Abstract
The present study focuses on the correlation between the expression pattern of β-catenin (component of Wnt signaling), ΔNp63 (proliferation marker), and Notch 1 (transmembrane receptor) in oral squamous cell carcinoma. The study also aims to investigate the interaction between β-catenin and ΔNp63 in oral cancer. Furthermore, we also analyzed the prognostic significance of β-catenin, ΔNp63, and Notch 1 in oral squamous cell carcinoma. Immunohistochemical analysis of β-catenin, ΔNp63, and Notch 1 were done in 62 cases of oral squamous cell carcinoma. Co-immunoprecipitation analysis was done to study the possible interaction between β-catenin and ΔNp63 in oral cancer. Kaplan–Meier method was used to estimate overall and disease-free survival, and the Log-rank test was used to compare the resulting curves. Statistically significant positive correlation was found between the localization of β-catenin and the expression of ΔNp63 (p = 0.001**, r s = 0.427), whereas, no significant association was found between the expression pattern of β-catenin and Notch 1. Interestingly, interaction between β-catenin and ΔNp63 was observed in oral carcinoma. Moreover, β-catenin and ΔNp63 may be related to worst survival in oral carcinoma. Statistically significant positive association between localization of β-catenin and expression of ΔNp63 suggests that they might have dependent roles in maintaining the proliferation of oral carcinoma cells. In addition, the downregulated expression of Notch 1 was related to invasion and differentiation status of oral carcinoma cells. Furthermore, β-catenin and ΔNp63 may be used as independent prognostic markers of oral carcinoma. On the other hand, interaction of β-catenin with ΔNp63 may be a key event in maintaining the proliferation of oral carcinoma cells. The present study indicates that β-catenin and ΔNp63 may be used as independent prognostic markers of oral carcinoma and the interaction of β-catenin with ΔNp63 may be a crucial event in regulating proliferation and differentiation of oral carcinoma cells, which may be used as a target for therapeutic implications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral cancer stands sixth in being the most common cancer worldwide [1]. Studying the molecular pathways in oral cancer facilitates the understanding of possible underlying mechanisms involved in oral squamous cell carcinoma. Studies on the combinatorial expression of proliferation and differentiation specific signaling molecules and the plausible correlation between them could provide more details into the complementing networks involved in oral carcinogenesis. Wnt and Notch are the two major pathways which regulate the proliferation and differentiation of keratinocytes. Recently, it was found that the expression of keratinocyte stem cell marker p63 activates β-catenin signaling in head and neck squamous cell carcinoma cell lines. In addition, cross-regulation between Notch and p63 was observed during the differentiation of keratinocyte. However, translating these in vitro results for therapy might not be possible since the correlation between these proteins in human oral squamous cell carcinoma tissues has not been studied yet. Based on this background, we have investigated the correlation between the expression pattern of β-catenin, ΔNp63, and Notch 1 in human oral squamous cell carcinoma which could reveal their possible relationship in oral cancer.
The transmembrane protein β-catenin is also a component of Wnt signaling which is involved in regulating cell proliferation by activating certain set of genes including c-Myc [2, 3]. In inactive state, β-catenin was phosphorylated by glycogen synthase kinase 3β (GSK3β) and undergoes degradation. Activation of Wnt signaling inhibits phosphorylation and subsequent degradation of β-catenin which leads to its cytoplasmic accumulation and translocation into the nucleus. The translocated β-catenin activates genes which are involved in inducing and maintaining proliferation of cells. Interestingly, deregulated β-catenin signaling was observed in several tumors [4]. The specific localization of β-catenin may influence its oncogenic activity, such as the nuclear localization of β-catenin is related to malignant transformation [5]. Previous study from our lab shows that the interaction of MUC1 (mucin-type glycoprotein) with β-catenin modulates Cyclin D1 expression in gastric cancer [6]. Recently, it was found that Wnt/β-catenin signaling is involved in the progression of oral dysplasia and also maintains the proliferative potential of cancer stem cells [2, 7].
p63, a member of the p53 family, contains several variant isoforms including TA isoforms (contain transactivation domain) and ΔN isoforms (lack transactivation domain). Since the expression of ΔNp63 was higher than TAp63 in several tumors including oral carcinoma and also it maintains the stem cell phenotype of squamous epithelial cells, we have selected ΔNp63 for this study [8–11]. Interestingly, altered expression of ΔNp63 was also found in oral epithelial dysplasia and it may play a role in the initiation of oral carcinogenesis [12, 13].
Notch is a single pass transmembrane protein which acts both as a receptor and as a transcription factor. After binding with its ligand, Notch undergoes successive cleavages initially by ADAM-type proteases and then by presenilin-dependent proteases. This results in the release of Notch intracellular domain which then translocates to the nucleus and activates the expression of target gene [14]. Notch signaling controls the fate of undifferentiated, proliferative cells, and the role of Notch signaling depends upon the cell-type. It was found that Notch induces abnormal proliferation in breast cancer, T-cell leukemia, and prostate cancers, whereas in skin cancer it acts as a tumor suppressor [15]. Even though different Notch receptors are present, we have selected Notch1 since it was found to influence p63 expression [16].
Cytokeratins are intermediate filament proteins, which are widely used as a diagnostic marker for several tumors. In stratified squamous epithelium, the expression of CK14 in the basal cells is associated with their proliferative potential. Interestingly, differential expression pattern of CK14 was observed in oral squamous cell carcinoma [17–19]. During the differentiation of mouse embryonic stem cells into keratinocytes, CK14 appears first and after stratification it remains in the basal layer, where highly proliferative cells are known to reside [20]. Therefore, we also studied the expression pattern of β-catenin and Notch 1 specifically in basal-like oral carcinoma cells.
Even though the expression of β-catenin and ΔNp63 were extensively studied in oral carcinoma, the correlation and interaction between these two proteins has not been studied yet. Therefore, we have investigated the possible correlation between β-catenin and ΔNp63 in oral carcinoma. We also investigated the expression pattern of Notch 1 and its possible correlation with β-catenin, ΔNp63, and histopathological factors to provide new insight into the expression pattern and possible role of Notch 1 in oral squamous cell carcinoma. Moreover, we used CK14 to identify the basal-like carcinoma cells and studied the co-expression of CK14 with β-catenin and CK14 with Notch 1 which could provide basic insight to study the crosstalk between these two pathways in basal-like oral carcinoma cells. On the other hand, the possible interaction between β-catenin and ΔNp63 was also analyzed in oral squamous cell carcinoma. Taken together, the present study focuses on the possible correlation between β-catenin, ΔNp63, and Notch 1 in oral squamous cell carcinoma tissues. The study also aims to investigate the co-localization of CK14 with β-catenin and CK14 with Notch 1 in oral carcinoma tissue and cell line. Furthermore, we also analyzed the prognostic significance of β-catenin, ΔNp63, and Notch 1 in oral squamous cell carcinoma.
Materials and methods
Tissues and cell line
We examined 62 oral carcinoma patients including 50 men and 12 women, aged between 40 and 70, with an average age of 55. The range of follow-up was 9 to 36 months with a mean follow-up period of 28.1 months. Biopsies of oral squamous cell carcinoma tissues from different anatomical sites including tongue (22), buccal mucosa (11), palate (4), gingiva (10), and floor of mouth (15) were obtained with proper consent from the patients attending the Department of Surgical Oncology, Government Royapettah Hospital, Chennai. Seven normal samples were obtained from the patients undergoing orthodontic surgery. All specimens were fixed in 10% buffered formalin and routinely processed for paraffin embedding. Sections were cut at 4 μm and mounted on coated glass slides. The staging of carcinoma was assessed on hematoxylin and eosin-stained sections according to the criteria illustrated by Pindborg et al. [21]. The study was initiated after obtaining clearance from the Hospital Medical Board with permission from the Directorate of Medical Education, Government of Tamil Nadu, India.
The cell-bearing slides of H314 cell line (derived from a poorly differentiated tumor of the floor of the mouth; a kind gift from Dr. Angela Hague) was used for co-immunolocalization analysis and for culture protocol refer Minter et al. [22].
Immunohistochemistry
Tissues were deparaffinized in xylene and rehydrated through graded alcohols and finally in distilled water. Tissues were then incubated in 0.3% hydrogen peroxide at room temperature for 30 min and then placed in 0.1 M sodium citrate buffer (pH 6.0), heated for 10 min in a microwave oven and cooled at room temperature for 20 min. The sections were then incubated with 3% bovine serum albumin (BSA) for 30 min at room temperature to block non-specific binding sites and then incubated (overnight at 4°C) with goat polyclonal anti-Notch 1 (Santa Cruz Biotech., USA; sc-6014), rabbit polyclonal anti-β-catenin (Santa Cruz Biotech., USA; sc-7199), and rabbit polyclonal ΔNp63 (a kind gift from Dr. James Direnzo, USA) [23] in a dilution of 1:200, 1:200, and 1:100, respectively. In addition, the immunohistochemical analysis of CK14 was done in 19 cases which shows intracellular expression of β-catenin using mouse monoclonal anti-CK14 (a kind gift from Dr. Birgitte Lane, Singapore; reactive with the c terminus of human cytokeratin 14 protein) antibody. Immunohistochemical staining was performed using the respective horseradish peroxidase conjugated secondary antibodies (Invitrogen, USA). The chromogen 3, 3′ diaminobenzidine was then used as a substrate for localizing the antibody binding. Counter-staining was performed with hematoxylin and mounted. Negative controls were incubated with phosphate-buffered saline (PBS) instead of the primary antibodies. The immunostaining was assessed by two independent observers according to the intensity of staining and the percentage of stained cells in more than three random areas of cancer tissue as examined in ×200 magnification. The staining was assessed as 0, no staining, or staining observed in less than 10% of carcinoma cells; 1+, mild heterogeneous expression in 10–75% of carcinoma cells; 2+, moderate heterogeneous expression in 10–75% of carcinoma cells; and 3+, intense homogeneous expression in more than 75% of carcinoma cells. The staining of β-catenin, ΔNp63, and Notch 1 were also graded as 0 + 1 (negative/mild; including categories 0 and 1+), 2 (moderate; including category 2+), and 3 (intense; including category 3+) for correlation analysis as performed by Pinilla et al. [24]. Additionally, the localization of β-catenin was considered as 0 + M, negative/membranous; C, cytoplasmic, and N, nucleus, to study the correlation of β-catenin localization with the expression of ΔNp63 and Notch 1. In case of disagreement, the slides were re-evaluated by the two observers and a consensus was reached after discussion.
Immunofluorescence
The co-localization of CK14 with β-catenin and CK14 with Notch 1 were studied in 19 cases of oral squamous cell carcinoma which showed intracellular expression of β-catenin in immunohistochemical analysis. Oral carcinoma tissues and H314 cell line fixed slides were deparaffinized, rehydrated, and fixed for 5 min. After washing with PBS, the cells were permeabilized with 0.1% Triton X-100, blocked for 30 min with PBS containing 2% BSA and then incubated with mouse monoclonal anti-CK14 and rabbit polyclonal anti-β-catenin antibodies for 1 h at room temperature. The primary antibodies were washed with PBS and incubated for 1 h with anti-rabbit FITC conjugated secondary antibody (Invitrogen, USA) for β-catenin and anti-mouse Alexa fluor 594 (Red) conjugated secondary antibody (Invitrogen, USA) for CK14. The carcinoma tissues were also incubated with anti-Notch 1 and anti-CK14 antibodies for 1 h at room temperature. The primary antibodies were washed with PBS and incubated for 1 h with anti-goat FITC conjugated secondary antibody for Notch 1 and anti-mouse Alexa fluor 594 (Red) conjugated secondary antibody for CK14. After incubation, the slides were washed with PBS, mounted, and examined under confocal fluorescence microscope (Leica TCS SP2, Leica Microsystems, Germany).
Western blot and co-immunoprecipitation
For western blot and co-immunoprecipitation analysis, we have selected 23 cases including normal (7), carcinoma cases showing membranous (7), cytoplasmic (7), and nuclear (2) expression of β-catenin in immunohistochemical analysis. Protein lysates were prepared as described by Ratovitski et al. [25] and the protein concentrations were determined as described by Lowry et al. [26]. Samples were separated on 10% SDS-PAGE, transferred to nitrocellulose membrane, incubated with anti-β-catenin, anti-GSK3 β, anti-Notch 1, and anti-ΔNp63 antibodies and were detected using Amersham ECL plus kit according to the manufacturer’s protocol. For co-immunoprecipitation, the immune complex of β-catenin was prepared as described by Patturajan et al. [27]. The immune complex was then incubated with 20 μl of protein-A-sepharose beads (Sigma) for 30 min and then centrifuged at 12,000 rpm for 1 min at 4°C. After five washes with homogenizing buffer, the pellets were resuspended in 30 μl of sample buffer, separated by SDS-PAGE, and transferred to nitrocellulose membrane. The immunoblotting was done by incubating with anti-GSK3β and anti-ΔNp63 antibodies and were detected using Amersham ECL plus kit according to the manufacturer’s protocol.
Statistical analysis
Acastat Statistical software, version 6.1[Acastat Software, USA] was used for statistical analysis. Chi-square test and Fisher’s exact test (for 2 × 2 tables) were used for group comparison studies and Spearman’s correlation analysis was performed to study the association between different groups. Kaplan–Meier method was used to estimate overall and disease-free survival, and the Log-rank test was used to compare the resulting curves. The multivariant survival analysis was performed using Cox proportional hazards regression model. Disease-free survival was calculated from the date of treatment to the date of recurrence or metastasis or disease related death. A case was censored at the date of death for patients who died from causes other than the original tumor and at the date of last examination for patients who lost the follow-up. Survival analysis was performed using SPSS software (SPSS for windows 14.0, SPSS Inc., Chicago, Illinois). For all statistical analysis the p value was considered significant when it was less than 0.05. Due to the exploratory nature of the study, we did not adjust for multiple tests.
Results
Expression of β-catenin, ΔNp63, and Notch 1 in oral squamous cell carcinoma tissues
β-catenin
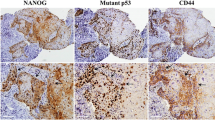
The immunodetection of β-catenin, ΔNp63, and Notch 1 are presented in Table 1. Among the 62 cases of oral squamous cell carcinoma, 12 were negative for β-catenin expression, whereas nine and 14 cases showed mild and moderate staining, respectively. Intense staining of β-catenin was observed in 27 cases of oral squamous cell carcinoma (Fig. 1a (a); Table 1). Interestingly, 17 cases showed absence of membranous and cytoplasmic staining for β-catenin, whereas, two cases showed focal nuclear β-catenin staining in carcinoma cells (Fig. 1a (m)). The expression of β-catenin was higher in poorly differentiated tumors than in moderate and well-differentiated tumors. Statistically significant difference was found between the histological type and β-catenin expression (χ 2 = 18.555; p = 0.001**). Lymph node metastasis positive cases showed a significant decrease in the expression of β-catenin when compared with lymph node metastasis negative cases (χ 2 = 6.421; p = 0.040*). In addition, significant difference was found between stage I–II, stage III–IV, and β-catenin expression (χ 2 = 6.704; p = 0.035*). However, the expression pattern of β-catenin did not show a significant difference between different anatomical sites (Table 2). In addition, β-catenin expression was significantly correlated with smoking (p = 0.042*) and tobacco chewing (p = 0.046*), whereas, statistically significant correlation was not reached between age, gender, alcohol consumption, and β-catenin expression (Supplement table 1).
Immunostaining of β-catenin, ΔNp63, and Notch 1 in oral squamous cell carcinoma. a Oral carcinoma tissues showing intense staining for β-catenin (a), nuclear staining for ΔNp63 (b), and moderate cytoplasmic staining for Notch 1 (c); nuclear expression of β-catenin in few oral carcinoma cells (m); serial sections of oral carcinoma tissue showing intracellular expression of β-catenin (n) and nuclear expression of ΔNp63 (o); serial sections of oral carcinoma tissue showing moderate cytoplasmic expression of Notch 1 (p) and nuclear expression of ΔNp63 (q); carcinoma cells showing membranous expression for Notch 1 (r). b Section of oral carcinoma tissue showing intracellular staining for β-catenin (d), intense staining for CK14 (e), and intense cytoplasmic and nuclear expression of β-catenin in CK14 expressing oral carcinoma cells (f). c Section of oral carcinoma tissue showing mild staining for Notch 1 (g), intense staining for CK14 (h), and mild staining of Notch 1 in CK14 positive carcinoma cells (i); H314 cell showing negative staining for Notch 1 (s), intense staining for CK14 (t), and absence of Notch 1 expression in CK14 positive carcinoma cell (u). d H314 cells showing intense staining for β-catenin (j), intense staining for CK14 (k), and intense β-catenin expression in CK14 positive H314 cells (l). Bar = 50 μm
ΔNp63
Intense nuclear staining of ΔNp63 was predominantly found in oral carcinoma tissues (Fig. 1a (b)). Out of the 62 cases, 14 cases showed intense staining for ΔNp63 and ten cases showed negative staining. Among the 38 remaining cases, 18 cases showed mild staining and 20 cases showed moderate staining for ΔNp63 (Table 1). ΔNp63 expression was significantly higher in stage III–IV than in stage I–II. ΔNp63 expression was also significantly correlated with the presence of lymph node metastasis (χ 2 = 7.201; p = 0.027*). Interestingly, the expression of ΔNp63 was higher in poorly differentiated tumors. Significant difference was found between stages, histological types, and expression of ΔNp63, whereas, no significant difference was found between ΔNp63 expression and location of tumors (Table 2). Interestingly, statistically significant correlation was found between alcohol consumption, smoking, tobacco chewing, and ΔNp63 expression, whereas, no significant correlation was found between age, gender, and ΔNp63 expression (Supplement table 1).
Notch 1
Majority of oral squamous cell carcinoma cases showed mild staining for Notch 1. In addition, moderate Notch 1 staining was also found in some cases of oral carcinoma. Interestingly, cytoplasmic expression of Notch 1 was predominantly observed in oral squamous cell carcinoma tissues (Fig. 1a (c)) with occasional membranous staining in some cases (Fig. 1a (r)). Among the 62 cases of oral squamous cell carcinoma, 16 cases showed negative staining for Notch 1, whereas 32 and 13 cases showed mild and moderate staining, respectively. Only one case showed intense staining for Notch 1 (Table 1). Interestingly, statistical significance between histological types and Notch 1 expression was observed (χ 2 = 18.024; p = 0.001**). Significant decrease in the expression of Notch 1 was also observed in stage III–IV when compared to stage I–II (χ 2 = 6.795; p = 0.033*), whereas, significant correlation was not found between Notch 1 expression and different location of tumors. Moreover, lymph node metastasis positive cases showed significant decrease in the expression of Notch 1 when compared to lymph node metastasis negative cases (χ 2 = 8.350; p = 0.015*; Table 2). In addition, significant correlation was not found between age, gender, smoking, alcohol consumption, tobacco chewing, and Notch 1 expression (Supplement table 1). Statistically significant difference was found between the expression of β-catenin, ΔNp63, and Notch 1 in oral squamous cell carcinoma samples (χ 2 = 41.107; p = <0.001**).
Association between β-catenin and ΔNp63 in oral squamous cell carcinoma
The immunostaining for β-catenin in oral carcinoma tissues was compared with ΔNp63 staining. Out of 62 cases, 12 cases showed negative/mild staining for both β-catenin and ΔNp63, whereas nine cases showed negative/mild and moderate staining for β-catenin and ΔNp63, respectively. Four moderately stained cases for β-catenin showed negative/mild staining for ΔNp63, whereas ten cases showed moderate staining for both β-catenin and ΔNp63. Among the 62 cases of oral squamous cell carcinoma, 14 cases showed intense staining for both β-catenin and ΔNp63, whereas one case showed intense and moderate staining for β-catenin and ΔNp63, respectively. Out of the 62 cases, 12 cases showed intense and negative/mild staining for β-catenin and ΔNp63, respectively. In addition, significant but weak positive correlation was found between the expression of β-catenin and ΔNp63 in oral squamous cell carcinoma samples (p = 0.01*, r s = 0.310; Table 3). Interestingly, intense staining of ΔNp63 was observed in cases showing cytoplasmic staining for β-catenin (Fig. 1a (n and o)). Moreover, significant positive correlation was found between the expression of ΔNp63 and the localization of β-catenin (p = 0.001**, r s = 0.427; Table 4).
Association between β-catenin and Notch 1 in oral squamous cell carcinoma
Among the 62 cases of oral squamous cell carcinoma, 13 cases showed negative/mild staining and two cases showed moderate staining for both β-catenin and Notch 1, Whereas, seven cases showed negative/mild and moderate staining for β-catenin and Notch 1, respectively. Out of the 62 cases, one case showed negative/mild and intense staining for β-catenin and Notch 1, respectively. Twelve moderately stained cases for β-catenin showed negative/mild staining for Notch 1. Among the remaining 27 cases, 23 cases showed intense and negative/mild staining for β-catenin and Notch 1, respectively, whereas, four intensely stained cases for β-catenin showed moderate staining for Notch 1. Interestingly, no significant association was found between β-catenin and Notch 1 in oral squamous cell carcinoma samples (χ 2 = 5.257; p = 0.262; Table 3). Moreover, significant correlation was not found between Notch 1 expression and localization of β-catenin (χ 2 = 1.803; p = 0.772; Table 4).
Correlation between ΔNp63 and Notch 1 in oral squamous cell carcinoma
The correlation between ΔNp63 and Notch 1 was also analyzed in 62 cases of oral squamous cell carcinoma. Out of the 62 cases, 24 cases showed negative/mild staining for both ΔNp63 and Notch1, whereas three cases showed negative/mild and moderate staining for ΔNp63 and Notch 1, respectively. Fourteen moderately stained cases for ΔNp63 showed negative/mild staining for Notch 1. Moderate staining for both ΔNp63 and Notch 1 was observed in six cases of oral squamous cell carcinoma. Out of 62 cases, one case showed negative/mild and intense staining for ΔNp63 and Notch 1, respectively. Ten intensely stained cases for ΔNp63 showed negative/mild staining for Notch 1; whereas, four cases showed intense and moderate staining for ΔNp63 and Notch 1, respectively. No significant association was found between the expression levels of ΔNp63 and Notch 1 in oral squamous cell carcinoma (χ 2 = 4.238; p = 0.375; Table 3).
Co-localization of β-catenin and Notch 1 with CK14 in oral squamous cell carcinoma
Co-immunolocalization of β-catenin and CK14 was studied in oral squamous cell carcinoma tissues and H314 cell line. Intense focal staining of CK14 was observed in both immunohistochemical (Fig. 2a (a)) and immunofluorescence (Fig. 2a (b)) analysis in 19 cases which shows intracellular expression of β-catenin. Oral carcinoma tissues and cell lines showed intense cytoplasmic as well as nuclear localization of β-catenin in cells expressing CK14 (Fig. 1b (f) and d (l)); whereas only mild and negative expression of Notch 1 was observed in CK14 expressing oral carcinoma and H314 cells (Fig. 1c (i) and (u)).
a Immunohistochemical (a) and immunofluorescence (b) analysis of CK14 in oral carcinoma tissues. Intense staining of CK14 was observed in oral carcinoma tissues showing intracellular expression of β-catenin. Bar = 50 μm. b (a) Western blot for β-catenin (∼92 kDa), (b) Western blot for ΔNp63 (∼82 kDa), (c) Western blot for Notch 1 (∼120 kDa), (d) Western blot for GSK3β (∼47 kDa), (e) protein lysates were immunoprecipitated with anti-β-catenin and immunoblotted with ΔNp63 (f) protein lysates were immunoprecitated with anti-β-catenin and immunoblotted with GSK3 β and (g) Western blot for β-actin (control)
Immunoblot analysis of β-catenin, ΔNp63, Notch 1, and GSK3β
Western blot analysis for β-catenin showed increased band intensity in carcinoma samples compared with normal samples (Fig. 2b (a)). Interestingly, ΔNp63 was detected only in carcinoma samples, whereas, band corresponding to ΔNp63 was not detected in normal samples (Fig. 2b (b)). In addition, the expression of Notch 1 was decreased in carcinoma samples, particularly in samples showing intracellular expression of β-catenin, compared with normal samples (Fig. 2b (c)). Moreover, strong band intensity for GSK3β was observed in normal and carcinoma samples showing membranous expression of β-catenin when compared with carcinoma samples showing cytoplasmic and nuclear localization of β-catenin (Fig. 2b (d)).
Co-immunoprecipitation analysis for β-catenin–GSK3β and β-catenin–ΔNp63 interactions
Comparison between interactions of β-catenin and GSK3β and β-catenin and ΔNp63 and localization of β-catenin are presented in Table 5. Interaction between β-catenin and GSK3β was observed in all cases of normal and carcinoma showing membranous expression of β-catenin. Interestingly, out of seven cases, where cytoplasmic expression of β-catenin was observed, only two cases showed interaction between β-catenin and GSK3β. In addition, the interaction between β-catenin and GSK3β was not observed in carcinoma samples showing nuclear expression of β-catenin (Fig. 2b (f)). Interestingly, the interaction between β-catenin and ΔNp63 was not observed in normal and carcinoma cases showing membranous expression of β-catenin. Out of seven cases, where cytoplasmic expression of β-catenin was observed, six cases showed interaction between β-catenin and ΔNp63. Moreover, the interaction between β-catenin and ΔNp63 was observed in carcinoma samples showing nuclear expression of β-catenin (Fig. 2b (e)).
Survival analysis
Survival analysis using Univariate Cox Proportional Hazards Regression model showed that high clinical stage, lymph node metastasis positive, high β-catenin expression, and high ΔNp63 expression indicate worst prognosis in overall and disease-free survival of 62 patients with oral squamous cell carcinoma (Table 6). Interestingly, clinical stage, β-catenin expression, and ΔNp63 expression were found to be independent prognostic factors in overall and disease-free survival by Multivariate Cox Proportional Hazards Regression model (Table 7).
Kaplan–Meier survival analysis (Table 8) showed that the survival of patients with increased β-catenin expression had poorer survival rates than those patients with reduced β-catenin expression in overall and disease-free survival (Figs. 3a and 4a). Furthermore, patients with increased ΔNp63 expression had worse survival than those with reduced ΔNp63 expression in overall as well as disease-free survival (Figs. 3c and 4c). Interestingly, β-catenin and ΔNp63 double positive cases showed statistically significant poor survival rates when compared with other cases (Figs. 3d and 4d). Moreover, the intracellular localization of β-catenin was significantly correlated with worst outcome in overall and disease-free survival (Supplement Fig. 1c, d). In addition, patients with decreased Notch 1 expression had a shorter survival time than those with increased Notch 1 expression (Figs. 3b and 4b). Moreover, patients with positive lymph node metastasis, high clinical stage and poor differentiation status showed significant shorter survival rates than those with negative lymph node metastasis, low clinical stage, and moderate and well differentiation status, respectively (Table 8).
Overall survival of patients with oral squamous cell carcinoma according to the expression of β-catenin (a), Notch 1 (b), ΔNp63 (c), and β-catenin + ΔNp63 (double positive) (d) calculated by the Kaplan–Meier method. B+/P + = β-catenin and ΔNp63 double positive cases; 0 + 1 = includes category 0 and 1+
Disease-free survival of patients with oral squamous cell carcinoma according to the expression of β-catenin (a), Notch 1 (b), ΔNp63 (c), and β-catenin + ΔNp63 (double positive) (d) calculated by the Kaplan–Meier method. B+/P + = β-catenin and ΔNp63 double positive cases; 0 + 1 = includes category 0 and 1+
Discussion
According to cancer stem cell hypothesis, only a small sub-population of cells termed “cancer stem cells” within the tumor have the ability to give rise to new tumors when injected into immunodeficient mice. Interestingly, stem-cell-related pathways including Wnt and Notch, which are the major pathways that regulate the self-renewal and differentiation of embryonic and adult stem cells, were found to be reactivated in cancer [7, 28, 29]. It was found that the Wnt/β-catenin signaling maintains the self-renewal potential of hematopoietic and neural stem cells and the activated β-catenin signaling upregulates Myc which blocks the differentiation of keratinocyte [4, 30]. In this study, we have investigated the expression pattern and the possible correlation between β-catenin, ΔNp63, and Notch 1 in oral squamous cell carcinoma. We also studied the co-localization of CK14 with β-catenin and CK14 with Notch-1 in oral squamous cell carcinoma tissues and H314 cell line to analyze the expression pattern of β-catenin and Notch 1 in basal-like oral carcinoma cells. Finally, we analyzed the prognostic significance of β-catenin, ΔNp63 and Notch 1 in oral squamous cell carcinoma.
Dysregulated expression of β-catenin was observed in several tumors and the loss of membranous β-catenin expression was found in oral squamous cell carcinoma. Moreover, the nuclear localization of β-catenin was found to be a key event which may be involved in the progression of oral dysplasia to carcinoma [2, 5]. In the present study, significant correlation was found between smoking, tobacco chewing, and β-catenin expression. This suggests that expression of β-catenin may be related to smoking and tobacco chewing in oral squamous cell carcinoma. As mentioned by Cai et al. [31], in this study the expression of β-catenin shows significant difference between stages, differentiation status, and lymph node metastasis. Moreover, consistent with previous studies by Ishida et al. [2] and Kobayashi et al. [5], we observed the absence of membranous and intense intracellular localization of β-catenin in some cases of oral carcinoma and H314 cell line. This suggests the possible involvement of activated β-catenin signaling in oral squamous cell carcinoma.
Consistent with findings of Nylander et al. [13], the present study also shows an intense staining pattern for ΔNp63 in oral squamous cell carcinoma tissues. This suggests the possible role of ΔNp63 in maintaining the proliferation of oral carcinoma cells. In this study, statistically significant difference was found between smoking, alcohol consumption, tobacco chewing, and ΔNp63 expression. This indicates that the expression of ΔNp63 may be related to the progression of oral carcinoma in high-risk populations. Recently, it was found that ΔNp63 inhibits GSK3β and induces β-catenin signaling [27]. In the present study, intense staining of ΔNp63 was predominantly observed in cases showing intracellular localization of β-catenin. In addition, ΔNp63 and β-catenin showed similar expression patterns in histological types of oral squamous cell carcinoma, particularly, in poorly differentiated tumors. Moreover, significant positive association was found between the expression pattern of ΔNp63 and the localization of β-catenin. These results suggest that β-catenin and ΔNp63 might have dependent roles in maintaining the proliferation of oral carcinoma cells. Even though, the interaction between β-catenin and ΔNp63 was found in cases showing intracellular expression of β-catenin, the interpretation of these results might not be precise without further studies on the molecular basis for the interaction between these two proteins in oral squamous cell carcinoma. However, the interaction of β-catenin with ΔNp63 may be a crucial event in oral carcinogenesis.
In this study, the cytoplasmic staining of Notch 1 was predominantly observed in oral carcinoma cells. Interestingly, Notch 1 expression was significantly reduced in stage III–IV and in lymph node metastasis positive cases. This suggests that the expression of Notch 1 might be related to invasion and metastasis of oral carcinoma cells. However, the expression of Notch 1 shows a significant difference between histological types, particularly, decreased expression of Notch 1 was observed in poorly and moderately differentiated tumors than in well-differentiated tumors. This suggests that Notch 1 may have a role in maintaining the differentiation status of oral carcinoma cells. Moreover, the expression of Notch 1 may not be related to age, gender, alcohol consumption, smoking, and tobacco chewing, since the statistical significance was not reached. In addition, Notch 1, β-catenin, and ΔNp63 showed similar expression pattern between different locations of tumors since the statistical significance was not reached.
Interestingly, Nguyen et al. showed that Notch 1 activation suppresses p63 expression through modulation of interferon responsive genes [16]. In this study, Notch 1 showed an inverse association with ΔNp63 in histological types of oral squamous cell carcinoma. In addition, statistically significant correlation was not found between the expression of ΔNp63 and Notch 1 in oral carcinoma tissues suggesting that ΔNp63 and Notch 1 may have independent roles in oral cancer.
Recently, it was found that the activation of Notch 1 induces the expression of p21, which is one of the key regulators of keratinocyte differentiation and inhibition of Notch signaling leads to squamous cell carcinoma formation [32, 33]. In addition, Notch 1 expression also suppresses β-catenin signaling in keratinocyte [32]. In the present study, even though the expression pattern of Notch 1 did not show a significant correlation with β-catenin, decreased expression of Notch 1 was found in cases showing intracellular expression of β-catenin. In addition, CK14 positive basal-like oral carcinoma and H314 cells also shows mild staining for Notch1 and intense staining for β-catenin. Even though the reason for this is unclear, the functional relationship between β-catenin, ΔNp63, and Notch 1 in keratinocytes suggests that the intracellular localization of β-catenin as well as increased expression of ΔNp63 may have some influence on Notch 1 expression in oral carcinoma cells, which needs further investigation.
As mentioned by Odajima et al. [34], the present study showed a significant correlation between increased β-catenin expression (particularly intracellular localization) and poor survival in oral squamous cell carcinoma. This suggests that impaired β-catenin expression may be related to the progression of oral squamous cell carcinoma. In addition, ΔNp63 overexpressing cases showed poor survival, which was consistent with the findings of Lo Muzio et al. [35]. This suggests that ΔNp63 may be related to aggressive biological behavior of oral carcinoma cells. Interestingly, β-catenin and ΔNp63 double positive cases showed worst survival when compared with other cases. This suggests that in combination with ΔNp63, β-catenin expression may maintain the proliferation of oral carcinoma cells and may be useful to identify patients at high risk for rapid and effective treatment. In addition, significant correlation was not found between overall survival, disease-free survival, and Notch 1 expression. This suggests that Notch 1 expression may not be related to survival of oral squamous cell carcinoma patients.
In the present study, univariate and multivariate analysis revealed the prognostic significance of β-catenin and ΔNp63 in oral squamous cell carcinoma as mentioned by Odajima et al. [34] for β-catenin and by Lo Muzio et al. [35] and Moergel et al. [36] for ΔNp63. This suggests that β-catenin and ΔNp63 may be used as independent prognostic markers of oral squamous cell carcinoma. Even though β-catenin and ΔNp63 help to determine stem/progenitor cells in oral squamous cell carcinoma tissues, further studies using β-catenin and ΔNp63 with well-established cancer stem cell markers and analysis on the tumor-inducing potential of β-catenin+/ΔNp63+ cells will provide more evidence on the relationship between β-catenin, ΔNp63, and oral cancer stem cells.
In conclusion, statistically significant positive association between the localization of β-catenin and the expression of ΔNp63 suggests that they might have dependent roles in maintaining the proliferation of oral carcinoma cells, whereas, the expression of ΔNp63 and Notch 1 may be independent events since no significant association was found between them. In addition, even though the reason for downregulated expression of Notch 1 in cases showing intracellular expression of β-catenin and also in CK14 positive basal-like oral carcinoma cells remains unclear, the result could pave a way to study the crosstalk between β-catenin and Notch 1 pathways in oral squamous cell carcinoma. Moreover, the overexpression of β-catenin and ΔNp63 may be related to the progression of oral squamous cell carcinoma and may be used as independent prognostic markers. On the other hand, the interaction of β-catenin with ΔNp63 may be a key event in maintaining the proliferation of oral carcinoma cells. Further studies with larger sample size, longer follow-up period, and on the molecular networks related to β-catenin as well as ΔNp63 could provide more details on using β-catenin and ΔNp63 as prognostic markers and as therapeutic targets of oral squamous cell carcinoma.
References
Warnakulasuriya S (2009) Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 45:309–316
Ishida K, Ito S, Wada N, Deguchi H, Hata T, Hosoda M, Nohno T (2007) Nuclear localization of beta-catenin involved in precancerous change in oral leukoplakia. Mol Cancer 6:62
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, Da Costa LT, Morin PJ, Vogelstein B, Kinzler KW (1998) Identification of c-MYC as a target of the APC pathway. Science 281:1509–1512
Barker N, Clevers H (2000) Catenins, Wnt signaling and cancer. Bioessays 22:961–965
Kobayashi M, Honma T, Matsuda Y, Suzuki Y, Narisawa R, Ajioka Y, Asakura H (2000) Nuclear translocation of beta-catenin in colorectal cancer. Br J Cancer 82:1689–1693
Udhayakumar G, Jayanthi V, Devaraj N, Devaraj H (2007) Interaction of MUC1 with beta-catenin modulates the Wnt target gene cyclinD1 in H. pylori-induced gastric cancer. Mol Carcino 46:807–817
Behbod F, Rosen JM (2005) Will cancer stem cells provide new therapeutic targets? Carcinogenesis 26:703–711
Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M (2001) p63 identifies keratinocyte stem cells. Proc Natl Acad Sci 98:3156–3161
Moll UM (2003) The role of p63 and p73 in tumour formation and progression: coming of age toward clinical usefulness. Clin Cancer Res 9:5437–5441
Parsa R, Yang A, McKeon F, Green H (1999) Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J Invest Dermatol 113:1099–1105
Di Como CJ, Urist MJ, Babayan I, Drobnjak M, Hedvat CV, Teruya-Feldstein J, Pohar K, Hoos A, Cordon-Cardo C (2002) p63 expression profiles in human normal and tumor tissues. Clin Cancer Res 8:494–501
Chen YK, Hsue SS, Lin LM (2005) Expression of p63 protein and mRNA in oral epithelial dysplasia. J Oral Pathol Med 34:232–239
Nylander K, Coates PJ, Hall PA (2000) Characterization of the expression pattern of p63 alpha and delta Np63 alpha in benign and malignant oral epithelial lesions. Int J Cancer 87:368–372
Allenspach EJ, Maillard I, Aster JC, Pear WS (2002) Notch signaling in cancer. Cancer Biol Ther 1:466–476
Brennan K, Brown AM (2003) Is there a role for Notch signaling in human breast cancer? Breast Cancer Res 5:69–75
Nguyen BC, Lefort K, Mandinova A, Antonini D, Devgan V, Della Gatta G, Koster MI, Zhang Z, Wang J, Tommasi di Vignano A, Kitajewski J, Chiorino G, Roop DR, Missero C, Dotto GP (2006) Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev 20:1028–1042
Su L, Morgan PR, Lane EB (1996) Keratin 14 and 19 expression in normal, dysplastic and malignant oral epithelia. A study using in situ hybridization and immunohistochemistry. J Oral Pathol Med 25:293–301
Presland RB, Dale BA (2000) Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit Rev Oral Biol Med 11:383–408
Fillies T, Jogschies M, Kleinheinz J, Brandt B, Joos U, Buerger H (2007) Cytokeratin alteration in oral leukoplakia and oral squamous cell carcinoma. Oncol Rep 18:639–643
Byrne C, Tainsky M, Fuchs E (1994) Programming gene expression in developing epidermis. Development 120:2369–2383
Pindborg JJ, Reichart PA, Smith CJ, der waal Van I (1997) Histological typing of cancer and precancer of the oral mucosa, 2nd edn. Heidelberg, Springer
Minter HA, Eveson JW, Huntley S, Elder DJE, Hague A (2003) The cyclooxygenase 2-selective inhibitor NS398 inhibits proliferation of oral carcinoma cell lines by mechanisms dependent and independent of reduced prostaglandin E2 synthesis. Clin Cancer Res 9:1885
Li H, Cherukuri P, Li N, Cowling V, Spinella M, Cole M, Godwin AK, Wells W, DiRenzo J (2007) Nestin is expressed in the basal/myoepithelial layer of the mammary gland and is a selective marker of basal epithelial breast tumors. Cancer Res 67:501–510
Pinilla MR, Peralto JLR, Hitt R, Sanchez JJ, Verde LS, Alameda F, Ballestin C, Cespedes MS (2005) β-Catenin, Nf-kB and FAS protein expression are independent events in head and neck cancer: study of their association with clinical parameters. Cancer Lett 230:141–148
Ratovitski EA, Patturajan M, Hibi K, Trink B, Yamaguchi K, Sidransky D (2001) p53 associates with and targets ΔNp63 into a protein degradation pathway. Proc National Acad Sci 98:1817–1822
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Patturajan M, Nomoto S, Sommer M, Fomenkov A, Hibi K, Zangen R, Poliak N, Califano J, Trink B, Ratovitski E, Sidransky D (2002) ΔNp63 induces β-catenin nuclear accumulation and signaling. Cancer Cell 1:369–379
Tysnes BB, Bjerkvig R (2007) Cancer initiation and progression: involvement of stem cells and the microenvironment. Biochim Biophys Acta 1775:283–297
Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111
Pelengaris S, Littlewood T, Khan M, Elia G, Evan G (1999) Reversible activation of c-Myc in skin: induction of a complex neoplastic phenotype by a single oncogenic lesion. Mol Cell 3:565–577
Cai Z-G, Shi X-J, Gao Y, Wei M-J, Wang C-Y, Yu G-Y (2008) β-catenin expression pattern in primary oral squamous cell Carcinoma. Chin Med J 121:1866–1870
Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, Aster JC, Krishna S, Metzger D, Chambon P, Miele L, Aguet M, Radtke F, Dotto GP (2001) Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J 20:3427–3436
Proweller A, Tu L, Lepore JJ, Cheng L, Lu MM, Seykora J, Millar SE, Pear WS, Parmacek MS (2006) Impaired notch signaling promotes de novo squamous cell carcinoma formation. Cancer Res 66:7438–7444
Odajima T, Sasaki Y, Tanaka N, Kato-Mori Y, Asanuma H, Ikeda T, Satoh M, Hiratsuka H, Tokino T, Sawada N (2005) Abnormal β-catenin expression in oral cancer with no gene mutation: correlation with expression of cyclin D1 and epidermal growth factor receptor, ki-67 labeling index, and clinicopathological feature. Hum Pathol 36:234–241
Lo Muzio L, Santarelli A, Caltabiano R, Rubini C, Pieramici T, Trevisiol L, Carinci F, Leonardi R, De Lillo A, Lanzafame S, Bufo P, Piattelli A (2005) P63 overexpression associates with poor prognosis in head and neck squamous cell carcinoma. Hum Pathol 36:187–194
Moergel M, Abt E, Stockinger M, Kunkel M (2010) Overexpression of p63 is associated with radiation resistance and prognosis in oral squamous cell carcinoma. Oral Oncol 46:667–671
Acknowledgments
We acknowledge “UGC-Meritorious Research Fellowship Programme” for financial assistance. We are also grateful to Dr. Birgitte Lane (Institute of Medical Biology, Singapore) and Dr. James Direnzo (Dartmouth Medical School, USA) for providing anti-CK14 and anti-ΔNp63 antibodies, respectively. We would like to thank Dr. Angela Hague (Department of Oral and Dental Science, University of Bristol, UK) for providing H314 cell line used in this study. We also thank Dr. Mary Lilly (Professor of Pathology, Royapettah Government Hospital, Chennai, India) for her support throughout this study. Finally, we would like to acknowledge Dr. R. Ravanan (Associate Professor, Department of Statistics, Presidency College, Chennai, India) for his kind help in reviewing and revising the statistical analysis.
Conflict of interest
We have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplement Fig. 1
Overall survival of patients with oral squamous cell carcinoma according to stage (a) and beta catenin localization (c); disease-free survival of patients with oral squamous cell carcinoma according to stage (b) and beta catenin localization (d) calculated by the Kaplan–Meier method. Intra. intracellular. (JPEG 127 KB)
Supplement Table 1
Correlation of β-catenin, ΔNp63, and Notch 1 expression with clinical parameters (DOC 27 kb)
Rights and permissions
About this article
Cite this article
Ravindran, G., Devaraj, H. Aberrant expression of β-catenin and its association with ΔNp63, Notch-1, and clinicopathological factors in oral squamous cell carcinoma. Clin Oral Invest 16, 1275–1288 (2012). https://doi.org/10.1007/s00784-011-0605-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-011-0605-0