Abstract
Objectives
The aim of this study was to evaluate the physical properties and cytotoxicity of a novel root-end filling material (EPC) which is made from epoxy resin and Portland cement as a mineral trioxide aggregate (MTA) substitute.
Materials and methods
EPC, developed as a root-end filling material, was compared with MTA and a mixture of AH Plus sealer and MTA (AMTA) with regard to the setting time, radio-opacity, and microleakage. Setting times were evaluated using Vicat apparatus. Digital radiographs were taken to evaluate the aluminium equivalent radio-opacity using an aluminium step wedge. Extracted single-rooted teeth were used for leakage test using methylene blue dye. After canal shaping and obturation, the apical 3-mm root was resected, and a root-end cavity with a depth of 3 mm was prepared. The root-end cavities were filled with MTA, AMTA, and EPC for 15 specimens in each of three groups. After setting in humid conditions for 24 h, the specimens were tested for apical leakage. For evaluation of the biocompatibility of EPC, cell (human gingival fibroblast) viability was compared for MTA and Portland cement by MTT assay, and cell morphological changes were compared for MTA and AH Plus by fluorescence microscopy using DAPI and F-actin staining. The setting time, radio-opacity, and microleakage were compared using one-way ANOVA and Scheffe’s post hoc comparison, and the cytotoxicity was compared using the nonparametric Kruskal–Wallis rank sum test. Statistical significance was set at 95 %.
Results
EPC had a shorter setting time and less microleakage compared with MTA (p < 0.05). EPC showed 5-mm aluminium thickness radio-opacity and similar biocompatibility to MTA.
Conclusions
Under the conditions of this study, EPC, a novel composite made from a mixture of epoxy resin and Portland cement, was found to be a useful material for root-end filling, with favourable radio-opacity, short setting time, low microleakage, and clinically acceptable low cytotoxicity.
Clinical relevance
The novel root-end filling material would be a potentially useful material for a surgical endodontic procedure with favourable properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mineral trioxide aggregate (MTA), which was developed in the 1990s as a root-end filling material, has increased in the diversity of its clinical applications [1]. The sealing ability and biocompatibility of MTA have been demonstrated previously in numerous studies [1–3], and these factors have made it possible to expand its application into other fields, including pulpotomy, apexification, apexogenesis, and revascularization [2–7]. However, certain shortcomings, such as long setting time and poor handling characteristics, still encourage the development of new MTA substitutes [8–10].
A considerable variation in the setting time of MTA (ProRoot MTA; Dentsply Tulsa Dental, Johnson City, TN, USA) has been reported in the literature. Although the manufacturer claims that its setting time is 4–6 h, it has been found to range from 140 min [8] to 3 h [11], and even to 72 h [12]. A long setting time requires additional visits to dental clinics for certain clinical procedures, such as apexification and pulpotomy, and particularly for apical surgery, in which any unset material may be washed out by the tissue fluid and blood in the surgical field and consequently may lead to microleakage and treatment failure. To overcome these shortcomings, extensive research has been carried out with the aim of enhancing the setting procedure, including the development of application instruments and a search for MTA substitutes [13–15].
Researchers have attempted to reduce the setting time using certain additives, thereby reducing washout and enhancing manipulation characteristics while maintaining the physical characteristics of the original MTA [13, 14, 16]. Recent research has investigated the physical and biological properties of Portland cement as an MTA substitute because it has a similar composition to MTA, and favourable results have been reported [17, 18]. Epoxy resin materials are commonly used in root canal treatment as a root canal sealer during obturation, and these materials show favourable properties in relation to leakage prevention and biological responses [19]. Among these materials, AH Plus sealer (Dentsply DeTrey GmbH, Konstanz, Germany) is well known and has reasonable biocompatibility and good sealing ability [19, 20].
We predicted that a mixture of AH Plus and MTA (AMTA) may enhance the handling characteristics of MTA and, based on this assumption, we have compared AMTA with a newly developed root-end filling material (EPC), which is composed mainly of epoxy resin and Portland cement (the main ingredients of AH plus and MTA, respectively). Thus, the aim of this study was to evaluate the setting time, radio-opacity, microleakage, and cytotoxicity of the EPC as a novel root-end filling material.
Materials and methods
EPC was made from epoxy resin (Dong Yang Epoxy Co., Bucheon, Korea), type II Portland cement (SsangYong Cement Co., Seoul, Korea), and barium sulphate (Junsei Chemical Co., Japan), with a 1:1:1 wt% mixing ratio. AH Plus sealer was mixed with MTA powder in a 1:1 wt% ratio for the AMTA specimen. MTA was mixed using hand instruments, according to the manufacturer’s instructions with a mixing ratio of 3:1 (powder/liquid).
Setting time
The setting times of EPC, MTA, and AMTA were measured with five specimens for each group using Vicat apparatus (DMJ system, Busan Korea). A Vicat needle of 1-mm diameter and 300-g capacity was loaded on the mixed material every 5 min. The initial setting time was defined as the time at which the needle could not penetrate the mixed material to a 5-mm depth. The final setting time was indicated when there was no indentation mark on the material surface [21]. The mixed material was stored in a sealed box to prevent the effects of humidity.
Radio-opacity
Using washer rings of 1-mm thickness and 8-mm inner diameter, specimens (n = 10) were prepared to determine the aluminium equivalent radio-opacity using an aluminium step wedge (1- to 10-mm thickness) made of pure aluminium. The specimens were radiographed using a digital x-ray sensor (Schick Technologies Inc., Long Island City, NY, USA) with an exposure condition of 60 kV, 2 mA, and 0.08 s from a 10-cm distance. The digital radiograph images were evaluated for aluminium wedge equivalent thickness (millimeters aluminium) using Adobe Photoshop 7.0 (Adobe Systems Incorporated, San Jose, CA, USA).
Microleakage
Forty-nine extracted single-root teeth were used for the leakage test. Clinical crowns were removed using a low-speed diamond disc (Horico Diamond Disk 350; Pfingst & Company, Inc., South Plainfield, NJ, USA). Working length was measured, and the root canals were instrumented using ProTaper Universal and ProFile NiTi file systems (Dentsply Maillefer, Ballaigues, Switzerland) with the #35/0.06 sizes. All canals were filled with gutta-percha, and access cavities were filled with glass ionomer cement (Ketac-Fil; 3M ESPE, St. Paul, MN, USA). Then, the filled roots were sectioned at the level of 3 mm from the apical foramen. Entire root surfaces were coated with multi-layer nail varnish for complete sealing. The root-end cavity was prepared using a cylindrical diamond bur (SF41; MANI, Tochigi, Japan), 3 mm in length and 1 mm in diameter. Two negative control specimens did not have a root-end cavity. For the leakage comparison, root specimens were randomly divided into three groups. Each group had 15 specimens. The root-end cavity was filled with one of MTA, AMTA, or EPC. The EPC and AMTA groups were filled by injecting using the Centrix syringe (C-REZ syringe and tip; Centrix Dental Corporation, Shelton, CT, USA) and finished with an alcohol ball. The MTA group was filled using hand instruments and finished with wet cotton. Two positive control specimens had no root-end filling.
Root-end-filled specimens were stored for 24 h to set the filled materials in humid conditions. Then, they were immersed in 1 % methylene blue dye solution for 72 h to compare the apical leakage. After irrigation and drying, the specimens were sectioned vertically across the root-end filling material using a low-speed diamond disc to evaluate the leakage along the axial wall of the root-end cavity. The leakage was scored using digital photographs as follows: score 0—no dye leakage on the axial wall, score 1—dye leakage on up to one third of the axial wall, score 2—dye leakage on up to two thirds of the axial wall, score 3—dye leakage along the whole length of the axial wall, and score 4—dye leakage on the coronal end of the filled material.
Cytotoxicity
For evaluation of the cytotoxicity of EPC, cell viability was compared for MTA and Portland cement using an MTT assay. Specimens were prepared with a weight of 0.2 g and with the same mixing ratio as described above, and separate specimens were permitted to set for 2, 4, 8, and 24 h in each group. The specimens were disinfected using 70 % alcohol.
Serum extracts were obtained from specimens in each setting time group in 1 mL of cell growth media, and the medium was changed every 24 h, and thus, three time-dependent (0–24, 24–48, and 48–72 h) extracted media samples were obtained. The extracts were diluted four times and added to human gingival fibroblast (HGF) culture. Using 100 μL of diluted extract medium, 1 × 104 HGF cells were cultured in a 96-well plate for 72 h in an incubator (Water-Jacketed CO2 Incubator; Thermo Fisher Scientific Inc., Waltham, MA, USA) at 37 °C with 5 % CO2. Negative control groups had no sample extracts. After a 72-h incubation, the storage medium was replaced with MTT solution, and the plates were incubated at 37 °C for an additional 4 h. The resulting formazan crystals were dissolved by removing the culture medium and adding 100 μL of MTT solvent (dimethyl sulfoxide). Cell viability was evaluated by spectrophotometry as being proportional to the absorbance measured at 570 nm using an ELISA microplate reader (Sunrise™ reader; TECAN, Männedorf, Switzerland).
Cell morphologic evaluation
For evaluation of cell morphological change, additional samples (EPC, MTA, and AH Plus) were mixed as described previously and according to the manufacturer’s instructions. Each sample was hardened for 4 h. The material samples were secreted for 24 h in the medium. The extract medium was diluted four times by mixing with fresh medium. HGF cells (cell suspension of 5 × 104 cells) were grown in 8-well chamber slides (Lab-Tek™; Lab-Tek International, Christchurch, New Zealand) for 24 h. The sample was washed briefly with phosphate-buffered saline (PBS), fixed with paraformaldehyde, and permeabilized with PBS containing 0.05 % Triton X-100 (Sigma-Aldrich, Saint Louis, MO, USA). Cells were blocked for 30 min using an image iT FX signal enhancer (Invitrogen I36933). Cells were then probed with rhodamine-phalloidin (1:100, Invitrogen R415) for 45 min to reveal the distribution of F-actin filaments. DAPI (Invitrogen P36931) staining solution was then added to visualize the nuclei. The samples were incubated for 5 min and rinsed several times in PBS. Excess buffer was drained from the coverslip, and the samples were mounted. The samples were viewed using a fluorescence microscope (Carl Zeiss Meditec Inc., Dublin, CA, USA) with appropriate filters. Rhodamine-phalloidin binds F-actin, revealing the distribution of actin filaments. DAPI stains DNA, thereby delineating the nuclei.
The setting time, radio-opacity, and microleakage results were compared statistically using one-way ANOVA and Scheffe’s post hoc comparison, and the cytotoxicity was compared using the nonparametric Kruskal–Wallis rank sum test using PASW Statistics 18 (SPSS Inc., Chicago, IL, USA). Statistical significance was set at p = 0.05.
Results
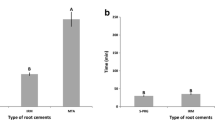
EPC had the shortest setting time among the experimental groups (p < 0.05). MTA had a final setting time of approximately 10 h, and EPC had a mean setting time of 83 min. EPC and AMTA had lower leakage scores than MTA (p < 0.05, Table 1). AMTA showed the highest radio-opacity, 9.7 mm Al, whereas EPC and MTA showed 5.2 and 5.7 mm Al, respectively (p < 0.05).
Cell viabilities from MTT assays using 24-h extract media from 2-, 4-, 8-, and 24-h setting materials are presented in Fig. 1. EPC showed similar cell viabilities to the MTA and Portland cement samples with different setting times. When HGFs were grown on media containing the extracts of EPC and MTA set for 4 h, no recognizable differences in cell morphology from those of the negative controls were observed by fluorescence microscopy. With DAPI staining, cells treated with EPC, MTA, and AH Plus had similar morphological appearance to the negative control. Rhodamine-phalloidin stained F-actin stress fibres very intensely. The actin fibres were organized in linear bundles throughout the EPC-treated cells, to the same extent as those treated with MTA and AH Plus, as well as the untreated controls (Fig. 2).
Discussion
It has been demonstrated by numerous studies that MTA has favourable sealing ability and biocompatibility, and proper radio-opacity as a root-end filling material [1, 8, 11]. However, MTA also has shortcomings, in particular a long setting time, poor handling characteristics, and high cost [8, 13]. The first of these, long setting time, may lead to a clinical problem with washout during setting [9] because, for example, the root-end filling material may be exposed to tissue fluid and blood for a considerable time; consequently, many attempts have been made to overcome this drawback. The second of these factors, handling characteristics, is important because apical surgery has a limited visual field and instrumental accessibility, and thus, the material characteristics of manipulation are very important for clinical applications. MTA has a granular consistency and has a short effective working time, which can lead to handling difficulties [13, 15, 16]. Although specially designed devices and instruments have been introduced to overcome these poor handling characteristics, it is still difficult to place the material easily into an apical root cavity.
To overcome these shortcomings, numerous attempts have been made to improve handling and setting time by the addition of certain setting catalysts [14, 16]. The addition of 1 % methylcellulose as an anti-washout ingredient has given MTA similar handling properties to zinc oxide eugenol cement [13]. The addition of NaOCl gel, K-Y Jelly, and 5 % CaCl2 to MTA has effectively reduced the setting time and improved manipulation [22]. Mixing MTA with calcium lactate gluconate solution instead of water has also been reported as a method for reducing setting time and to efficiently improve manipulation [23].
In the present study, we tested the possibility of mixing MTA with AH Plus as a product in itself and also developed a composite material made of epoxy resin and Portland cement as a novel root-end filling material. Barium sulphate was added to generate practical radio-opacity for postoperative evaluation.
Portland cement has been studied extensively as a replacement for MTA because of its relatively lower cost, similar chemical composition, and, in particular, the fact that it is similar in biocompatibility to MTA [9, 10, 17, 18, 24]. It has been reported that Portland cement and MTA have similar physical and mechanical properties [9]. Cytological and histological responses from both materials, following implantation into the bone of an experimental animal, showed similar results without significant differences [17].
Root canal sealer is a representative dental material made of epoxy resin. Among the various types available, AH Plus has been reported to have the lowest genotoxicity and cytotoxicity [19, 20]. It has been reported that AH Plus causes limited tissue response or inflammation and has the lowest cytotoxicity when compared with recently introduced sealers made from methacrylate-based resin, such as EndoREZ or Epiphany [20, 25]. Thus, in this study, AH Plus was added to MTA to generate another root-end filling material with improved handling properties and biocompatibility compared with the original MTA. On the basis of this idea, EPC was also developed by mixing epoxy resin and Portland cement, which are the main ingredients of AH Plus and MTA, respectively.
EPC and AMTA were shown to have favourable handling properties, which enable clinicians to inject the material into root-end cavities using a syringe. Then, the root-end filling could be finished with the use of alcohol sponges because EPC and AMTA had appropriate consistency and plasticity. In the present study, not only the handling characteristics but also the leakage prevention was better than those of MTA. This might result from the adhesive sealing effect of epoxy resin, which was used as a base material for EPC [26, 27]. The results of advanced leakage prevention were similar to the results which were obtained by testing mixed MTA with methacrylate resin [28]. However, noticeably, it was reported that a dye leakage test method is no longer regarded as a reliable method to assess the sealing ability of dental material due to the decoloration effect [29]. Thus, in this study, we used the methylene blue dye method with the positive and negative controls that shows constant dye staining as all or none for minimizing the methodological shortcomings. But it is highly recommended to compare again the microleakage by use of protein or bacterial leakage test in the future. In the present study, the final setting time of MTA, measured by a Vicat device, was approximately 10 h, which is longer than previously reported (164 min) [8, 11]. It is assumed that the previous results refer the initial setting time. We suggest that it is more appropriate to compare the final setting time because the hydraulic cement could be washed out by humidity or tissue fluid before final setting, even after initial setting has occurred. Furthermore, the mixing ratio, manipulation method, working time, and condensation pressure might have an effect on setting time [12, 21, 30]. In this study, EPC showed the shortest setting time while MTA had the longest. MTA is usually reported to have a longer setting time than Portland cement, resulting from the comparatively lower levels of sulphur and tricalcium aluminate in MTA [31]. The longer setting time of AMTA compared with EPC may result from the setting time of AH Plus, which the manufacturer claims to be 8 h. The setting time of AMTA was approximately 1 h shorter than that of AH Plus, which may be due to the MTA powder mixed in. EPC had a relatively shorter setting time than the other materials, and would thus be of clinical benefit in overcoming the shortcomings of MTA. The short setting time may be the result of the epoxy resin, and could be further shortened by the addition of Portland cement (as powder) and barium sulphate.
The ISO Standard 6876:2001 [32] establishes 3 mm Al as the minimum radiopacity for root sealing materials. The radio-opacity of EPC (5.2 mm Al), as determined by using aluminium equivalent thickness, was similar to that of MTA and was found to be suitable for clinical use [33]. AMTA showed the highest radio-opacity, due to the presence of AH Plus, which has a high radio-opacity of approximately 13.6 mmAl. In this study, EPC contained barium sulphate with approximately 33 % weight ratio, and it is therefore necessary to carry out further studies with different mixing ratios or alternative ingredients, such as bismuth oxide [24, 34].
MTA and Portland cement require water for the hydration reaction [35]. However, in EPC, the expected by-product of calcium hydroxide during the setting of MTA and Portland cement, which may have a biological advantage as a result of high alkaline content, might be limited or could not be produced in the absence of water. Meanwhile, in the cytotoxicity test, EPC showed a favourable result in relation to cell viability, comparable with MTA or Portland cement. Generally, cell viability of over 80 % compared with the control group can be regarded as favourable [36]. In this study, all of the media extracts had over 100 % cell viability, regardless of the setting time of the specimens.
Methodologically, different groups were designed for cytological tests from other mechanical tests. Basically, we tried to compare EPC and MTA (two groups) without AMTA because EPC is a potential root-end filling material for MTA substitutes. Here, we added a Portland cement group as a positive control which is highly related to the MTA (80 % of MTA is Portland cement) and also the ingredient of the EPC.
Cellular morphologic destructions can be detected by fluorescence microscopy using stains, such as DAPI, that bind to nucleic acids. In the present study, cells exposed to EPC and MTA showed very similar morphologies, following DAPI staining, and the cells were as intact as in the negative control group (Fig. 2). In contrast, some of the nuclei of the AH Plus extract-treated HGFs did not display the typical morphology, but rather a strikingly creased or partially condensed nuclear morphology was observed.
We also employed rhodamine-conjugated phalloidin to visualize F-actin filaments. Changes in actin fibres are readily observed by fluorescence staining, indicating that intracellular changes in F-actin fibres occur before any gross morphological changes become evident [37]. Both EPC- and MTA-treated HGF cells exhibited original straight actin cables extending from the perinuclear region to the cell periphery, similar to those in untreated cells. These favourable results regarding cell viability and morphological changes suggest that the novel cement EPC has low cytotoxicity and favourable biocompatibility. However, further biocompatibility tests using periodontal ligament cells instead of HGF cells would be valuable because it has been reported that periodontal ligament cells can grow on MTA [38].
On the basis of the physical and biological results, the novel root-end filling material EPC would be useful in clinical application. Further research with in vivo tests is strongly recommended before clinical usage.
Conclusions
Under the limited conditions in this study, the novel root-end filling material EPC was shown to have the favourable characteristics of short setting time, proper radio-opacity, and clinically acceptable cytotoxicity. On the basis of the physical and biological results, EPC would be useful in clinical application.
References
Camilleri J, Pitt Ford TR (2006) Mineral trioxide aggregate: a review of the constituents and biological properties of the material. Int Endod J 39:747–754
Ford TR, Torabinejad M, Abedi HR, Bakland LK, Kariyawasam SP (1996) Using mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc 127:1491–1494
Salako N, Joseph B, Ritwik P, Salonen J, John P, Junaid TA (2003) Comparison of bioactive glass, mineral trioxide aggregate, ferric sulfate, and formocresol as pulpotomy agents in rat molar. Dent Traumatol 19:314–320
Bakland LK (2000) Management of traumatically injured pulps in immature teeth using MTA. J Calif Dent Assoc 28:855–858
Witherspoon DE, Ham K (2001) One-visit apexification: technique for inducing root-end barrier formation in apical closures. Pract Proced Aesthet Dent 13:455–460
Banchs F, Trope M (2004) Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod 30:196–200
Holden DT, Schwartz SA, Kirkpatrick TC, Schindler WG (2008) Clinical outcomes of artificial root-end barriers with mineral trioxide aggregate in teeth with immature apices. J Endod 34:812–817
Chng HK, Islam I, Yap AU, Tong YW, Koh ET (2005) Properties of a new root-end filling material. J Endod 31:665–668
Islam I, Chng HK, Yap AU (2006) Comparison of the physical and mechanical properties of MTA and portland cement. J Endod 32:193–197
Camilleri J (2008) The physical properties of accelerated Portland cement for endodontic use. Int Endod J 41:151–157
Torabinejad M, Hong CU, McDonald F, Pitt Ford TR (1995) Physical and chemical properties of a new root-filling material. J Endod 21:349–353
Sluyk SR, Moon PC, Hartwell GR (1998) Evaluation of setting properties and retention characteristics of mineral trioxide aggregate when used as a furcation perforation repair material. J Endod 24:768–771
Ber BS, Hatton JF, Stewart GP (2007) Chemical modification of ProRoot MTA to improve handling characteristics and decrease setting time. J Endod 33:1231–1234
Wiltbank KB, Schwartz SA, Schindler WG (2007) Effect of selected accelerants on the physical properties of mineral trioxide aggregate and portland cement. J Endod 33:1235–1238
Asgary S, Shahabi S, Jafarzadeh T, Amini S, Kheirieh S (2008) The properties of a new endodontic material. J Endod 34:990–993
Huang TH, Shie MY, Kao CT, Ding SJ (2008) The effect of setting accelerator on properties of mineral trioxide aggregate. J Endod 34:590–593
Saidon J, He J, Zhu Q, Safavi K, Spångberg LS (2003) Cell and tissue reactions to mineral trioxide aggregate and Portland cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 95:483–489
Ribeiro DA, Duarte MA, Matsumoto MA, Marques ME, Salvadori DM (2005) Biocompatibility in vitro tests of mineral trioxide aggregate and regular and white Portland cements. J Endod 31:605–607
Leyhausen G, Heil J, Reifferscheid G, Waldmann P, Geurtsen W (1999) Genotoxicity and cytotoxicity of the epoxy resin based root canal sealer AH plus. J Endod 25:109–113
Scarparo RK, Grecca FS, Fachin EV (2009) Analysis of tissue reactions to methacrylate resin-based, epoxy resin-based, and zinc oxide-eugenol endodontic sealers. J Endod 35:229–232
American Society for Testing and Materials (2004) Standard test method for time of setting of hydraulic cement by Vicat needle. ASTM C191-04. American Society for Testing and Materials, West Conshohocken
Kogan P, He J, Glickman GN, Watanabe I (2006) The effects of various additives on setting properties of MTA. J Endod 32:569–572
Hsieh SC, Teng NC, Lin YC et al (2009) A novel accelerator for improving the handling properties of dental filling materials. J Endod 35:1292–1295
Coomaraswamy KS, Lumley PJ, Hofmann MP (2007) Effect of bismuth oxide radioopacifier content on the material properties of an endodontic Portland cement-based (MTA-like) system. J Endod 33:295–298
Al-Hiyasat AS, Tayyar M, Darmani H (2010) Cytotoxicity evaluation of various resin based root canal sealers. Int Endod J 43:148–153
Oquntebi BR, Shen C (1992) Effect of different sealers on thermoplasticized Gutta-percha root canal obturations. J Endod 18:363–366
De Almeida WA, Leonardo MR, Tanomaru Filho M, Silva LA (2000) Evaluation of apical sealing of three endodontic sealers. Int Endod J 33:25–27
Kim JC, Kim MR, Ko HJ, Won KY (2009) Apical microleakage of MTA with 4-META/MMA & TBB resin as a root-end filling material. J Kor Acad Cons Dent 34:371–376
Wu MK, Kontakiotis EG, Wesselink PR (1998) Decoloration of 1 % methylene blue solution in contact with dental filling materials. J Dent 26:585–589
Fridland M, Rosado R (2003) Mineral trioxide aggregate (MTA) solubility and porosity with different water-to-powder ratios. J Endod 29:814–817
Dammaschke T, Gerth HU, Züchner H, Schäfer E (2005) Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent Mater 21:731–738
International Organization for Standardization. ISO 6876–2001: Dental root sealing materials
Shah PMM, Chong BS, Sidhu SK, Ford TR (1996) Radiopacity of potential root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 81:476–479
Saliba E, Abbassi-Ghadi S, Vowles R, Camilleri J, Hooper S, Camilleri J (2009) Evaluation of the strength and radiopacity of Portland cement with varying additions of bismuth oxide. Int Endod J 42:322–328
Camilleri J (2007) Hydration mechanism of mineral trioxide aggregate. Int Endod J 40:462–470
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Van Cruchten S, Van Den Broeck W (2002) Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol 31:214–223
Samara A, Sarri Y, Stravopodis D, Tzanetakis GN, Kontakiotis EG, Anastasiadou E (2011) A comparative study of the effects of three root-end filling materials on proliferation and adherence of human periodontal ment fibroblasts. J Endod 37:865–870
Acknowledgments
This work was supported by clinical research grant from Pusan National University Hospital (2011).
Conflict of interest
The authors deny any conflicts of interest related to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, SJ., Chung, J., Na, HS. et al. Characteristics of novel root-end filling material using epoxy resin and Portland cement. Clin Oral Invest 17, 1009–1015 (2013). https://doi.org/10.1007/s00784-012-0782-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-012-0782-5