Abstract
Objectives
The aim was to test the properties of experimental calcium silicate/calcium phosphate biphasic cements with hydraulic properties designed for vital pulp therapy as direct pulp cap and pulpotomy.
Methods
CaSi-αTCP and CaSi-DCDP were tested for ion-releasing ability, solubility, water sorption, porosity, ability to nucleate calcium phosphates, and odontoblastic differentiation—alkaline phosphatase (ALP) and osteocalcin (OCN) upregulation—of primary human dental pulp cells (HDPCs).
Results
The materials showed high Ca and OH release, high open pore volume and apparent porosity, and a pronounced ability to nucleate calcium phosphates on their surface. HDPCs treated with CaSi-αTCP showed a strong upregulation of ALP and OCN genes, namely a tenfold increase for OCN and a threefold increase for ALP compared to the control cells. Conversely, CaSi-DCDP induced a pronounced OCN gene upregulation but had no effect on ALP gene regulation.
Conclusions
Both cements showed high biointeractivity (release of Ca and OH ions) correlated with their marked ability to nucleate calcium phosphates. CaSi-αTCP cement proved to be a potent inducer of ALP and OCN genes as characteristic markers of mineralization processes normally poorly expressed by HDPCs.
Clinical relevance
Calcium silicate/calcium phosphate cements appear to be attractive new materials for vital pulp therapy as they may provide odontogenic/dentinogenic chemical signals for pulp regeneration and healing, and dentin formation in regenerative endodontics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulp therapy is designed to maintain the pulp vitality of a tooth affected by caries, traumatic injury, or other damage. Vital pulp procedures require the use of biomaterials to form a protective layer over the exposed vital pulp in direct pulp cap procedures (on the floor of cavities after removal of deep carious lesions or after traumatic exposure) or in pulpotomies (on the radicular pulp after removal of the coronal tissue). These protective materials should possess specific bio-properties—like biointeractivity (release of biologically relevant ions), apatite-forming ability, biocompatibility, and bioactivity—to promote pulp cell activity and pulp healing, and the formation of new reparative dentin.
Conventional calcium hydroxide (proposed in 1930)-based materials are currently used in clinical practice for reparative dentinogenesis due to their capability to release calcium (Ca) and hydroxyl (OH) ions [1]. However, high solubility and fast dissolution are typical drawbacks of conventional calcium hydroxide materials making them clinically inadequate in bleeding sites.
Calcium silicate-based (CaSi) cements, conventionally known in dentistry as mineral trioxide aggregate (MTA) cements, are hydraulic self-setting materials, mainly composed of dicalcium and tricalcium silicates with peculiar intrinsic properties suitable for their clinical use [1–5]. They form calcium hydroxide during their hydration process and set in the presence of blood and other biological fluids, ensuring a good sealing [6] also correlated to their expansion [7]. They are biointeractive (Ca and OH releasing), bioactive (apatite-forming), and biocompatible materials able to release biologically relevant ions potentially acting as epigenetic signals [1, 5, 8] toward human stem [9, 10] and pulp [11–15] cells inducing a positive biological response.
Recently, CaSi cements have received growing attention for their ability to induce the formation of a hard dentine bridge in vivo after their direct application on healthy human pulp. In addition, they provided good clinical outcomes when used for both direct and indirect pulp capping [2, 3].
Alpha tricalcium phosphate (alpha-TCP) is a very biocompatible material with a high chemical reactivity. Previous studies showed that the addition of alpha-TCP increases the apatite-forming ability of calcium silicate cements [16, 17] and supports a biomimetic microenvironment favorable to the survival and differentiation of orofacial mesenchymal stem cells and can potentially promote tissue regeneration in peri-apical bone defects [10].
Alpha tricalcium phosphate is an important metastable (Ca/P = 1.5) component of self-setting cements for biomaterial applications due to its ability to hydrolyze quickly to hydroxyapatite in aqueous media [18, 19]. The mechanisms involved in this hydrolysis and phase conversion of alpha-TCP have already been described as Ca3(PO4)2 + H2O → Ca9(OH)(HPO4)(PO4)5 [18–22]. When exposed to aqueous solution, alpha-TCP supplies Ca2+ and PO4 3− and, as a result of consecutive dissolution-precipitation reactions, Ca-deficient apatite crystals precipitate and interlock [23]. After immersion of alpha-TCP powder in deionized water for 30 days at room temperature, most of the alpha-TCP phase is converted into a poorly crystallized apatite structure [18].
Dicalcium phosphate dihydrate (DCPD) is a safe material used as a mineral dietary supplement (identified as E341 according to the European classification of food additives) and to prepare osteotransductive bone cements [24]. DCPD possesses two very attractive properties: It is basically insoluble in water, but it converts into apatite after contact with water.
Mixing a CaSi cement with alpha-TCP or DCPD seems very promising to optimize the biological properties of each component.
This study aimed to screen the chemical and physical properties—in terms of ion release, solubility, water sorption, porosity, and ability to nucleate calcium phosphates—of two experimental biphasic cements containing a calcium silicate and a calcium phosphate (alpha-TCP or DCPD) component. Their effects on odontoblastic differentiation were tested on primary human dental pulp cells (HDPCs) cultured with the cement extracts. The upregulation of alkaline phosphatase (ALP) and osteocalcin (OCN) after 24-h culture was examined by quantitative real-time PCR.
Materials and methods
The calcium silicate (CaSi) powder prepared by melt-quenching technique and milling procedures [25] and composed of dicalcium silicate, tricalcium silicate, tricalcium aluminate, and calcium sulfate (Aalborg, Denmark) was thermally and mechanically treated and then milled with alpha tricalcium phosphate (alpha-TCP; Ca3(PO4)2) or dicalcium phosphate dihydrate (DCPD; CaHPO4 · 2H2O) (Sigma-Aldrich, Steinheim, Germany) using 1:1 w/w. Two experimental biphasic calcium silicate/calcium phosphate powders CaSi-αTCP and CaSi-DCPD were obtained. Cement pastes were prepared using a liquid to powder ratio (L/P) of 0.33. The mixing liquid was Dulbecco’s phosphate-buffered saline (DPBS) [10].
The calcium phosphate powders and the experimental cements before their hydration and after setting (24-h hydration in 10 mL of deionized water i.e. the same condition than in physical tests) have been submitted to X-ray diffraction (XRD) analysis by an X-ray diffractometer (X’PertPRO, PANalytical, Almelo, The Netherlands) with X’Celerator detector. The Cu X-ray source was set at accelerating voltage of 40 kV and the current in the electron beam at 40 mA. Specimens were scanned from 3° to 60° 2θ with step size of 0.05°, time/step 200 s.
Powder Diffraction Files (PDF) of the ICDD (International Centre for Diffraction Data), American Standard Test Method (ASTM C 1365), and National Institute of Standards and Technology (NISTIR 5755) were used to identify the XRD peaks.
Biointeractivity: alkalizing activity (pH of soaking water), calcium, and phosphate release
The freshly mixed pastes were placed in PVC moulds (8.0 ± 0.1 mm diameter and 1.6 ± 0.1 mm thickness) to prepare material disks (n = 10 for each material). Disks filled with unset freshly mixed cement were immediately immersed in 10 mL of deionized water (pH 6.8) in polypropylene-sealed containers and stored at 37 °C. The soaking water was collected and replaced at six endpoints (3 and 24 h and 3, 7, 14, and 28 days). The collected water was analyzed for pH and Ca ion release by a potentiometric method under magnetic stirring at room temperature (24 °C).
The pH was measured using a selective temperature-compensated electrode (Sen Tix Sur WTW, Weilheim, Germany) connected to a multiparameter laboratory meter (inoLab 750 WTW, Weilheim, Germany) previously calibrated with standard solutions.
The amount of ions released was measured using a Eutech Calcium probe (Eutech instruments Pte Ldt, Singapore) and Quantofix Phosphate (Macherey-Nagel GmbH & Co. KG, Düren, Germany). Cumulative calcium release was calculated separately for each of the ten samples of material by adding up the amounts released at the six different endpoints. Then, the mean and standard deviations were calculated.
Physical properties: porosity, water sorption, solubility, and setting time
The freshly mixed paste was placed in the moulds (n = 10 for each material) and allowed to set (at 37 °C and 99 % relative humidity) for a period equal to 70 % of the final setting time.
Each sample was weighed to determine the initial mass (I) and immediately immersed vertically in 20 mL of distilled water and placed at 37 °C, and the mass while suspended in water (S) was determined. After 24-h immersion, the specimens were removed from the water, and the excess water from the surface from each sample was removed using a moistened filter paper and the saturated mass (M) was recorded. Finally, the samples were dried at 37 °C until the weight was stable and the final dry mass (D) was measured. Each weight measurement was repeated three times using an analytical balance (Bel Engineering series M, Monza, Italy) and determined to the nearest 0.001 g.
The exterior volume V (V = M − S), the volume of open pores V OP (V OP = M − D), the volume of the impervious portion V IP (V IP = D − S), and the apparent porosity P (P = [(M − D) / V] × 100) were calculated in cubic centimeter or in percentage, following Archimedes’ principle (and according to ASTM C373-88). Water sorption A (A = [(M − D) / D] × 100) and solubility S (S = [(I − D) / D] × 100) were calculated as percentages of the original weight [25].
The final setting time was determined using a Gilmore needle weighing 453.6 g with a tip diameter of 1.06 mm, in accordance with ASTM standard C266-07. The freshly mixed cement pastes were placed in a mould measuring 10 mm in diameter and 2 mm thick and then stored at 37 °C and 95 ± 5 % relative humidity. The setting time was recorded when no indentation was caused by the needle.
Statistical analysis
The results were analyzed using two-way ANOVA followed by RM Student-Newman-Keuls test (p < 0.05) or Friedman’s test (non-parametric repeated measures comparisons, p < 0.05) for phosphorous leaching data. Different letters represent statistically significant differences in the same line (capital letters) or in the same column (small letters).
Apatite-forming ability by environmental microscopy and microanalysis (ESEM-EDX) of the surface
Freshly prepared materials were immediately immersed vertically in 20 mL of Hank’s Balanced Salt Solution (HBSS, Lonza Walkersville, Inc., Walkersville, MD, USA) and stored at 37 °C for 1, 7, and 28 days [1, 5]. The HBSS was renewed weekly.
The surface of each damp sample was examined in wet conditions without any previous preparation [4, 5] using an environmental scanning electron microscope (ESEM, Zeiss EVO 50; Carl Zeiss, Oberkochen, Germany) connected to a secondary electron detector for energy dispersive X-ray analysis (EDX; Oxford INCA 350 EDS, Abingdon, UK) with computer-controlled software (Inca Energy Version 18), at low vacuum (100 Pa), accelerating voltage of 20 kV, working distance 8.5 mm, 0.5 wt% detection level, 133 eV resolution, amplification time 100 μs, measuring time: 600 s for element mapping and 60 s for spectra.
The elemental X-ray microanalysis (wt% and at%) with ZAF correction was performed in full frame to analyze entire areas showed in the pictures (EDX area scan). The Ca/P ratio was calculated from the atomic data obtained.
Cell test
Primary HPC culture
Human pulp-derived cells (HPCs) were obtained from human third molars freshly extracted from healthy patients, with proper informed consent. As previously described [26], pulp tissue was minced into small tissue pieces and cultured in Dulbecco’s minimal essential medium (DMEM) supplemented with 10 % fetal calf serum (FCS), 2 mmol/L glutamine, 100 U/mL of penicillin, and 100 mg/mL of streptomycin. In all experiments, cells were pooled and used between passage 2 and passage 6 [27].
Extract preparation and cell proliferation by alamarBlue assay
Cement samples were prepared and stored at 37 °C for 3 h and then immersed in extraction vehicle (DMEM with 10 % FCS) for 24 h at 37 °C in accordance with ISO recommendations (ISO 7405 clause 6, ISO 10993-5 clause 4 and ISO 10993-12 clause 10). A higher extraction ratio (1.9 cm2/mL expressed as surface area of the sample/extractant volume or 0.3 g sample/mL expressed as mass of the sample/extractant volume) higher than that suggested by ISO 10993-12 clause 10 was used.
HPCs (5 × 104 cells/well) were seeded in 96-multiwell plates. After 24 h at 37 °C, 0.2 mL of pure extract of each cement was added to the wells. Cells incubated in complete medium formed the untreated control group. Cell proliferation of four replicate samples was assessed by alamarBlue assay (AB) at 1, 3, 7, and 14 days.
AlamarBlue assay uses a visible blue fluorogen probe resazurin, which is reduced to a red fluorescent compound (resorufin) by cellular redox enzymes of the mitochondrial respiratory chain. Viable cells continuously convert resazurin to resorufin, thereby generating a quantitative measure of viability and proliferation [28].
The AB assay was performed according to the manufacturer’s protocol (BioSource International, Camarillo, CA, USA). At predetermined culture intervals (1, 3, 7, 14 days), 200 μL of alamarBlue dye were added directly into culture media at a final concentration of 10 % and the plates were returned to the incubator for 4 h. As a negative control, AB was added to the medium without cells. The percentage AB reduction was calculated from the values of optical density at 540 and 590 nm using the manufacturer’s formula. Each experiment was performed three times (n = 3) in quadruplicate. Statistical analysis was performed by the nonparametric one-way ANOVA test (with a p value <0.05 considered significant).
Total RNA: extraction and reverse transcription
100,000 HPC cells were seeded in six multiwell plates, and when the cells reached 80 % of confluence, they were exposed to the pure extract of each cement. Cells incubated in complete medium (untreated cells) formed the control group. After a 24-h period, cells were detached from the plates and homogenized with Qiashredder (Qiagen cat. No. 79654). Total RNA was extracted by the guanidinium thiocyanate method [29]. One micrograms of total RNA was subjected to cDNA synthesis for 1 h at 37 °C using the “Ready-to-go You-Prime First-Strand Beads” kit (Amersham Pharmacia Biotech, Piscataway, NJ, cod. 27-9264-01) in a reaction mixture containing 0.5 μg oligodT (Amersham Pharmacia Biotech cod. 27-7610-01).
Gene expression by qRT-PCR analysis
Alkaline phosphatase (ALP) and osteocalcin (OCN) gene expression were evaluated at 1 day by real-time quantitative PCR (qRT-PCR) analysis. qRT-PCR analysis was performed on cell lysate using StepOne base (Applied Biosystems, Foster City, CA, USA) and the 5-exonuclease assay (TaqMan technology).
Complementary DNA (cDNA) transcripts from RNA, synthesized as described above, were used to quantitatively measure the amplification of DNA using fluorescent probes.
Briefly, real-time PCR was performed in 48-well optical reaction plates with cDNA equivalent to 100 ng of RNA in a volume of 25-μL reaction containing × 1 TaqMan Gene Expression Master Mix (Applied Biosystem cat. No. 1411192), optimized concentrations of FAM-labeled probe, and specific forward and reverse primers for ALP and OCN genes from Assay on Demand (Applied Biosystems).
The values were normalized to the β-actin expression and converted into fold change that expresses a change as the ratio between an initial value and a final value emphasizing a change rather than the absolute values. Results were expressed as fold increase over the control values and analyzed using a comparative method. qRT-PCR data represent the means of three independent experiments, and each experiment was performed in triplicate. Statistical analysis was performed by the nonparametric one-way ANOVA test (with a p value <0.05 considered significant).
Results
Alkalizing activity (pH of soaking water) and ion release
Both materials induced the alkalization of the soaking water that showed statistically significant changes over time but was still present at 28 days (Table 1A). The pH of the soaking water was strongly basic (pH 11–11.5) at short times (until 24 h) then significantly decreased at 3 days and after 14 days. At 28 days, the pH approached neutrality (pH ≈ 8).
Both materials released Ca and the release significantly decreased with the soaking time, in particular at 1–3 days (Table 1B, C).
No statistically significant differences were found between CaSi-αTCP and CaSi-DCPD at any time, but a statistical significance was noted for calcium release at 3 h towards the control CaSi cement.
Traces of phosphate were released from CaSi-αTCP and CaSi-DCPD but not from CaSi (Table 1D).
XRD characterization
The XRD analyses of αTCP starting powder showed the pattern of αTCP (according to PDF 9-348) with traces of βTCP (PDF 9-169) (Fig. 1a) as a minor phase; the XRD pattern of DCPD powder indicated pure brushite (according to PDF 9-77) (Fig. 1b).
XDR patterns of starting powders (alpha-TCP, DCPD, and CaSi), anhydrous CaSi-αTCP and CaSi-DCPD cements and 24-h hydrated cements. Alpha-TCP starting material showed to be mainly alpha-TCP with traces of beta-TCP. XRD pattern of DCPD showed pure brushite. Diagram of the unhydrated CaSi powder showed mainly tricalcium silicate alite (Ca3SiO5) and the presence of dicalcium silicate belite (Ca2SiO4 larrnite) and calcium carbonate (CaCO3 aragonite). DRX patterns of the unhydrated CaSi-alpha-TCP and CaSi-DCPD powders showed the phases of the starting powders. Calcium silicate hydrates and portlandite (hydration products) were detected in the diagrams of the 24-h hydrated cements together with the same phases detected in the anhydrous cements
Diagrams of the unhydrated CaSi powder (Fig. 1a, b) showed mainly tricalcium silicate alite (Ca3SiO5, PDF 1-73-599) and the presence of dicalcium silicate belite (Ca2SiO4 larrnite, PDF 1-83-461) and calcium carbonate (CaCO3 aragonite, PDF 1-75-2230).
Unhydrated CaSi-αTCP (Fig. 1a) and CaSi-DCPD (Fig. 1b) cements generated patterns displaying the same reflexes found in their constitutive materials, namely alite, larrnite, calcium carbonate, and αTCP or DCPD, respectively. Diagram comparison of αTCP powder with unhydrated CaSi-αTCP cement shows no decrease in the intensities of the αTCP diffraction peaks, no mechanically induced phase transformation (as formation of amorphous TCP phase), i.e. no reduction in crystallinity, no peaks broadening, and no increase of the background below the peaks occurred after the milling process. Similar results emerge from the diagram comparison of DCPD starting powder with unhydrated CaSi-DCPD cement.
XRD pattern of the 24-h hydrated cements (Fig. 1a, b) showed the same phases detected in the anhydrous cements together with the presence of hydration products as portlandite (Ca(OH)2, PDF 1-87-673) and calcium silicate hydrates (Ca2SiO4 · H2O, PDF 11-211; CaH4Si2O7, PDF 12-739). In addition, hydrated CaSi-αTCP cement showed traces of DCPD.
Physical properties
Significantly higher porosity values were measured for CaSi-DCPD, whereas no statistical differences were obtained between CaSi-αTCP and CaSi (Table 2A).
Statistically significant differences among all the materials were found for water sorption, solubility, and setting time (Table 2B). CaSi-DCPD showed higher water sorption and solubility than either CaSi or especially CaSi-αTCP. CaSi-αTCP was markedly less soluble and absorbed considerably less water.
CaSi-αTCP showed a shorter final setting time (approx. 3 h), whilst CaSi-DCPD had a longer final setting time (approx. 4 h) than the CaSi control cement.
Apatite-forming ability
The ESEM image of freshly mixed CaSi-αTCP cement (Fig. 2) displayed a finely granular surface, and EDX analyses (see spectrum) revealed Ca, Si, P, Na, K, and S elements.
Morphochemical characterization of CaSi-αTCP cement showing ESEM pictures, EDX spectra of the whole area, and the elemental X-ray microanalysis (compositional wt% and at%) performed on freshly prepared paste and on the 28-day aged surface. The ESEM image of the freshly mixed cement displayed a finely granular surface, and EDX analyses (microchemical spectrum and semiquantitative compositional analysis) revealed Ca, Si, P, Na, K, and S elements. After 28-day soaking in HBSS, the reflexes of the silicate component Si decreased in intensity, whilst P element increased. The surface was uniformly covered by a coating composed of globular CaP particles (spherulites 1–3 μ size). The semiquantitative compositional analysis showed that the Ca/P atomic ratio decreased with time from approx. 4.68 in freshly mixed paste to approximately 2.9 at 28 days
After 28-day soaking in HBSS, the surface was uniformly covered by a coating of globular CaP precipitates. The semiquantitative compositional analysis (see element tables) showed that the Ca/P atomic ratio decreased with time, from approx. 4.68 in freshly mixed paste to approximately 2.9 at 28 days in relation to the increase in P content.
Freshly mixed CaSi-DCPD cement (Fig. 3) showed a surface containing interspersed granules 3–10 μ wide, and mainly displayed Ca, P, and Si (see spectrum).
Morphochemical ESEM-EDX characterization and semiquantitative compositional analysis of CaSi-DCPD cement. Freshly mixed CaSi-DCPD cement showed a uniform surface containing interspersed granules 3–10 μ wide and mainly displayed Ca, P, Si, Cl, and traces of S, Na, and K. After 28-day soaking in HBSS, a thick dense layer of globular CaP precipitates was present on the surface, and the reflex of the Si component almost disappeared. The intensity of P reflexes increased with soaking and consequently the Ca/P atomic ratio decreased from 2.36 to approx. 1.44 at 28 days
After 28-day soaking in HBSS, a thick dense layer of globular CaP precipitates formed on the surface and the Ca/P atomic ratio (see element tables) decreased from 2.36 to approx. 1.44 at 28 days owing to the decrease in intensity of the Si reflex together with the increase in P content.
Freshly mixed CaSi cement (Fig. 4) showed a finely granular surface with sparse granules 5–10 μ wide, and revealed mainly Ca, Si, K, S, Na, and traces of Al and Mg (see spectrum).
ESEM-EDX analyses of freshly mixed CaSi cement. ESEM displayed a finely granular surface with sparse granules 5–10 μ wide. EDX detected mainly Ca, Si, K, S, Na, and traces of Al and Mg. After 28 days in HBSS, EDX revealed a decrease in Si intensity and the appearance of P. ESEM showed a thin layer of fine/small globular CaP particles (spherulites ≤1 μ size) with a Ca/P atomic ratio of approx. 2.76
After ageing in HBSS, a decrease in intensity of the Si reflex together with an appearance of the P reflex was noted (see spectrum), globular CaP deposits (≤1 μ size) layered the surface, and the Ca/P atomic ratio (see element tables) was approx. 2.76.
Effect on cell proliferation
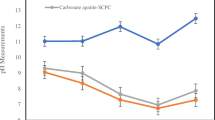
An increase in cell viability was observed in a time-dependent manner in all groups for the test periods (Fig. 5a). In particular, cell proliferation rates were similar in untreated cells and HPCs treated with CaSi and CaSi-DCDP extracts up to 3, 7, and 14 days with a significant increase of about 50 % compared to the data at 1 day. In contrast, CaSi-αTCP caused a lower (about 15 %) increase in cell proliferation after 3 and 7 days compared to untreated, CaSi, and CaSi-DCPD groups.
(a) Proliferation of HPCs exposed to cement extracts (alamarBlue assay at 1, 3, 7, 14 days). The number of viable cells was correlated with the magnitude of dye reduction and was expressed as percentage AB reduction. Results represented mean and SD calculated from at least three independent experiments in quadruplicate. *P < 0.05 indicates statistically significant differences between cements and the untreated control group. (b) ALP gene expression in HPC cells treated for 24 h with different cement extracts. The values were expressed as mRNA fold change and were normalized to β-actin expression (as the ratio between an initial value and a final value and analyzed using a comparative method). The data represent the mean and standard deviations of four independent experiments. Statistical analysis was performed by the nonparametric one-way ANOVA test; *P < 0.05 indicates statistically significant differences between cements and the untreated control group. (c) OCN gene expression at 24 h in HPC cells treated with different cement extracts. The values were expressed as mRNA fold change and were normalized to β-actin expression (as the ratio between an initial value and a final value and analyzed using a comparative method). The data represent the mean and standard deviations of four independent experiments. Statistical analysis was performed by the nonparametric one-way ANOVA test; *P < 0.05 indicates statistically significant differences between cements and untreated control group
Cement extracts showed a cell proliferation comparable to the untreated cell population after a time period of 14 days. Nevertheless, after 14 days, HDPCs stimulated with CaSi-αTCP displayed a lower viability than cells stimulated by different cement extracts.
Effect on gene expression
A fold increase of ALP and OCN messenger RNA (mRNA) in HPCs treated with CaSi, CaSi-αTCP, and CaSi-DCPD was observed (Fig. 5b, c). The relative level of gene expression was normalized against β-actin expression (messenger RNA signal), and the control was set as 1.0.
ALP and OCN genes were upregulated in HPCs treated with CaSi-αTCP cement: The ALP gene was upregulated threefold (Fig. 5b), and the OCN gene was upregulated more than tenfold compared to untreated cells and HPCs treated with different cement extracts (Fig. 5c).
Moreover, HPCs treated with CaSi and CaSi-DCPD cements caused no statistical changes in the regulation of ALP and OCN gene expression compared to untreated cells (Fig. 5b, c).
Discussion
Due to their chemical [30, 31] and biological [32–34] properties, calcium phosphate components have been introduced in the composition of experimental calcium silicate-calcium phosphate cements to obtain Ca and P releasing materials for direct pulp capping procedures able to provide positive epigenetic chemical signals to the cells involved in pulp-dentin reparative processes.
The ability to release calcium and hydroxide ions is a key factor for successful vital pulp capping therapy in regenerative endodontics because of the action of calcium on pulp cell differentiation and hard tissue mineralization [11, 12, 35–38].
Like conventional calcium hydroxide cements (pH 10–12) [39], calcium silicate (MTA) cements exert an initial caustic irritative effect on the surrounding tissues due to their basic pH. However, the biological outcome in direct pulp capping procedures is the formation of a reactionary/regenerative (tertiary) dentin layer (known as “bridging”) by the odontoblasts activated by the chemical insult and by the stimulating effect of the calcium ions released.
Calcium hydroxide suspensions (pH 12–13) not only possess a strong bactericidal effect but also cause necrosis, whereas calcium hydroxide cements and MTA do not cause necrosis in the remaining pulp tissue [39]. The alkalinity of the tissue is the basis for the desired tissue reaction and bridging effect [39]. Several studies demonstrated that both calcium hydroxide and calcium silicate-based materials share their properties to stimulate reparative hard tissues [2, 39–41]. The present study found no statistical differences between the experimental materials in terms of calcium release or alkalinizing activity at any time (Table 1).
It is well known that calcium ions are necessary for the differentiation and mineralization of pulp cells [35], and Ca ions specifically modulate osteopontin and bone morphogenetic protein-2 levels during pulp calcification [42]. In addition, the eluted Ca ions increase the proliferation of human dental pulp cells in a dose-dependent manner [12, 43], and Ca release enhances the activity of pyrophosphatase, which helps to maintain dentine mineralization and the formation of a dentine bridge. Moreover, hydroxide ions stimulate the release of ALP and BMP-2, which participate in the mineralization process [44].
Alpha-TCP was previously introduced in small amounts (5 wt%) in an experimental MTA-like formulation and showed an increased apatite-forming ability [16, 17] and the property to support the growth and differentiation of human orofacial mesenchymal stem cells [10].
A pilot study (Table 3) on ion releasing properties was performed using different calcium phosphate powders such as alpha-TCP and beta-TCP, hydroxyapatite (HA), DCPA, and DCPD to select two materials for the present study.
The formulation of the experimental cements used in the present study included large amounts of calcium phosphates, and the rapid formation of spherulites of calcium phosphate on the cement surface was markedly evident at ESEM-EDX examination (see Figs. 2 and 3).
Alpha-TCP and DCDP are biodegradable compounds that can act as phosphate sources due to their hydrolysis and progressive dissolution rate. DCPD is quite soluble in water due to the presence of structural water; in particular, DCPD is the most soluble calcium orthophosphate salt at pH >8.2 [45], and its solubility further increases at pH 12.7 or above [46]. Alpha-TCP is also reactive in aqueous systems given its high specific energy, and it can be hydrolyzed to a mixture of other calcium phosphates [18]. The solubility in water (expressed in g/L) at 25 °C is ≈ 0.088 for DCPD and ≈ 0.0025 for alpha-TCP [31], and at 37 °C expressed as − log(Kps) is 6.63 for DCPD and 25.5 for alpha-TCP. The CaSi component creates a strongly alkaline pH inside the slurry system. The solubility phase diagrams at alkaline pH show the higher solubility of DCPD than alpha-TCP in the range 8.5 < pH < 12 [23, 31], and difference is more marked for the leaching of P than Ca as at that pH range the release of P by alpha-TCP is very low [23].
In the present study, the experimental cements showed a less caustic effect than Ca(OH)2 [1] and alkalinizing activity similar to the conventional MTA cements [1, 5] but retained the ability to provide calcium ions and resulted in an improved ability to nucleate calcium phosphate deposits. For these reasons, the cements can be proposed for vital pulp therapy in regenerative endodontics. Unexpectedly, only small amounts of phosphate were released into the soaking solution. This finding could be correlated with a partial hydrolysis in other calcium phosphates and/or the incorporation of the released phosphorus ions in the cement structure. Gandolfi et al. [25] recently demonstrated that phosphate ions bind to the silanol groups of the calcium silicate hydrogel with the rapid formation of apatite inside the cement mass [16, 17], favored by the alkaline environment inside the cement.
After mixing the CaSi-CaP powder with water, the calcium phosphate component may undergo a (partial) dissolution and reprecipitation processes inside the hydrating cement mass leading to different reactions. (a) The calcium and phosphate ions released inside the calcium-silicate-hydrate (C-S-H) gel (CaO-SiO2-H2O system) can re-precipitate calcium phosphate, mainly apatite due to the high pH (i.e. high OH− availability) in the cement mass. CSH silanol groups (Si-OH) can bind to Ca2+ or PO4 3− and HPO4 2− ions and trigger the nucleation of apatite [25]. (b) A calcium-silicate-phosphate-hydrate C-S-P-H gel (CaO-SiO2-PO4/HPO4-H2O system) can form inside the calcium phosphate-containing CaSi-αTCP and CaSi-DCPD cements [16, 17, 25, 47] and consequently explain the reduced leaching of P ions from the cement. (c) Calcium phosphate in the cement formulation could also potentially react inside the hydrating cement mass by consuming calcium hydroxide with the formation of calcium silicate phosphate hydrate (CSPH) gel, with a decrease of the calcium hydroxide content and alkalinity of the cement. However, this study found no reduction of alkalinizing activity for either CaSi-αTCP or CaSi-DCPD, despite DCPD that can rapidly react with calcium hydroxide.
Apatites precipitate in fairly alkaline pHs, and the CaSi slurry generates an especially suitable environment for apatite nucleation. In the hydrated CaSi-DCPD system, the DCPD likely reacts with the Ca(OH)2 originated from the hydration of calcium silicates leading to the formation of calcium-deficient apatite, following the reaction 6CaHPO4 · 2H2O + 3Ca(OH)2 → Ca9(HPO4)(PO4)5 (OH) + 17 H2O in which the Ca/P ratio increases with the reaction time. The high alkalinity inside the cement slurry favors the reaction and raises the Ca/P ratio [46]. The hydrolytic conversion of αTCP to calcium-deficient apatite in the system CaSi-αTCP follows the reaction 3Ca(PO3) + H2O → 9Ca2+ + OH− + HPO4 2− + 5PO4 3− → Ca9(HPO4)(PO4)5 (OH) occurring in the 7.5 < pH < 9 [21].
In this study, the experimental CaSi-αTCP and CaSi-DCPD cements demonstrated higher porosity (both open and impervious porosity), solubility, and water sorption than ProRoot MTA that showed a water sorption of 14.73 %, a solubility of 10.89 %, and an apparent porosity of 29.36 % [1].
The solubility and porosity together with ion release and CaP nucleation concerning Ca(OH)2 and all the main commercial calcium silicate (MTA) cements have been published in a detailed study [1]. Such properties depend on factors such as the nature of the network structure and the mineral particles responsible for water sorption and solubility as well the permeability of the material to water diffusion (i.e. porosity). The pronounced ion release can be correlated with the apparent porosity: the large open pore volume and water sorption provided a broad wet biointeractive surface for the release of the calcium and hydroxyl ions involved in the formation of a calcium phosphate coating.
The mechanism of apatite nucleation on calcium silicate MTA cements in phosphate-containing solutions has been summarized by Gandolfi et al. in 11 steps [47]. In the present study, the Ca/P atomic ratio of the coating formed on the cement surfaces approached that of biological carbonated apatites where a considerable amount of carbonate ions (CO3 2−) are replaced by PO4 3− and/or OH− ions forming Ca-rich apatite type B (CO3 substituting for PO4) or type A (CO3 substituting for OH). It should be emphasized that the Ca/P atomic ratio obtained by the EDX area scan depends on factors such as the hydrolysis/dissolution and precipitation processes of the calcium phosphate component and also on the underlying CaSi-CaP cement. Actually, the Ca/P atomic ratios of freshly mixed cement pastes (4.68 for CaSi-alpha-TCP and 2.28 for CaSi-DCPD) were high due to the Ca contribution of the calcium silicate component (the Ca/Si ratio of commercial MTAs is 3–4) [1, 5].
The experimental CaSi-αTCP and CaSi-DCPD cements displayed longer setting times than ProRoot MTA that showed a final setting time of 170 min [7]. Several inorganic phosphate salts are used to delay the hydration and setting of calcium silicate (Portland) cements due to the adsorption of phosphate ions at the surface of the cement particles [48]. However, the low content (5–10 wt%) of some inorganic phosphate compounds may reduce the setting time [7] and enhance the structural integrity [49].
Many studies have demonstrated that calcium silicate-based materials share their stimulating properties on various cell lines involved in mineralization processes including mesenchymal stem cells [9, 10] dental pulp cells [11–14, 38, 42], cementoblasts [34, 50], and osteoblasts [51–54], and on the reparative events of hard tissues. The ability of these materials to release calcium and the elution of hydroxide ions is a key factor in mineralization.
Direct contact with MTA activates and differentiates human dental pulp cells [11], while MTA stimulates gene expression [13] and induces the proliferation of pulp cells in vitro [11, 12]. Both freshly mixed and premixed (24-h setting at 37 °C) MTA tested with undifferentiated pulp cells showed a significant decrease in cell number in the G1 phase (interval) and a slight but significant increase in the S phase (DNA replication) and G2 phase (growth and protein synthesis), demonstrating that MTA induces proliferation and not apoptosis [11]. Interestingly, pulp cell growth on 24-h set calcium silicate cements showed a significant increase in S and G2 phases and no increase in the bubG1 (cell death) phase [14]. Calcium silicate cement was a more effective promoter of cell proliferation than beta-TCP [15], as silicon-based materials possess the capacity to promote cell proliferation and differentiation with Si ions stimulating the entry of cells into S and G2 phases [55]. The Si/Ca molar ratio of a calcium silicate cement affects its in vitro cell behavior: A higher Si content enhances the expression of cell attachment, proliferation, and differentiation [56].
Successful clinical results were obtained in terms of inflammatory response and hard tissue bridge formation when MTA and Ca(OH)2-based cements were used for pulp capping in human teeth. However, pulp healing with calcium hydroxide was slower than that of MTA, and a lower response of calcium hydroxide was observed for dentin bridge formation compared with MTA [57].
Calcium phosphate cements appeared superior to pure calcium hydroxide as direct pulp-capping agents [58] because of their superior biocompatibility, transformation into apatite over time, and ability to induce bridge formation with no superficial tissue necrosis [59]. Tricalcium phosphate caused a strong inflammatory response and demonstrated the highest percentage of reparative dentin formation compared with calcium hydroxide in in vivo pulp capping [60]. A recent study demonstrated the biocompatibility and odontogenicity of a fast-setting αTCP cement developed for pulp capping [61]. Unfortunately, bacterial infection represents an important clinical complication when calcium phosphate ceramic is used as a pulp capping agent [62]. Conversely, calcium silicate (MTA) materials like Ca(OH)2 cements possess early (up to 15–30 days) antimicrobial/bacteriostatic action due to their alkalinizing activity (leaching of hydroxyl ions). Their high pH provides a stimulus for tooth repair [39, 44] and exerts an antimicrobial activity against common endodontic pathogens [63–65] probably due to protein denaturation and consequent damage to DNA and cytoplasmic membranes [66].
During the reparative process, cell proliferation is an important stage preceding the deposition of newly formed tissues. Our results indicate that all experimental cements tested in the present study modulated HPC cell proliferation during the experimental periods. In particular, cell growth in the presence of CaSi and CaSi-DPDC extracts was similar to that of the untreated group during the 14-day study. Conversely, within the first week, HPCs cultured in the presence of CaSi-αTCP extracts showed a lower proliferation than untreated cells, CaSi, and CaSi-DPDC groups. The reduced proliferation observed during the first 7 days in HPCs exposed to CaSi-αTCP might be the result of cell involvement in the commitment process. Calcium and silicon ions released from bioactive glasses have been shown to stimulate osteoblasts and HPC proliferation [51, 55, 67, 68]. Moreover, calcium silicate cements enhance the expression of mineralization proteins such as osteocalcin and bone sialoprotein [69–71].
ALP gene expression is an early marker of mineralizing cell differentiation. An increase in the specific activity of ALP in bone cells reflects a shift to a more differentiated state. Therefore, ALP plays a crucial role in the initiation of matrix mineralization, and ALP gene expression is downregulated after the start of mineralization [68, 72]. OCN is the most abundant non-collagenous bone-matrix protein characteristic and is a later marker of osteoblast and pulp cell differentiation [70, 71, 73, 74]. The property of calcium silicate cements to enhance the expression of ALP and OCN has been widely demonstrated [13, 14]. ALP and OCN gene expression in pulp cells began earlier during culture in the presence of CaSi-αTCP extract compared to the other groups.
The present study showed that the experimental cements tested released high amounts of calcium ions within the first 24 h that might represent a stimulatory signal for HPCs. qRT-PCR assay results on HPCs stimulated with CaSi and CaSi-DCPD did not show changes in mRNA fold increase for ALP genes and a slight upregulation of OCN gene expression. Conversely, HPCs treated with CaSi-αTCP showed a threefold upregulation of ALP mRNA and a tenfold increase in OCN mRNA levels significantly different from the other groups. These results suggest that the chemical composition of the tested cements may affect the regulation of the HPC phenotype through epigenetic DNA regulation and gene activation. Our data identify the CaSi-αTCP cement as an inducer of genes such as ALP and OCN, normally silent or poorly expressed in HPCs but characteristic markers of mineralization. This property may play a significant role in inducing pulp tissue to form new reparative dentine when exposed to CaSi-αTCP.
Conclusions
Experimental calcium silicate-calcium phosphate cements are biointeractive bioactive materials able to release/provide biologically relevant ions. These ions act as epigenetic chemical signals potentially involved in the activation and differentiation of mineralizing dentine-forming cells responsible for pulp healing and regeneration and reparative/reactionary dentinogenesis [75–78].
The high biointeractivity (release of Ca and OH ions) of these cements is correlated with their marked ability to nucleate calcium phosphates and pronounced open porosities and apparent porosity. CaSi-αTCP cement proved to be a potent inducer of ALP and OCN genes as characteristic markers of mineralization processes normally poorly expressed by HPCs.
Hydraulic calcium silicate-calcium phosphate cements appear to be attractive new materials for vital pulp therapy able to provide odontogenic/dentinogenic chemical signals for pulp regeneration and healing, and dentin formation in regenerative endodontics.
References
Gandolfi MG, Siboni F, Botero T, Bossù M, Riccitiello F, Prati C (2014) Calcium silicate and calcium hydroxide materials for pulp capping: biointeractivity, porosity, solubility and bioactivity of current formulations. J Appl Biomater Funct Mater, in press; doi: 10.5301/jabfm.5000201.
Parirokh M, Torabinejad M (2010) Mineral trioxide aggregate: a comprehensive literature review—part III: clinical applications, drawbacks, and mechanism of action. J Endod 36:400–413
Okiji T, Yoshiba K (2009) Reparative dentinogenesis induced by mineral trioxide aggregate: a review from the biological and physicochemical points of view. Int J Dent 2009:1–12
Gandolfi MG, Van Landuyt K, Taddei P, Modena E, Van Meerbeek B, Prati C (2010) ESEM-EDX and Raman techniques to study MTA calcium-silicate cements in wet conditions and in real-time. J Endod 36:851–857
Gandolfi MG, Taddei P, Modena E, Siboni F, Prati C (2013) Biointeractivity-related vs chemi/physisorption-related apatite precursor-forming ability of current root end filling materials. J Biomed Mater Res B Appl Biomater 101:1107–1123
Gandolfi MG, Prati C (2010) MTA and F-doped MTA cements used as sealers with warm gutta-percha. Long-term sealing ability study. Int Endod J 43:889–901
Gandolfi MG, Iacono F, Agee K, Siboni F, Tay F, Pashley DH, Prati C (2009) Setting time and expansion in different soaking media of experimental accelerated calcium-silicate cements and ProRoot MTA. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:e39–e45
Gandolfi MG (2012) A new method for evaluating the pulpward diffusion of Ca and OH ions through coronal dentin into the pulp. Iranian Endod J 7:189–197
D’Antò V, Di Caprio MP, Ametrano G, Simeone M, Rengo S, Spagnuolo G (2010) Effect of mineral trioxide aggregate on mesenchymal stem cells. J Endod 36:1839–1843
Gandolfi MG, Shah SN, Feng R, Prati C, Akintoye SO (2011) Biomimetic calcium-silicate cements support differentiation of human orofacial mesenchymal stem cells. J Endod 37:1102–1108
Moghadame-Jafari S, Mantellini MG, Botero TM, McDonald NJ, Nor JE (2005) Effect of ProRoot MTA on pulp cell apoptosis and proliferation in vitro. J Endod 31:387–391
Takita T, Hayashi M, Takeichi O, Ogiso B, Suzuki N, Otsuka K, Ito K (2006) Effect of mineral trioxide aggregate on proliferation of cultured human dental pulp cells. Int Endod J 39:415–422
Paranjpe A, Smoot T, Zhang H, Johnson JD (2011) Direct contact with Mineral Trioxide Aggregate activates and differentiates human dental pulp cells. J Endod 37:1691–1695
Chen CC, Shie MY, Ding SJ (2011) Human dental pulp cell responses to new calcium silicate-based endodontic materials. Int Endod J 44:836–842
Liu CH, Hung C Jr, Huang TH, Lin CC, Kao CT, Shie MY (2014) Odontogenic differentiation of human dental pulp cells by calcium silicate materials stimulating via FGFR/ERK signaling pathway. Mater Sci Eng C 43:359–366
Gandolfi MG, Taddei P, Tinti A, Dorigo De Stefano E, Prati C (2011) Alpha-TCP improves the apatite-formation ability of calcium-silicate hydraulic cement soaked in phosphate solutions. Mater Sci Eng C 31:1412–1422
Taddei P, Tinti A, Gandolfi MG, Rossi PL, Prati C (2009) Ageing of calcium silicate cements for endodontic use in simulated body fluids: a micro-Raman study. J Raman Spectrosc 40:1858–1866
Yubao L, Xingdong Z, de Groat K (1997) Hydrolysis and phase transition of alpha-tricalcium phosphate. Biomaterials 18:737–741
TenHuisen KS, Brown PW (1998) Formation of calcium-deficient hydroxyapatite from a-tricalcium phosphate. Biomaterials 19:2209–2217
Fernandez E, Ginebra MP, Boltong MG, Driessens FCM, Ginebra J, De Maeyer EAP, Verbeeck RMH, Planell JA (1996) Kinetic study of the setting reaction of a calcium phosphate bone cement. J Biomed Mater Res 32:367–374
Driessens FCM, Wolke JGC, Jansen JA (2012) A new theoretical approach to calcium phosphates, aqueous solutions and bone remodeling. J Austr Ceram Soc 48:144–149
Ginebra MP, Fernandez E, Driessens FCM, Planell JA (1999) Modeling of the hydrolysis of α-tricalcium phosphate. J Amer Ceram Soc 82:2808–2812
Ishikawa K (2008) Calcium phosphate cement. In Bioceramics and Their Clinical Application; Kokubo T. Ed.; CRC Press: New York, NY, USA; pp. 438-463.
Driessens FC, Planell JA, Boltong MG, Khairoun I, Ginebra MP (1998) Osteotransductive bone cements. Proc Inst Mech Eng H: J Eng Med 212:427–435
Gandolfi MG, Taddei P, Siboni F, Modena E, Ciapetti G, Prati C (2011) Development of the foremost light-curable calcium-silicate MTA cement as root-end in oral surgery. Chemical-physical properties, bioactivity and biological behaviour. Dent Mater 27:e134–e157
Spagnuolo G, D’Antò V, Valletta R, Strisciuglio C, Schmalz G, Schweikl H, Rengo S (2008) Effect of 2-hydroxyethyl methacrylate on human pulp cell survival pathways ERK and AKT. J Endod 34:684–688
Lee DH, Kim NR, Ahn SJ, Yang HC (2006) Effect of passage number on human dental pulp cell proliferation and differentiation. Biomater Res 10:74–77
Borra RC, Lotufo MA, Gagioti SM, Barros Fde M, Andrade PM (2009) A simple method to measure cell viability in proliferation and cytotoxicity assays. Braz Oral Res 23:255–262
Holland PM, Abramson RD, Watson R, Gelfand DH (1991) Detection of specific polymerase chain reaction product by utilizing the 5’-3’ exonuclease activity of Thermus aquaticus DNA polymerase. Proc Natl Acad Sci U S A 88:727–780
Chow LC (2010) Next generation calcium phosphate-based biomaterials. Dent Mater J 28:1–10
Fernández E, Gil FJ, Ginebra MP, Driessens FCM, Planell JA, Best SM (1999) Calcium phosphate bone cements for clinical applications. Part I: solution chemistry. J Mater Sci: Mater Med 10:169–176
Lee SK, Lee SK, Lee SI, Park JH, Jang JH, Kim HW, Kim EC (2010) Effect of calcium phosphate cements on growth and odontoblastic differentiation in human dental pulp cells. J Endod 36:1537–1542
Eyckmans J, Roberts SJ, Bolander J, Schrooten J (2013) Mapping calcium phosphate activated gene networks as a strategy for targeted osteoinduction of human progenitors. Biomaterials 34:4612–4621
Hakki SS, Bozkurt BS, Gandolfi MG, Prati C, Belli S (2013) The response of cementoblasts to calcium phosphate resin-based and calcium silicate-based commercial sealers. Int Endod J 46:242–252
Schröder U (1985) Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation, and differentiation. J Dent Res 64:541–548
Scarano A, Manzon L, Di Giorgio R, Orsini G, Tripoli D, Piatelli A (2003) Direct capping with four different materials in humans: histological analysis of odontoblast activity. J Endod 29:729–734
Lopez-Cazaux S, Bluteau G, Magne D, Lieubeau B, Guicheux J, Alliot-Licht B (2006) Culture medium modulates the behaviour of human dental pulp-derived cells: technical note. Europ Cell Mater 17:35–42
Mizuno M, Banzai Y (2008) Calcium ion release from calcium hydroxide stimulated fibronectin gene expression in dental pulp cells and the differentiation of dental pulp cells to mineralized tissue forming cells by fibronectin. Int Endod J 41:933–938
Schmalz G (2009) Calcium hydroxide cements. In: Schmalz G, Arenholt-Bindslev D (eds) Biocompatibility of dental materials, vol Chapter 6.5. Springer, Verlag Berlin, pp 166–176
Pitt Ford TR, Torabinejad M, Abedi HR, Bakland LK, Kariyawasam SP (1996) Using mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc 127:1491–1494
Mente J, Geletneky B, Ohle M, Koch MJ, Ding PGF, Wolff D, Dreyhaupt J, Martin N, Staehle HJ, Pfefferle T (2010) Mineral trioxide aggregate or calcium hydroxide direct pulp capping: an analysis of the clinical treatment outcome. J Endod 36:806–813
Rashid F, Shiba H, Mizuno N, Mouri Y, Fujita T, Shinohara H, Ogawa T, Kawaguchi H, Kurihara H (2003) The effect of extracellular calcium ion on gene expression of bone-related proteins inhuman pulp cells. J Endod 29:104–107
Clapham DE (1995) Calcium signaling. Cell 80:259–268
Okabe T, Sakamoto M, Takeuchi H, Matsushima K (2006) Effects of pH on mineralization ability of human dental pulp cells. J Endod 32:198–201
Takagi S, Chow LC, Ishikawa K (1998) Formation of hydroxyapatite in new calcium phosphate cements. Biomaterials 19:1593–1599
Chow LC, Eanes ED (2001) Solubility of calcium phosphates. In: Chow LC, Eanes ED (eds) Octacalcium phosphate. Monogr Oral Sci. Karger, Basel, pp 94–111
Gandolfi MG, Taddei P, Tinti A, Prati C (2010) Apatite-forming ability of ProRoot MTA. Int Endod J 43:917–929
Collepardi MM (1995) Water reducers/retarders, chapter 6 in Concrete admixtures handbook. Properties, science and technology. Ed. Ramachandran VS, pp 286-409
Ma W, Brown PW (1994) Effect of phosphate additions on the hydration of Portland cement. Adv Cem Res 6:1–12
Hakki SS, Bozkurt SB, Hakki EE, Belli S (2009) Effects of Mineral Trioxide Aggregate on cell survival, gene expression associated with mineralized tissues, and biomineralization of cementoblasts. J Endod 35:513–519
Sun J, Wei L, Liu X, Li J, Li B, Wang G, Meng F (2009) Influences of ionic dissolution products of dicalcium silicate coating on osteoblastic proliferation, differentiation and gene expression. Acta Biomater 5:1284–1293
Jung GY, Park YJ, Han JS (2010) Effects of HA released calcium ion on osteoblast differentiation. J Mater Sci Mat Med 21:1649–1654
Nakamura S, Matsumoto T, Sasaki J, Egusa H, Lee KY, Nakano T, Sohmura T, Nakahira A (2010) Effect of calcium ion concentrations on osteogenic differentiation and hematopoietic stem cell niche-related protein expression in osteoblasts. Tissue Eng Part A 16:2467–2473
Matsumoto S, Hayashi M, Suzuki Y, Suzuki N, Maeno M, Ogiso B (2013) Calcium ions released from mineral trioxide aggregate convert the differentiation pathway of C2C12 Cells into osteoblast lineage. J Endod 39:68–75
Shie MY, Ding SJ, Chang HC (2011) The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater 7:2604–2614
Shie MY, Chang HC, Ding SJ (2012) Effects of altering the Si/Ca molar ratio of a calcium silicate cement on in vitro cell attachment. Int Endod J 45:337–345
Accorinte MLR, Holland R, Reis A, Bortoluzzi MC, Murata SS, Dezan E, Souza V, Loguercio Dourado A (2008) Evaluation of Mineral Trioxide Aggregate and Calcium Hydroxide cement as pulp-capping agents in human teeth. J Endod 34:1–6
Chaung HM, Hong CH, Chiang CP, Lin SK, Kuo YS, Lan WH, Hsieh CC (1996) Comparison of calcium phosphate cement mixture and pure calcium hydroxide as derect pulp-capping. J Formos Med Assoc 95:545–550
Yoshimine Y, Maeda K (1995) Histologic evaluation of tetracalcium phosphate-based cement as a direct pulp-capping agent. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 79:351–358
Chohayeb AA, Adrian JC, Salamat K (1991) Pulpal response to tricalcium phosphate as a capping agent. Oral Surg, Oral Med, Oral Pathol Endod 71:343–345
Lee JB, Park SJ, Kim HH, Kwon YS, Lee KW, Min KS (2014) Physical properties and biological/odontogenicy effects of and experimentally developed fast-setting alpha-tricalcium phosphate-based pulp capping material. BMC Oral Health 14:87–97
Boone ME, Kafrawy AH (1979) Pulp reaction to a tricalcium phosphate ceramic capping agent. Oral Surg Oral Med Oral Pathol Endod 47:369–371
Estrela C, Bammann LL, Estrela CR, Silva RS, Pécora JD (2000) Antimicrobial and chemical study of MTA, Portland cement, calcium hydroxide paste, Sealapex and Dycal. Braz Dent J 11:3–9
McHugh CP, Zhang P, Michalek S, Eleazer PD (2004) pH required to kill Enterococcus faecalis in vitro. J Endod 30:218–219
Al-Hezaimi K, Al-Hamdan K, Naghshbandi J, Oglesby S, Simon JHS, Rotstein I (2005) Effect of white-colored Mineral Trioxide Aggregate in different concentrations on Candida albicans in vitro. J Endod 31:684–686
Siqueira JF Jr, Lopes HP (1999) Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J 32:361–369
Ding S-J, Shie M-Y, Wang C-Y (2009) Novel fast-setting calcium silicate bone cements with high bioactivity and enhanced osteogenesis in vitro. J Mater Chem 19:1183–1190
An S, Gao Y, Ling J, Wei X, Xiao Y (2012) Calcium ions promote osteogenic differentiation and mineralization of human dental pulp cells: implications for pulp capping materials. J Mater Sci: Mater Med 23:789–795
Wu BC, Huang SC, Ding SJ (2013) Comparative osteogenesis of radiopaque dicalcium silicate cement and white-colored mineral trioxide aggregate in a rabbit femur model. Materials 6:5675–5689
Min KS, Kim HI, Park HJ, Pi SH, Hong CU, Kim EC (2007) Human pulp cells response to Portland cement in vitro. J Endod 33:163–166
Shen Q, Sun J, Wu J, Liu C, Chen F (2010) An in vitro investigation of the mechanical-chemical and biological properties of calcium phosphate/calcium silicate/bismutite cement for dental pulp capping. J Biomed Mater Res B Appl Biomater 94:141–148
Eleniste PP, Huang S, Wayakanon K, Largura HW, Bruzzaniti A (2014) Osteoblast differentiation and migration are regulated by dynamin GTPase activity. Int J Biochem Cell Biol 46:9–18
Lim WH, Liu B, Cheng D, Hunter DJ, Zhong Z, Ramos DM, Williams BO, Sharpe PT, Bardet C, Mah SJ, Helms JA (2014) Wnt signaling regulates pulp volume and dentin thickness. J Bone Miner Res 29:892–901
Min KS, Lee S-I, Lee Y, Kim E-C (2009) Effect of radiopaque Portland cement on mineralization in human dental pulp cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:e82–e86
Schmalz G, Smith AJ (2014) Pulp development, repair, and regeneration: challenges of the transition from the traditional dentistry to biologically based therapies. J Endod 40:S2–S5
Cooper PR, Holder MJ, Smith AJ (2014) Inflammation and regeneration in the dentin-pulp complex: a double-edged sword. J Endod 40:S46–S51
Simon SRJ, Tomson PL, Berdal A (2014) Regenerative endodontics: regeneration or repair? J Endod 40:S70–S75
Prati C, Gandolfi MG (2015) Calcium silicate bioactive cements: biological perspectives and clinical applications. Dent Mater 31:351–370
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gandolfi, M.G., Spagnuolo, G., Siboni, F. et al. Calcium silicate/calcium phosphate biphasic cements for vital pulp therapy: chemical-physical properties and human pulp cells response. Clin Oral Invest 19, 2075–2089 (2015). https://doi.org/10.1007/s00784-015-1443-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-015-1443-2