a link between Stainless Steel Crowns and gingivitis, the patient’s level of oral hygiene with Stainless Steel Crowns has a significant effect on the gingival index [44] so that swelling observed in both our present and previous studies could be due to a combination of both Stainless Steel Crowns and the patient’s gingival index.
Compared with MTA, Biodentine offers many advantages (shorter setting time, enhanced compressive strength, micro-hardness, and lower cost) and represents a promising material for use in pulpotomies. However, long-term results must be evaluated. Although this study shows preliminary short-term follow-up results in primary molar pulpotomies, our findings highlight the different strengths in methodology: all molars were treated under rubber dam isolation and restored with stainless steel crowns, which have excellent marginal adaptation that makes them the restoration of choice for this treatment. In addition, all molars were clinically and radiographically re-evaluated in two follow-ups (at 6 months and 12 months), with a high recall rate in the second evaluation (93 %).
To date, there is just one clinical study evaluating the effectiveness of the use of Biodentine during pulpotomies of primary molars after 6 months of follow-up [45]. However, there are many disparities in methodology with the present study: the authors do not explain how many operators performed the pulpotomies and how the clinical and radiographic evaluations were performed. No total success rate of the materials was calculated, and the authors think their failures might be due to iatrogenic errors like “poorly adapted stainless steel crowns, a thin base, voids in the cement and areas of residual caries or coronal pulp tissue.” These could be solved with a postoperative radiograph after pulpotomy and placement of the stainless steel crowns.
Overall, our results are in line with this paper comparing MTA to Biodentine in primary teeth pulpotomies which also showed that both materials were equally efficient in this clinical indication [45].
This is the first randomized clinical trial to evaluate the performance of Biodentine as a pulp-dressing agent during primary molar pulpotomy with a follow-up of 12 months. The preliminary results obtained using Biodentine as a pulpotomy agent after 12 months of follow-up are promising; however, further studies with a larger sample size and a longer follow-up period are necessary.
References
Fuks AB (2008) Vital pulp therapy with new materials for primary teeth: new directions and treatment perspectives. J Endod 34(7 Suppl):S18–S24
Definitions AAPD, Policies OH, Guidelines C (2013) Guideline on pulp therapy for primary and immature permanent teeth. AAPD Reference Manual 35:222–229
Chen JW, Jorden M (2010) Materials for primary tooth pulp treatment: the present and the future. Endod Topics 23:41–49
Smaïl-Faugeron V, Courson F, Durieux P, Muller-Bolla M, Glenny AM, Fron Chabouis H (2014) Pulp treatment for extensive decay in primary teeth. Cochrane Database Syst Rev 6;8:CD003220.
Shayegan A, Petein M, Abbeele AV (2008) Beta-tricalcium phosphate, white mineral trioxide aggregate, white Portland cement, ferric sulfate, and formocresol used as pulpotomy agents in primary pig teeth. Oral Surg Oral Med Oral Pathol 105:536–542
Sakai VT, Moretti AB, Oliveira TM, Fornetti AP, Santos CF, Machado MA, et al. (2009) Pulpotomy of human primary molars with MTA and Portland cement: a randomised controlled trial. Br Dent J 207:E5discussion 128-129
Torabinejad M, Parirokh M (2010) Mineral trioxide aggregate: a comprehensive literature review—part II: leakage and biocompatibility investigations. J Endod 36:190–202
Accorinte MLR, Loguercio AD, Reis A, et al. (2008) Response of human dental pulp capped with MTA and calcium hydroxide powder. Oper Dent 33:488–495
Tomson PL, Grover LM, Lumley PJ, Sloan AJ, Smith AJ, Cooper PR (2007) Dissolution of bio-active dentine matrix components by mineral trioxide aggregate. J Dent 35:636–642
Laurent P, Camps J, About I (2012) Biodentine(TM) induces TGF-beta1 release from human pulp cells and early dental pulp mineralization. Int Endod J 45:439–448
Begue-Kirn C, Smith AJ, Ruch JV, et al. (1992) Effects of dentin proteins, transforming growth factor beta 1 (TGF beta 1) and bone morphogenetic protein 2 (BMP2) on the differentiation of odontoblast in vitro. Int J Dev Biol 36:491–503
Shirvani A, Asgary S (2014) Mineral trioxide aggregate versus formocresol pulpotomy: a systematic review and meta-analysis of randomized clinical trials. Clin Oral Investig 18:1023–1030
Lin PY, Chen HS, Wang YH, Tu YK (2014) Primary molar pulpotomy: a systematic review and network meta-analysis. J Dent 42:1060–1077
Stringhini Junior E1, Vitcel ME, Oliveira LB (2015) Evidence of pulpotomy in primary teeth comparing MTA, calcium hydroxide, ferric sulphate, and electrosurgery with formocresol. Eur Arch Paediatr Dent 16:303–312
Grech L, Mallia B, Camilleri J (2013) Investigation of the physical properties of tricalcium silicate cement-based root-end filling materials. Dent Mater 29:e20–e28
Shayegan A, Jurysta C, Atash R, Petein M, Abbeele AV (2012) Biodentine used as a pulp-capping agent in primary pig teeth. Pediatr Dent 34:e202–e208
Nowicka A, Wilk G, Lipski M, Kołecki J, Buczkowska-Radlińska J (2015) Tomographic evaluation of reparative dentin formation after direct pulp capping with Ca(OH)2, MTA, biodentine, and dentin bonding system in human teeth. J Endod 41:1234–1240
Schulz KF, Altman DG, Moher D; CONSORT Group (2011) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Int J Surg 9:672–677
Definitions AAPD, Policies OH, Guidelines C (2012) 2013 Guideline on use of local anesthesia for pediatric dental patients. AAPD Reference Manual 34:183–189
Huth KC, Paschos E, Hajek-Al-Khatar N, et al. (2005) Effectiveness of 4 pulpotomy techniques–randomized controlled trial. J Dent Res 84:1144–1148
Doyle TL, Casas MJ, Kenny DJ, Judd PL (2010) Mineral trioxide aggregate produces superior outcomes in vital primary molar pulpotomy. Pediatr Dent 32:41–47
Mielke Jr CH, Kaneshiro MM, Maher IA, Weiner JM, Rapaport SI (1969) The standardized normal Ivy bleeding time and its prolongation by aspirin. Blood 34(2):204–215
HP Compaq LA2205wg 22-inch Widescreen LCD Monitor Espeficiations. http://www8.hp.com/h20195/v2/GetPDF.aspx/c04110977.pdf Accessed 28 July 2015.
Agamy HA, Bakry NS, Mounir MM, Avery DR (2004) Comparison of mineral trioxide aggregate and formocresol as pulp-capping agents in pulpotomized primary teeth. Pediatr Dent 26:302–309
Farsi N, Alamoudi N, Balto K, Mushayt A (2005) Success of mineral trioxide aggregate in pulpotomized primary molars. J Clin Pediatr Dent 29:307–311
Holan G, Eidelman E, Fuks AB (2005) Long-term evaluation of pulpotomy in primary molars using mineral trioxide aggregate or formocresol. Pediatr Dent 27:129–136
Moretti AB, Sakai VT, Oliveira TM, Fornetti AP, Santos CF, Machado MA, et al. (2008) The effectiveness of mineral trioxide aggregate, calcium hydroxide and formocresol for pulpotomies in primary teeth. Int Endod J 41:547–555
Sonmez D, Sari S, Cetinbas T (2008) A comparison of four pulpotomy techniques in primary molars: a long-term follow-up. J Endod 34:950–955
Ansari G, Ranjpour M (2010) Mineral trioxide aggregate and formocresol pulpotomy of primary teeth: a 2-year follow-up. Int Endod J 43:413–418
Zealand CM, Briskie DM, Botero TM, Boynton JR, Hu JC (2010) Comparing grey mineral trioxide aggregate and diluted formocresol in pulpotomized human primary molars. Pediatr Dent 32:393–399
Zanini M, Sautier JM, Berdal A, Simon S (2012) Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J Endod 38:1220–1226
Laurent P, Camps J, De Méo M, Déjou J, About I (2008) Induction of specific cell responses to a Ca(3)SiO(5)-based posterior restorative material. Dent Mater 24:1486–1494
De Rossi A, Silva LA, Gatón-Hernández P, Sousa-Neto MD, Nelson-Filho P, Silva RA, de Queiroz AM (2014) Comparison of pulpal responses to pulpotomy and pulp capping with biodentine and mineral trioxide aggregate in dogs. J Endod 40:1362–1369
Parirokh M, Torabinejad M (2010) Mineral trioxide aggregate: a comprehensive literature review–part III: clinical applications, drawbacks, and mechanism of action. J Endod 36:400–413
Koubi G, Colon P, Franquin JC, Hartmann A, Richard G, Faure MO, Lambert G (2013) Clinical evaluation of the performance and safety of a new dentine substitute, Biodentine, in the restoration of posterior teeth—a prospective study. Clin Oral Investig 17:243–249
Tran XV, Gorin C, Willig C, Baroukh B, Pellat B, Decup F, Opsahl Vital S, Chaussain C, Boukpessi T (2012) Effect of a calcium-silicate-based restorative cement on pulp repair. J Dent Res 91:1166–1171
Mathieu S, Jeanneau C, Sheibat-Othman N, Kalaji N, Fessi H, About I (2013) Usefulness of controlled release of growth factors in investigating the early events of dentin-pulp regeneration. J Endod 39:228–235
Bhavana V, Chaitanya KP, Gandi P, Patil J, Dola B, Reddy RB (2015) Evaluation of antibacterial and antifungal activity of new calcium-based cement (Biodentine) compared to MTA and glass ionomer cement. J Conserv Dent 18:44–46
Koruyucu M, Topcuoglu N, Tuna EB, Ozel S, Gencay K, Kulekci G, Seymen F (2015) An assessment of antibacterial activity of three pulp capping materials on Enterococcus faecalis by a direct contact test: an in vitro study. Eur J Dent 9:240–245
Camps J, Déjou J, Rémusat M, About I (2000) Factors influencing pulpal response to cavity restorations. Dent Mater 16:432–440
Fernandez CC, Martinez SS, Jimeno FG, Lorente Rodriguez AI, Mercade M (2013) Clinical and radiographic outcomes of the use of four dressing materials in pulpotomized primary molars: a randomized clinical trial with 2-year follow-up. Int J Paediatr Dent 23:400–407
Eidelman E, Holan G, Fuks AB (2001) Mineral trioxide aggregate vs. formocresol in pulpotomized primary molars: a preliminary report. Pediatr Dent 23:15–18
Erdem AP, Guven Y, Balli B, et al. (2011) Success rates of mineral trioxide aggregate, ferric sulfate, and for- mocresol pulpotomies: a 24-month study. Pediatr Dent 33:165–170
Sharaf AA, Farsi NM (2004) A clinical and radiographic evaluation of stainless steel crowns for primary molars. J Dent 32:27–33
Niranjani K, Prasad MG, Vasa AA, Divya G, Thakur MS, Saujanya K (2015) Clinical evaluation of success of primary teeth pulpotomy using Mineral Trioxide Aggregate(®), Laser and Biodentine(TM)—an in vivo study. J Clin Diagn Res 9:ZC35–ZC37
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
There were no conflicts of interests.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Cuadros-Fernández, C., Lorente Rodríguez, A.I., Sáez-Martínez, S. et al. Short-term treatment outcome of pulpotomies in primary molars using mineral trioxide aggregate and Biodentine: a randomized clinical trial. Clin Oral Invest 20, 1639–1645 (2016). https://doi.org/10.1007/s00784-015-1656-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-015-1656-4