Abstract
Horseradish peroxidase was reconstituted with cobalt tetradehydrocorrin, rHRP(Co(TDHC)), as a structural analog of cobalamin coordinated with an imidazolate-like His residue, which is generally seen in native enzymes. In contrast to the previously reported cobalt tetradehydrocorrin-reconstituted myoglobin, rMb(Co(TDHC)), the HRP matrix was expected to provide strong axial ligation by His170 which has imidazolate character. rHRP(CoII(TDHC)) was characterized by EPR and its reaction with reductants indicates a negative shift of its redox potential compared to rMb(Co(TDHC)). Furthermore, aqua- and CN-forms of Co(III) state were prepared. The former species was obtained by oxidation of rHRP(CoII(TDHC)) with K3[Fe(CN)6]. The cyanide-coordinated Co(III) species in the latter was prepared by ligand exchange of rHRP(CoIII(OH)(TDHC)) with exogenous cyanide upon addition of KCN. The 13C NMR chemical shift of cyanide in rHRP(CoIII(CN)(TDHC)) was determined to be 121.8 ppm. IR measurements show that the cyanide of rHRP(CoIII(CN)(TDHC)) has a stretching frequency peak at 2144 cm−1. The 13C NMR and IR measurements indicate strong coordination of cyanide to CoIII(TDHC) relative to rMb(CoIII(CN)(TDHC)). Thus, the extent of π-back donation from the cobalt ion to the cyanide ion is relatively high in rHRP(CoIII(CN)(TDHC)). The pK 1/2 values of rHRP(CoIII(OH)(TDHC)) and rHRP(CoIII(CN)(TDHC)) are the same (pK 1/2 = 3.2) as determined by a pH titration experiment, indicating that cyanide ligation does not affect Co–His ligation, whereas cyanide ligation weakens the Co–His ligation in rMb(CoIII(CN)(TDHC)). Taken together, these results indicate that HRP reconstituted with cobalt tetradehydrocorrin is a suitable cobalamin-dependent enzyme model with imidazolate-like His residue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cobalamin, a vitamin B12-like cobalt corrinoid complex, is an important cofactor in metalloenzymes which catalyze essential reactions such as methyl group transfer and 1,2-rearrangements in biological systems [1–9]. These reactions are often catalyzed via organometallic species known as methylcobalamin and adenosylcobalamin. For example, methionine synthase, a representative cobalamin-dependent methyltransferase widely found in prokaryotes and eukaryotes, is responsible for the methylation of homocysteine to yield methionine. The enzyme requires N 5-methyltetrahydrofolate as a methyl group donor and the methyl group is transferred from tetrahydrofolate to homocysteine via the formation and heterolytic cleavage of the CH3–Co bond in the cobalamin cofactor (Fig. 1). The crystal structure of the cobalamin-binding domain in methionine synthase was revealed by Ludwig and co-workers and it was found that the axial ligand of cobalamin is a protein-derived histidine residue and not the benzimidazole moiety of cobalamin (Fig. 1) [5]. It is likely that the state of axial ligation in terms of the base-off/His-on coordination regulates the reactivity of the CH3–Co bond in the methyl transfer reaction. In the catalytic cycle, two key intermediates have been proposed: a highly nucleophilic Co(I) species with a tetra-coordinate complex and a methylated Co(III) species [1, 2]. In contrast, the adenosylcobalamin-dependent enzymes such as methylmalonyl-CoA mutase and glutamate mutase promote homolytic Co–C bond cleavage, leading to a protein-bound cofactor in the CoII state and an organic 5-deoxyadenosyl radical for 1,2-rearrangement [7–9]. This difference in the reactivity of the organometallic species is remarkable, considering that methylcobalamin and adenosylcobalamin cofactors generally share an identical metal complex structure. Obviously, a prominent role of the surrounding protein has been anticipated in the process of triggering Co–C bond activation and enzymatic catalysis [10–15]. Furthermore, characteristic imidazolate-like histidine ligation to the cobalamin cofactor is observed in the abovementioned methionine synthase as well as in methylmalonyl-CoA mutase and glutamate mutase [16–20]. Over the past decades, several model systems have been reported using cobalamin and its derivatives as well as cobalt corrinoid complexes [20–28]. Previously, our group reported the structure, reactivity and physicochemical properties of myoglobin (Mb) reconstituted with Co(TDHC), (TDHC = 8,12-dicarboxyethyl-1,2,3,7,13,17,18,19-octamethyltetradehydrocorrin), rMb(Co(TDHC)), in efforts to develop a useful protein-based model of cobalamin-dependent methionine synthase [29–32]. Further replication of the coordination state of the native enzyme promises a more sophisticated model system. Thus, this paper demonstrates the physicochemical properties of Co(TDHC) in a different hemoprotein matrix, horseradish peroxidase (HRP) as shown in Scheme 1. In the case of HRP, His170, an axial ligand for the native heme in the native form, serves as a histidine ligand with strong imidazolate character induced by a hydrogen bonding network which includes Asp247 as a hydrogen acceptor (Fig. 1) [33–38]. Such imidazolate ligation is often found in native cobalamin-dependent enzymes. Here, we report preparation, characterization and physicochemical properties of HRP reconstituted with Co(TDHC), rHRP(Co(TDHC)).
Experimentals
Instruments
UV–Vis spectral measurements were carried out with a Shimadzu UV-3150 or UV-2550 double-beam spectrophotometer, or a Shimadzu BioSpec-nano spectrometer. 13C NMR spectra were collected with a JEOL JNM-ECS400 (100 MHz) spectrometer. EPR spectroscopy was performed with a Bruker EMXmicro spectrometer. IR spectra were recorded with a JASCO FT/IR-6200 spectrometer using an SL-2 demountable cell unit (optical path length = 0.025 mm, Pier Optics Co., Ltd., Japan) with CaF2 windows. ICP-OES (inductively coupled plasma optical emission spectroscopy) was performed with a Shimadzu ICPS-7510 emission spectrometer. CD spectra were recorded with a JASCO J820AC spectrometer. The pH measurements were made with an F-52 Horiba pH meter.
Materials
All reagents of the highest guaranteed grade available were obtained from commercial sources and were used as received unless otherwise indicated. Distilled water was demineralized using a Millipore Integral-3 apparatus. Synthesis of cobalt tetradehydrocorrin, Co(TDHC), was described in our previous report [29]. HRP was purchased from Sigma-Aldrich (Type VI, salt-free lyophilized powder: P8375) and used without further purification. The apoprotein was prepared according to Teale’s method [39, 40]. The reconstituted protein, rHRP(CoII(TDHC)), was obtained by the conventional method [35–38]. A cobalt standard solution for ICP-OES was purchased from Wako Pure Chemical Industries.
Incorporation of CoII(TDHC) into apoHRP
A CoII(TDHC) solution (0.65 mM, 0.15 mL, 2 eq.) in 50 mM sodium phosphate buffer (pH 7.0) was added dropwise into 0.5 mL of a solution of apoHRP (0.1 mM) with gentle shaking on an ice bath after equilibrating for 10 min at ambient temperature. To remove the excess cofactor, the protein containing CoII(TDHC) was purified using a HiTrap™ desalting column (5 mL GE Healthcare) with 50 mM sodium phosphate buffer (pH 7.0). The eluted fractions were collected to yield rHRP(CoII(TDHC)) (33 µM, 1 mL, 66%). The obtained protein solution was stored in the dark at 4 °C. The UV–Vis and CD spectra of the obtained protein are shown in Fig. 2, S1 and Table 1. The molar coefficient at 513 nm was determined to be 16.4 mM−1 cm−1 by ICP-OES measurement.
EPR measurement
EPR measurements were carried out at the X-band (9.35 GHz) microwave frequency with 2.00 mM of microwave power, 100 kHz field modulation and 5.00 G of modulation amplitude. During EPR measurements, the sample temperature was maintained at 100 K or 114 K using the liquid N2 vapor. The rHRP(CoII(TDHC)) solution (0.33 mM) in 50 mM sodium phosphate buffer (pH 7.0) was placed in a 5-mm tube. The sample was quickly frozen in a cold pentane bath chilled with liquid N2.
Reaction of rHRP(CoII(TDHC)) with reductants
The following procedures were performed in a glove box (O2 < 0.1 ppm). Into a solution of rHRP(CoII(TDHC)) (60 µM, 0.6 mL) in 50 mM sodium phosphate buffer (pH 7.0), sodium dithionite or Ti(III) citrate (0.18 M, 20 µL, 100 eq.) in the same buffer was added. UV–Vis spectra were recorded before and after the reaction (Fig. 3). For the EPR measurements, the concentrated protein solution (0.33 mM) was utilized (Fig. S2).
UV–Vis absorption spectral changes of rHRP(CoII(TDHC)) upon addition of a dithionite and b Ti(III) citrate as reductants under N2 atmosphere. Spectra before and after reactions are represented as solid and dashed lines, respectively. The spectra were measured in 50 mM sodium phosphate buffer at pH 7.0 at 25 °C
Preparation of rHRP(CoIII(OH)(TDHC))
Into a solution of rHRP(CoII(TDHC)) (33 μM, 1 mL, 0.33 μmol) in 50 mM sodium phosphate buffer at pH 7.0 was added a solution of K3[Fe(CN)6] (0.1 M, 33 µL, 33 μmol, 100 eq.). After equilibrating at 4 °C under aerobic conditions for 1 h, the protein was purified using a HiTrap™ desalting column (5 mL, GE Healthcare) with 50 mM sodium phosphate buffer at pH 7.0 to yield rHRP(CoIII(OH)(TDHC)) (28 µM, 1 mL, 85%). The protein solution was stored in the dark at 4 °C. UV–Vis spectral data of the protein are shown in Fig. 4 and Table 1.
Preparation of rMb(CoIII(CN)(TDHC))
Into a solution of rHRP(CoIII(OH)(TDHC)) (28 μM, 0.5 mL, 0.014 μmol) in 50 mM sodium phosphate buffer at pH 7.0 was added a solution of KCN (50 mM, 2.8 μL, 0.14 μmol, 10 eq.). After equilibrating at 4 °C for 0.5 h, the protein was purified using a HiTrap desalting column (5 mL, GE Healthcare) with 50 mM sodium phosphate buffer at pH 7.0 to yield rHRP(CoIII(CN)(TDHC)) (15 µM, 0.5 mL, 54%). The protein solution was stored in the dark at 4 °C. UV–Vis spectral data of the protein are shown in Fig. 4 and Table 1.
Relaxation analysis of cyanide binding
In general, it is difficult to determine the affinity of cyanide for aqua-Co(III) complexes, because the ligand-exchange process is too slow to detect by conventional titrimetric measurements at 25 °C, pH 7.0 [41]. Thus, the binding constant of cyanide was determined by a relaxation analysis (Fig. 5) [41]. Into a solution of rHRP(CoIII(OH)(TDHC)) (10 μM) in 50 mM sodium phosphate buffer at pH 7.0 was added a solution of 0.10 M KCN in the same buffer to control the final concentrations of KCN (0.10, 0.30, 0.6, 0.9 and 1.2 mM) and then UV–Vis spectra were recorded at 25 °C for 30 min. The concentrations of the cyanide ion were determined using the following Henderson–Hasselbach equation:
where the pH and pK a values are 7.0 and 9.219 at 25 °C, respectively; C 0 is the initial concentration of KCN. The spectral changes occurring over 30 min upon addition of KCN were observed. The transient relaxation kinetics are represented by the following equation:
where L denotes an exogenous ligand; k on and k off represent rate constants for the association and dissociation of L, respectively. Absorbance changes in the transient relaxation were analyzed as the pseudo-first-order reaction to determine the observed rate constants, k obs (Fig. S3). The rate constants at various cyanide concentrations were evaluated by the following equation [41]:
The binding constant of cyanide for rHRP(CoIII(OH)(TDHC)) was determined using the following equation:
NMR measurements of rHRP(CoIII(CN)(TDHC))
Into a solution of rHRP(CoII(TDHC)) (2 mM) in 50 mM sodium phosphate buffer at pH 7.0 was added a solution of normal or 13C-enriched KCN (0.1 M, 33 μL, 3.3 μmol, 2 eq.) and then the mixture was equilibrated at 4 °C under aerobic conditions for 30 min. After concentration to 0.8 mL using an Amicon Ultra-4 centrifugal filter (10 kDa) (GE Healthcare), the protein solution was passed through a HiTrap desalting column (GE Healthcare) equilibrated with 50 mM sodium phosphate buffer at pH 7.0. After addition of D2O (final concentration of D2O: 10% v/v), the solution was concentrated to 0.4 mL and transferred to an NMR microtube (Shigemi Co., Ltd., Hachioji, Japan). The 13C NMR measurements of the proteins were carried out at 4 °C over 102,383 scans.
FT-IR measurements of rHRP(CoIII(CN)(TDHC))
Each solution containing 13C-enriched or normal cyanide bound to the cobalt complex (3 mM) in 50 mM sodium phosphate buffer at pH 7.0 was prepared. A series of FT-IR spectra was accumulated (50 scans) at 25 °C with 4.0 cm−1 resolution.
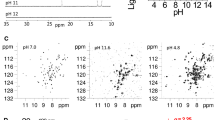
Determination of pK 1/2 values
UV–Vis spectra of rHRP(CoII(TDHC)), rHRP(CoIII(OH)(TDHC)), and rHRP(CoIII(CN)(TDHC)) (12–14 μM) in 50 mM sodium phosphate buffer at 25 °C were measured under conditions of varying pH values which were adjusted by incremental addition of aqueous solutions of 1–12 M HCl. The pH values of the solution were recorded before and after each spectroscopic measurement. The pK 1/2 values were determined from the data which are fitted to the following Henderson–Hasselbach equation for multi-proton transfer process [42]:
where Z is absorbance at a certain pH; α is the number of proton in the transfer process; A acid and A neutral are absorbances of the acid and neutral forms, respectively.
Results and discussion
Reconstitution of HRP with CoII(TDHC)
Incorporation of the CoII(TDHC) cofactor into apoHRP using the conventional method yields rHRP(CoII(TDHC)) [35–38, 40]. Interestingly, addition of two equivalents of CoII(TDHC) cofactor to apoHRP is necessary to complete the reconstitution according to the titration experiment (Fig. S1a). Here, it is noted that the two methyl substituents at the C1- and C19-positions located on the opposite faces of the corrinoid ring provide two enantiomers, (1R,19R)-TDHC and (1S,19S)-TDHC, in a 1:1 ratio. In the case of rMb(CoII(TDHC)), a small excess (~1.3 eq.) is sufficient to complete the reconstitution. X-ray crystal structure analysis shows that the ratio of occupancy of the enantiomers rMb(CoII((1R,19R)-TDHC)) and rMb(CoII((1S,19S)-TDHC)) is 13:7 [29]. The abovementioned requirement for two equivalents of CoII(TDHC) cofactor may be an indication that apoHRP selectively binds one of the CoII(TDHC) enantiomers of CoII(TDHC) because the heme pocket of HRP accurately recognizes the position of the methyl groups in contrast to the heme pocket of Mb. The UV–Vis absorption spectrum of rHRP(CoII(TDHC)) in 50 mM phosphate buffer at pH 7.0 in Fig. 2a and Table 1 has a sharp absorption band at 513 nm, which is similar to the absorption at 510 nm in rMb(CoII(TDHC)), indicating His ligation to the cobalt center [29]. CD spectral changes in the far-UV region occurring with addition of the cofactor indicate an increasing negative Cotton effect at 222 nm in Fig. S1b. This observation indicates successful recovery of α-helixes in the protein as the reconstitution proceeds. Furthermore, a typical spectrum was observed in the EPR measurement of rHRP(CoII(TDHC)) (Fig. 2b). The interaction of the cobalt nucleus (I = 7/2) with the unpaired electron in a pentacoordinate low-spin d 7 configuration of Co(II) results in hyperfine splitting of the signal into an octet centered at g z = 1.99, and the presence of a 14N (I = 1) ligand attached to the axial position of the CoII(TDHC) complex shows superhyperfine splitting of each component of the octet into a triplet [29]. This provides support for our proposal that the cobalt atom coordinates to the Nε2-atom of the His170 imidazole ring as an axial ligand.
Reduction of rHRP(CoII(TDHC))
The reaction of rHRP(CoII(TDHC)) with a large excess of dithionite does not produce a UV–Vis spectral change (Fig. 3a). In contrast, the same reaction of rMb(CoII(TDHC)) or CoII(TDHC) yields the Co(I) species. However, Ti(III) citrate, a stronger reductant, reacts with rHRP(CoII(TDHC)) to produce the characteristic absorption band of the Co(I) species at 524 nm (Fig. 3b). Furthermore, the characteristic Co(II) signals in the EPR spectrum of rHRP(Co(TDHC)) almost disappear upon the addition of excess Ti(III) citrate (Fig. S2), supporting the nearly quantitative formation of the Co(I) species. From these results, the Co(I)/Co(II) redox potential is estimated to be in a range between −0.4 and −0.8 V vs NHE for rHRP(Co(TDHC)) [43]. Compared with the previously reported potential for rMb(Co(TDHC)) (−0.13 vs NHE) [29], a large negative shift of the potential is observed as the cofactor is incorporated into the HRP matrix, indicating stronger ligation by the imidazolate-like His170. The Co(I)/Co(II) redox potential of cobalamin in methionine synthase was previously determined to be −0.50 vs NHE [44]. This value is within the expected range of the present system, suggesting that rHRP(Co(TDHC)) is a suitable model for the native enzyme when we focus on the Co(I)/Co(II) redox potential.
Preparation of rHRP(CoIII(OH)(TDHC) by oxidation of rHRP(CoII(TDHC))
Addition of K3[Fe(CN)6] to a solution of rHRP(CoII(TDHC)) in 50 mM phosphate buffer at pH 7.0 leads to a new set of absorption maxima (λ max) at 635 nm and 691 nm with disappearance of the 513 nm absorption (Fig. 4; Table 1). It is speculated that these new absorption bands are derived from an intermolecular charge transfer transition for the cobalt–axial ligand bond of the CoIII(TDHC) species [22] and are generally similar to absorption bands of rMb(CoIII(OH2)(TDHC)). Although increasing the pH value of the oxidized protein solution was expected to induce spectral changes derived from deprotonation of a water molecule coordinated to the cobalt atom, no changes were observed in reconstituted HRP. This indicates that there is an axially ligated hydroxy group at the Co center in the oxidized protein even under neutral conditions. Considering that the pK a value of a coordinated H2O molecule in rMb(Co(OH2)(TDHC)) is 7.5, the second coordination sphere involving distal His42 and Arg38 in reconstituted HRP (Fig. 1) appears to stabilize the hydroxy species with hydrogen bonding.
Cyano-Co(III) complex obtained by ligand exchange
The coordinated hydroxy anion in rHRP(CoIII(OH)(TDHC)) is exchangeable with a cyanide ion upon addition of a solution of KCN, giving red-shifted absorption maxima at 650 and 713 nm (Fig. 4; Table 1). These maxima are similar to maxima observed at 652 and 716 nm for rMb(CoIII(CN)(TDHC)). This observation provides support for the formation of a monocyano-adduct of CoIII(TDHC) with His170 coordination in the heme-binding site of HRP. The affinity of cyanide for rHRP(CoIII(OH)(TDHC)) was determined by relaxation analysis and is indicated by a K value of 1.3 × 106 M−1 at pH 7.0 (Fig. 5), which is slightly higher than that of rMb(CoIII(CN)(TDHC)) (K = 8.6 × 105 M−1), whereas the K value is significantly lower than that of cyanocobalamin (K ≥ 1012 M−1) [45].
NMR spectral analysis
Figure 6 shows the 13C NMR spectra of rHRP(CoIII(CN)(TDHC)), in which Fig. 6a was obtained from a sample with 13C-enriched KCN. The differential spectrum (Fig. 6a, b) gives a broad peak at 121.8 ppm assigned as 13C-enriched cyanide bound to the cobalt center in the HRP matrix (Fig. 6c). Interestingly, only one peak was observed although rMb(CoIII(CN)(TDHC)) has two peaks in the 13C NMR differential spectrum because of the mixture of the two cofactor enantiomers in the asymmetric protein matrix (vide supra). This difference supports our hypothesis that apoHRP selectively binds one enantiomer of Co(TDHC).
Chemical shifts of the cyanide peak in cyano-cobalt corrinoids are listed in Table 2. In comparison with rMb(CoIII(CN)(TDHC)), benzimidazole-deligated cyanocobalamin (CNCbl(base-off)) and CoIII(CN)2(TDHC), the 13C-cyanide peak of rHRP(CoIII(CN)(TDHC)) undergoes a down-field shift whereas the peak shows an up-field shift compared to dicyanocobalamin, (CN)2Cbl. Interestingly, the chemical shift of the 13C-cyanide peak of rHRP(CoIII(CN)(TDHC)) is generally consistent with the chemical shift seen in CNCbl(base-on). Taken together, these findings suggest that the stronger σ-donation from the cyanide ion to the cobalt ion in the HRP matrix relative to rMb(CoIII(CN)(TDHC)) is possibly due to the Co–CN bond of rHRP(CoIII(CN)(TDHC)) being similar to that of CNCbl(base-on).
IR spectral analysis
Figure 7 shows IR spectra of rHRP(CoIII(13CN)(TDHC)) and rHRP(CoIII(12CN)(TDHC)). The 13CN/12CN-differential spectrum clearly includes the typical isotope shift [The ratio of the wavenumber (ν 13CN/ν 12CN = 0.98) is consistent with the root square of inverse ratio of the reduced mass (μ 12CN/μ 13CN = 0.96)]. The stretching frequency of the cyanide ion in rHRP(CoIII(12CN)(TDHC)) which is assigned to the band at 2144 cm−1 is lower than that of rMb(CoIII(12CN)(TDHC)). The frequency is higher than that of both the mono- and dicyanocobalamins and Co(CN)2(TDHC) (Table 3), indicating a relatively weaker Co–CN bond. In contrast, the lower stretching frequency of the cyanide ion in rHRP(CoIII(CN)(TDHC)) is due to strong π-back donation from the cobalt atom to the cyanide ion, indicating that the Co–CN bond is strengthened relative to that of rMb(CoIII(CN)(TDHC)).
Determination of pK 1/2 values for reconstituted HRPs
The pK 1/2 value for each reconstituted HRP, which represents the pH value corresponding to a 50% loss of the cofactor from the HRP matrix via the cleavage of the metal–N(His170) bond, was determined by fitting the titration curve (Fig. 8; Table 4). From the UV–Vis spectral changes of rHRP(CoIII(OH)(TDHC)) and rHRP(CoII(TDHC)) which occur when the pH decreases, the pK 1/2 values were determined to be 3.2 and 3.3, respectively. rHRP(CoII(TDHC)) has a smaller pK 1/2 value than rMb(CoII(TDHC)), whereas the rHRP(CoIII(OH)(TDHC)) has the same pK 1/2 value as rMb(CoIII(OH2)(TDHC)), indicating that the imidazolate ligation in the HRP matrix provides more stable binding of the Co(II) species compared to imidazole ligation in the Mb matrix. Interestingly, the pK 1/2 value of rHRP(CoIII(CN)(TDHC)) is the same as that of rHRP(CoIII(OH)(TDHC)), while the pK 1/2 value of rMb(CoIII(CN)(TDHC)) is 2.3 units higher than that of rMb(CoIII(H2O)(TDHC)). These results indicate that axial ligation of imidazolate-like His170 remains significantly strong even when the strongly electron-donating cyanide ligand is bound in the opposite axial position.
Conclusion
HRP reconstituted with cobalt tetradehydrocorrin replicates the imidazolate-like His ligation which is common to a number of cobalamin-dependent enzymes. The reconstituted CoII protein was characterized by UV–Vis absorption, CD and EPR spectroscopy to confirm His170-ligation to CoII in the HRP matrix. The redox potential for Co(I)/Co(II) in reconstituted HRP is negatively shifted relative to similarly reconstituted myoglobin, indicating strong ligation by the imidazolate-like His residue. In addition, 13C NMR and IR studies of rHRP(CoIII(CN)(TDHC)) indicate that cyanide interacts more strongly with CoIII(TDHC) relative to reconstituted myoglobin. These results also indicate that the imidazolate-like His170 axial ligand exerts a push effect on the cobalt center. The hydroxy species of CoIII(TDHC) also appears to be stabilized possibly due to the second coordination sphere in the distal site of HRP matrix compared with the Mb matrix. Thus, compared to rMb(Co(TDHC)), the Co(II) cofactor in rHRP(Co(TDHC)) is relatively stabilized and the Co(III) species interacts with cyanide strongly due to the imidazolate ligation between the axial His and Co center. Investigations of model reactions of native enzymes using the present system are now in progress.
References
Kräutler B (2009) In: Sigel A, Sigel H, Sigel RKO (eds) Metal ions in life science. RSC, Cambridge, pp 1–51
Kräutler B, Puffer B (2012) In: Kadish KM, Smith KM, Guilard R (eds) Handbook of porphyrin science. World Scientific, Singapore, pp 131–263
Kräutler B, Ostermann S (2003) In: Kadish KM, Smith KM, Guilard R (eds) The Porphyrin handbook. Academic Press, San Diego, pp 229–276
Gruber K, Puffer B, Kräutler B (2011) Chem Soc Rev 40:4346–4363
Drennan CL, Huang S, Drummond JT, Matthews RG, Ludwig ML (1994) Science 266:1669–1674
Matthews RG (2001) Acc Chem Res 34:681–689
Brown KL (2005) Chem Rev 105:2075–2150
Buckel W, Golding BT (1996) Chem Soc Rev 25:329–337
Dong S, Padmakumar R, Maiti N, Banerjee R, Spiro TG (1998) J Am Chem Soc 120:9947–9948
Friedrich P, Baisch U, Harrington RW, Lyatuu F, Zhou K, Zelder F, McFarlane W, Buckel W, Golding BT (2012) Chem Eur J 18:16114–16122
Brooks AJ, Fox CC, Marsh ENG, Vlasie M, Banerjee R, Brunold TC (2005) Biochemistry 44:15167–15181
Brooks AJ, Vlasie M, Banerjee R, Brunold TC (2004) J Am Chem Soc 126:8167–8180
Bucher D, Sandala GM, Durbeej B, Radom L, Smith DM (2012) J Am Chem Soc 134:1591–1599
Masuda J, Shibata N, Morimoto Y, Toraya T, Yasuoka N (2000) Structure 8:775–788
Calafat AM, Marzilli LG (1993) J Am Chem Soc 115:9182–9190
Jarrett JT, Choi CY, Matthews RG (1997) Biochemistry 36:15739–15748
Jarrett JT, Amaratunga M, Drennan CL, Scholten JD, Sands RH, Ludwig ML, Matthews RG (1996) Biochemistry 35:2464–2475
Matthews RG, Koutmos M, Datta S (2008) Curr Opin Struct Biol 18:658–666
Mancia F, Keep NH, Nakagawa A, Leadlay P, McSweeney S, Rasmussen B, Diat O, Evans PR (1996) Structure 4:339–350
Reitzer R, Gruber K, Jogl G, Wagner UG, Bothe H, Buckel W, Kratky C (1999) Structure 7:891–902
Murakami Y, Aoyama Y, Tokunaga K (1980) J Am Chem Soc 102:6736–6744
Liu C-J, Thompson A, Dolphin D (2001) J Inorg Biochem 83:133–138
Dommaschk M, Thoms V, Schütt C, Näther C, Puttreddy R, Rissanen K, Herges R (2015) Inorg Chem 54:9390–9392
Sonnay M, Fox T, Blacque O, Zelder F (2016) Chem Sci 7:3836–3842
Zipp CF, Michael JP, Fernandes MA, Nowakowska M, Dirr HW, Marques HM (2015) Inorg Chem Commun 57:15–17
Galezowski W (2005) Inorg Chem 44:1530–1546
Hisaeda Y, Masuko T, Hanashima E, Hayashi T (2006) Sci Technol Adv Mater 7:655–661
Murakami Y, Kikuchi J, Hisaeda Y, Hayashida O (1996) Chem Rev 96:721–758
Hayashi T, Morita Y, Mizohata E, Oohora K, Ohbayashi J, Inoue T, Hisaeda Y (2014) Chem Commun 50:12560–12563
Morita Y, Oohora K, Sawada A, Doitomi K, Ohbayashi J, Kamachi T, Yoshizawa K, Hisaeda Y, Hayashi T (2015) Dalton Trans 45:3277–3284
Morita Y, Oohora K, Mizohata E, Sawada A, Kamachi T, Yoshizawa K, Inoue T, Hayashi T (2016) Inorg Chem 55:1287–1295
Morita Y, Oohora K, Sawada A, Kamachi T, Yoshizawa K, Hisaeda Y, Hayashi T (2017) Inorg Chem. doi:10.1021/acs.inorgchem.6b02482
Gajhede M, Schuller DJ, Henriksen A, Smith AT, Poulos TL (1997) Nat Struct Biol 4:1032–1038
Dawson H (1988) Science 240:433–439
Tamura M, Asakura T, Yonetani T (1972) Biochim Biophys Acta 268:292–304
DiNello RK, Dolphin DH (1981) J Biol Chem 256:6903–6912
Fruk L, Muller J, Niemeyer CM (2006) Chem Eur J 12:7448–7557
Matsuo T, Murata D, Hisaeda Y, Hori H, Hayashi T (2007) J Am Chem Soc 129:12906–12907
Teale FWJ (1959) Biochim Biophys Acta 35:543
Hayashi T (2010) In: Kadish KM, Smith KM, Guilard R (eds) Handbook of porphyrin science. World Scientific, Singapore, pp 1–69
Neya S, Suzuki M, Hoshino T, Kawaguchi AT (2013) Inorg Chem 52:7387–7393
Matsuo T, Dejima H, Hirota S, Murata D, Sato H, Ikegami T, Hori H, Hisaeda Y, Hayashi T (2004) J Am Chem Soc 126:16007–16017
Reijerse EJ, Sommerhalter M, Hellwig P, Quentmeier A, Rother D, Laurich C, Bothe E, Lubitz W, Friedrich CG (2007) Biochemistry 46:7804–7810
Banerjee RV, Harder SR, Ragsdale SW, Matthews RG (1990) Biochemistry 29:1129–1135
Hayward GC, Hill HA, Pratt JM, Vanston NJ, Williams RJ (1965) J Chem Soc 6485–6493
Kenneth LB (1999) In: Banerjee R (ed) Chemistry and biochemistry of B12. Wiley, New York, pp 197–237
Brown KL, Hakimi MJ (1984) Inorg Chem 23:1756–1764
Murakami Y, Aoyama Y, Nakanishi S (1976) Inorg Nucl Chem Lett 12:809–812
Pratt JM (1999) In: Banerjee R (ed) Chemistry and biochemistry of B12. Wiley, New York, pp 73–112
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research provided by JSPS KAKENHI Grant Numbers JP15H05804, JP24655051, JP15H00944, JP22105013, and JP16H00837, the JSPS Japanese–German Graduate Externship, and JST PRESTO (JPMJPR15S2).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oohora, K., Tang, N., Morita, Y. et al. Cobalt tetradehydrocorrins coordinated by imidazolate-like histidine in the heme pocket of horseradish peroxidase. J Biol Inorg Chem 22, 695–703 (2017). https://doi.org/10.1007/s00775-017-1458-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-017-1458-z