Abstract
Tetracyclines coordinate metal(II) ions under physiological conditions forming chelate complexes with their ketoenolate moiety at rings B and C. These metal(II) complexes are the biologically relevant molecules conferring the antibiotic character of the drug by inhibiting ribosomal protein biosynthesis in prokaryotes. The Tet repressor, TetR, is the molecular switch for tetracycline resistance determinants in gram-negative bacteria. TetR controls transcription of a gene encoding the integral membrane protein TetA, which mediates active efflux of a tetracycline–metal(II) cation, [MeTc]+, by equimolar antiport with a proton. We evaluated distinct characteristics of the metal binding by crystal structure determination of TetR/[MeTc]+ complexes and of association equilibrium constants of [MeTc]+ and TetR/[MeTc]+ complexes. Various divalent metal ions bind to the same octahedral coordination site, defined by a histidine side chain of TetR, the tetracycline, and three water molecules. Whereas association constants for [MeTc]+ vary within 3 orders of magnitude, association of the [MeTc]+ cation to TetR is very similar for all measured divalent metals. Taking intracellular cation concentrations into account, it is evident that no other metal ion can compete with Mg2+ for TetR/[MeTc]+ complex formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The expression of tetracycline resistance in gram-negative bacteria is negatively regulated by the Tet repressor (TetR). This dimeric protein is a typical prokaryotic repressor, which binds in absence of the inducer tetracycline to specific operator-DNA sequences of the regulatory region of the Tet operon. Binding of the tetracycline–metal(II) cation [MeTc]+ to TetR causes conformational changes of the protein and dissociation of the specific operator/repressor complex [1–3]. This allows transcription of both the repressor gene tetR and, at higher drug concentrations, the resistance gene tetA [4]. The resistance protein TetA is responsible for the active efflux of the [MeTc]+ cation coupled to the uptake of a proton [5]. Variants of this type of resistance determinants were isolated from different bacteria and up to now 12 TetR sequences (classes A–E, G, H, J, 30, 31, 33, and Z) have been characterized [6]. The polypeptides of TetR classes B and D [TetR(B) and TetR(D)] show 65% sequence identity. Consequently, they have isostructural polypeptide folds and equivalent magnesium binding sites, as shown by crystal structure analysis of their tetracycline complexes [7].
Previous fluorescence measurements on TetR(B) established the importance of divalent cations (Mg2+, Ca2+, Ba2+, and Zn2+) for the biological function [8]. The functional role of the metal(II) ion, Me2+, is obvious from crystal structures of the TetR/[MgTc]+ complexes [7, 9]. The antibiotic binds as [MgTc]+ in a very deep tunnel-like pocket of TetR (Fig. 1), with Mg2+ octahedrally coordinated by chelating tetracycline (via β-diketonate O11, O12), TetR (imidazole Nε2 of His100) and three meridionally arranged water molecules (Fig. 2b). The aqua ligands are part of the extended [MgTc]+ recognition pattern of TetR. Two of these water molecules form hydrogen bonds to the carboxylate side chain of Glu147 and the third one is hydrogen-bonded to Thr103Oγ. The magnesium-binding side chains of His100 and Thr103 are part of the β-turn, which is at the end of a short α-helix in the DNA-binding conformation of TetR [1]. The conformational transition of α-helix to β-turn was neither observed in crystal structures of free TetR nor in TetR complexes with 7-chlorotetracycline (7ClTc) in the absence of divalent metal ions [10, 11]. Thus, magnesium coordination in the TetR binding site was identified as essential for the mechanism of induction by classic tetracyclines.
All-helical structure of the Tet repressor class D [TetR(D)] homodimer (lilac and blue helices) in complex with [Co7HTc]+ (Protein Data Bank entry 2VKE). The antibiotic (yellow van der Waals spheres and red metal ion) is completely buried in the recognition site of the core domain. The N-terminal three-helix bundles comprise the helix–turn–helix motifs of the DNA-binding domains. The N and the C terminus of one monomer are labeled
a Chemical structure of tetracycline (7HTc) and Mg2+ binding site. The common atom numbering of the tetracene framework is given, and the ring system is labeled A–D. Oxygens are numbered by the C atom they are bound to. b Coordination sphere of Mg2+ and Co2+ ions in the TetR(D) complex with tetracycline (7HTc) (Protein Data Bank entries 2TRT and 2VKE, respectively). The orientation of the complexes is based on the superposition of the corresponding TetR(D) monomers. The octahedral coordination geometry comprises the ketoenolate of tetracycline (O11 and O12), three water molecules, and the imidazole side chain of His100 of TetR(D). The complex with Mg2+ is shown in faint colors, the complex with Co2+ in darker colors
The [MgTc]+ complex has to be considered as the functional unit not only for TetR and TetA, but according to recent crystal structure analysis also for the elongation factor Tu [12] and for the prokaryotic ribosomal target, where the antibiotic binds and interferes with polypeptide elongation [13].
TetR interactions with tetracycline were studied by Raman resonance spectroscopy using Ni2+ instead of Mg2+ because it quenches fluorescence, a prerequisite for Raman resonance experiments [14]. We studied the metal binding site by X-ray crystal structure analysis of TetR(D) in complex with 7ClTc with Ni2+ ions and in complex with tetracycline (7HTc) with Co2+ ions. The affinity of different metal(II) ions for tetracycline and of the respective [MeTc]+ cations for TetR(D) was analyzed by determining their binding constants with spectroscopic methods.
Materials and methods
Proteins, tetracyclines, and divalent cations
TetR(D) was expressed and purified as described in [10]. Tetracyclines were purchased from Sigma in the form of their hydrochlorides and used as provided. Tetracycline was dissolved in degassed H2O and used for a maximum of 24 h. No oxidation was detected during this time by measuring the UV spectrum and binding constant to TetR. The concentration was determined by UV absorption of a dilution in 0.1 M HCl at 355 nm (ε 355nm = 13,320 M−1 cm−1 [8]). Divalent cations were provided as the salts MgCl2·6H2O, CaCl2·2H2O, Sr(NO3)2·2H2O, BaCl2·2H2O, MnSO4, (NH4)2Fe(SO4)2·6H2O, CoCl2·6H2O, NiSO4·6H2O, CuSO4·5H2O, ZnCl2, and CdCl2·2.5H2O (Merck or Fluka, purissima or pro analysi).
Crystallographic analyses
Yellow single crystals of the TetR(D)/[Mg 7ClTc]+ complex were grown using the hanging-drop vapor-diffusion method at 291 K. A mixture of 10 μl protein solution [9 mg/ml TetR(D), 2 mM 7ClTc, 2 mM MgCl2, 200 mM NaCl, and 10 mM tris(hydroxymethyl)aminomethane (Tris)/HCl, pH 8.0] and 5 μl reservoir solution [1.0 M (NH4)2SO4, 200 mM NaCl, and 20 mM Tris/HCl (pH 8.0)] were allowed to equilibrate against 1 ml reservoir solution [11]. These crystals (tetragonal space group I4122, a = b = 68.9 Å, c = 180.9 Å) were soaked overnight in their crystallization buffer containing 1 mM concentration of the corresponding metal(II)sulfates (Me2+ is Fe2+, Co2+, Ni2+, Zn2+, Cd2+) for Mg2+ → Me2+ exchange. X-ray diffraction data were collected at 277 K using a Marresearch imaging plate detector on a rotating anode X-ray generator (ENRAF NONIUS FR571, Cu Kα radiation, graphite monochromator). The data were processed using the HKL-2000 package [15]. Crystallographic computing was performed using the CCP4 program suite [16], and the program O for electron density map inspection and model building [17]. The sites of Fe2+, Co2+, Ni2+, Zn2+, and Cd2+ were located in difference Fourier maps, using observed structure factor amplitudes of the corresponding TetR(D)/[Me7ClTc]+ complex with phases and structure factor amplitudes calculated on the basis of the polypeptide model of the known TetR(D)/[Mg7ClTc]+ complex (Protein Data Bank entry 2TCT), excluding [Mg7ClTc]+ and all water molecules. The best diffraction data were obtained with a Ni2+-soaked crystal and were used for crystallographic structure refinement using REFMAC5 [18]. Statistics of this diffraction data set and the refined structure are given in Table 1.
Cocrystallization was used to obtain crystals of the repressor with Co2+ and tetracycline by the hanging-drop vapor-diffusion method at 298 K. Yellow single crystals of the TetR(D)/[Co7HTc]+ complex were grown mixing 2 μl protein solution [9 mg/ml TetR(D), 1 mM 7HTc, 25 mM Tris (pH 8.0), 100 mM NaCl] with the same volume of reservoir solution [200 mM NaCl, 0.8 M ammonium sulfate, 50 mM Tris/HCl (pH 7.5), 5 mM CoCl2] and equilibrated against 500 μl reservoir solution. These crystals are isomorphous to the previous crystals of TetR(D)/[MeTc]+. X-ray diffraction data were collected at 110 K (Cryostream, Oxford Cryosystems, Oxford, UK) using a Saturn92 CCD on a rotating anode X-ray generator (Micromax007, Cu Kα radiation, Osmic multilayer mirrors, Rigaku MSC, Kemsing, UK). Dry paraffin oil (Jena Bioscience, Jena, Germany) was used as a cryoprotectant. The data were processed using the CrystalClear package [19]. Crystallographic computing was performed using the CCP4 program suite [16], and the program COOT for electron density map inspection and model building [20]. To confirm the identity of the metal ion, the model without a metal ion was refined with REFMAC5, and the phases were used to calculate an anomalous map. All measured data up to 1.62-Å resolution were used for refinement, even though the data beyond 1.8 Å were incomplete and improved R free only little (by 0.001). Statistics of this diffraction dataset and the refined structure are also given in Table 1.
Structure alignment of homodimers of TetR complexes was performed with ALIGN [21] using the default option for superposing Cα atoms.
pK a values of tetracycline
UV spectra of 20 μM 7HTc were measured in solutions containing 150 mM NaCl plus HCl (pH 0–2), 100 mM malonate buffer (pH 3–6), or 100 mM Tris/glycine buffer (pH 7–9). Absorption at 300 and 315 nm was fitted using the Henderson–Hasselbalch equation with the pK a values and the absorption of the species given in Fig. 3 as parameters. The same buffers were used in the presence of 1 M MgCl2 to measure pK a values of the magnesium complexes. The high Mg2+ concentration was chosen to ensure complex formation.
a Titration of 7HTc in the presence (closed diamonds) and the absence (open diamonds) of magnesium ions. b Association constants K Ass(pH-dependent) for [MeTc]+ formation at different pH values (pK Ass = −log K Ass). c Protonation and complexation states of 7HTc. The pK a and K Ass values were calculated from the experiments shown in a and b. The association constants given in this figure are for the pure protonation states [K Ass(pH-independent)]. At pH 8.0, only 70% of the tetracycline molecules exist as 7HTcH−; the measured K Ass (given in Table 3) is thus correspondingly smaller
Equilibrium constants
The association constants for association of the metal ions to tetracycline (K Ass,MeTc) were obtained by measuring UV absorption (Cary50Bio, Varian) while titrating the tetracycline solution [approximately 10–20 μM in 100 mM Tris/HCl (pH 8.0), 150 mM NaCl] with Me2+ solutions (0.1–1,000 mM) (Me2+ is Mg2+, Ca2+, Sr2+, Ba2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+). For Fe2+ the pH of the buffer had to be lowered and a reducing agent added [100 mM N-(2-hydroxyethyl)piperazine-N′-ethanesulfonic acid/NaOH (pH 7.0), 150 mM NaCl, 10 mM NaPH2O2 (Riedel-de Haen, pro analysi)] to prevent oxidation and precipitation. The wavelength used for analysis was 390 nm; other wavelengths (for example, 415 nm when measuring at higher pH) yielded the same results. No kinetic effects were observed on the timescale of the titration (10 s to 10 min). The model used to fit the experimental data was based on the equilibrium
and mass balances
and
This results in
If only Tc− and [MeTc]+ contribute to the absorption, characterized by their respective ε Tc and ε MeTc, then
was used for fitting. Absorption of the metal ion itself extends the formula to
The parameters ε Tc, ε MeTc and K Ass (and where necessary ε Me) were fitted with a least-squares target in Excel or our own program (G.J.P.) to calculate errors.
TetR(D)/[MgTc]+ binding constants (K Ass,TetRMeTc) were obtained by fluorescence titration (fluorimeter LS50B, PerkinElmer) using at least one of the following three methods. The same buffer (50 mM Tris/HCl (pH 8.0), 150 mM NaCl) was used for all titrations (including measurements with Fe2+). In some cases slow equilibration was observed; therefore, a fixed time schedule was used for all titrations, adding the titrant every 2.5 min and measuring fluorescence at two consecutive readouts of 5 s each after 2 min.
Method A
Protein solution (0.05–1 μM) in buffer with sufficient Me2+ concentration to ensure complete [MeTc]+ formation was titrated with equal amounts of a tetracycline stock solution (3–10 μM) (cf. [22]). Background, [MeTc]+, and TetR/[MeTc]+ contribute to the fluorescence,
(i MeTc and i TetRMeTc are the relative fluorescence intensities of the respective species). With the simplification of
the ternary complex concentration can be explicitly calculated,
At lower metal ion concentration, [MeTc]+ dissociation has to be taken into account,
and [TetRMeTc] cannot be calculated explicitly anymore. Numerical solution, though, of the implicit equations shows that the above simplifications are valid at the metal concentrations used in this work.
Method B
Protein solution (0.05–1 μM) in buffer with 0.1–50 mM Me2+ and a fivefold to tenfold excess of 7HTc over protein was titrated with increasing amounts of Mg2+ (1.5-fold to twofold more in each step). Evaluation of these curves gives the preference of Me2+ binding over Mg2+ binding, according to the reaction
with
As discussed later, the corrected exchange constant, K Ex, was then calculated with
The average of these corrected exchange constants was used to calculate the association constants.
Method C
Protein solution (0.05–1 μM) in buffer with 0.1–10 mM Mg2+ and a fivefold to tenfold fold excess of 7HTc over protein was titrated with increasing amounts of Me2+ (1.5-fold to twofold increase in each step). Evaluation was done as for method B.
The choice of method was dictated by the fluorescence of the corresponding complex and absorption, quenching effects, and solubility of the divalent cation. Fluorescence quenching (Mn2+, Fe2+, Co2+, and Ni2+) prohibits use of method A. Method B was used for all Me2+, but the concentration of Fe2+ was limited by its fluorescence quenching effect and that of Mn2+ by its solubility. Method C was used only for a few metals to check the consistency of the other methods, since it is hampered by absorption and quenching effects of millimolar transition metal ion solutions.
Results
Structure of TetR(D) tetracycline complexes with various metal ions
It is possible to obtain TetR(D)/[Me7ClTc]+ complexes by cocrystallization of TetR(D) with Me2+ and 7ClTc, but some of the unchelated metal(II) ions precipitated the protein. Therefore, we first used soaked crystals for X-ray diffraction data collection. Owing to difficulties with crystal handling, the diffraction quality of most of the soaked crystals was limited to 3–3.5-Å resolution. Initial difference Fourier maps of datasets from TetR(D)/[Mg7ClTc]+ and the corresponding [Me7ClTc]+ complexes identified unambiguously the isomorphous exchange of the Mg2+ ion by other divalent metal ions (Me2+ is Fe2+, Ni2+, Co2+, Zn2+ and Cd2+). The cadmium compound was described as one of the heavy atom derivatives used for initial structure determination with multiple isomorphous replacement [7, 9]. The high sulfate concentration of the crystallization buffer for TetR/[MeTc]+ prohibited addition of heavier group II ions other than Mg2+ owing to the low solubility of calcium, strontium, and barium sulfate.
Structure of the TetR(D)/[Ni7ClTc]+ complex
We obtained complete diffraction data up to 2.2-Å resolution for a Ni2+-soaked crystal and performed crystallographic structure refinement to a final R factor of 14.6% (Table 1; Protein Data Bank entry code 2FJ1). The crystal structure is isomorphous and the geometry very similar to that of the Mg2+ complex as indicated by the root mean square deviation of 0.27 Å for the Cα atoms of the respective homodimers. The [Ni7ClTc]+ complex of TetR shows similar octahedral coordination geometry for the Ni2+ ion as known for the corresponding Mg2+ complex. Details of the coordination geometry are given in Table 2 and Fig. 2b.
Structure of the TetR(D)/[Co7HTc]+ complex
The complex of TetR(D) with tetracycline could be cocrystallized with Co2+. The overall structure also shows no difference from that of the corresponding Mg2+ complex (alignment of the Cα atoms of the respective dimers, 2TRT, and TetR(D)/[Co 7HTc]+, root mean square deviation 0.47 Å). Also, binding of the tetracycline as well as coordination of the metal ion are very well conserved. The Co2+ ion is octahedrally coordinated with angles between neighboring ligands ranging only from 78° to 98° (Mg2+ complex 71–114°). Metal–ligand distances are slightly larger for Co2+ (average 2.07 Å) than for Mg2+ (1.98 Å) but smaller than for Ni2+ (2.13 Å) (Table 2). The identity of the Co2+ ion is unambiguously confirmed by anomalous dispersion. Phases of the refined structure were applied to the anomalous differences to calculate an anomalous difference electron density map. The highest peak in this map indicated the Co2+ position with 40σ. This peak is not created by the bias of Co2+ being part of the model as phases calculated with REFMAC5 from a model before including any atom at this position also gave 33σ. The only other peaks above 3σ in the anomalous map are three chloride positions (5.5σ, 5σ, and 4σ) beside the sulfur atoms of Met120 (5.5σ), Met84 (3σ), and Met177 (3σ).
pK a values of tetracycline
Schneider [23] reports pK a values of 3.3–3.4, 6.7–7.7, and 8.7–9.5 for the three deprotonation steps of tetracycline. These data are notoriously difficult to measure, presumably owing to slow transitions (10 min to hours) between different conformers and tautomers [23]. We have remeasured these data to obtain values consistent with our conditions (buffer composition, timescale of measurement, detection method). The pK a values are in the same range as previously measured (Fig. 3c). The pK a values for the magnesium complexes are lower owing to the more positive charge. Values for pK a(7HTcH−) and pK a(Mg7HTcH+) are at the border of the pH range measured and were not calculated. Consequently, K Ass(Mg2+–7HTc2−) is only estimated.
Association constants for the [Me7HTc]+ complex
Formation of tetracycline complexes is strongly dependent on their protonation status [24–26]. The measurements reported here were performed at pH 8.0. The species forming the complexes at this pH is predominantly TcH− with two deprotonated groups, C(12)–OH (pK a 7.65) and C(3)–OH (pK a 3.27), and one protonated group, C(4)–NH(CH3)2 (pK a ~9.4). Protonation at C(12)–OH prevents complex formation and deprotonation at C(4)–NH(CH3)2 gives rise to a spectroscopically distinct complex. The pH dependency of the association constants K Ass,MgTc (Fig. 3b) can be explained by the model shown in Fig. 3c. Based on only the equilibria
the measured, pH-dependent K Ass(pH) is diminished by the fraction of 7HTcH−. This can be accounted for by calculating
from a pH-independent Mg2+–7HTc− association constant K Ass(pH-independent) (Fig. 3c). This explains the pH-dependent changes observed in the range pH 5.5–8.5 (best fitted with K Ass(pH-independent) = 3,310 M−1 when fixing pK a(7HTcH2) = 7.65). At higher pH not only Tc2− and [MgTc]+ can form (Fig. 3c), but also [Mg2Tc]2+, which both result in deviation from the predicted curve towards stronger complex formation (lower pK Ass). Chelate complex formation with Mg2+ occurs via the 1,3-diketoenolate moiety at positions 11 and 12 [27, 28]. For Ca2+ at pH 7 and a variety of other divalent cations this probably holds true; for Ca2+ at pH 8.5 and softer cations, which also easily accept nitrogen as a ligand, coordination to O1 or N4 has also been described [23]. In the TetR/[MeTc]+ complex structures these alternative binding modes are never observed. We determined the association constants for association of tetracycline to different metal ions at pH 8.0 (K Ass,MeTc; Table 3, Fig. 4a) measured by the change of tetracycline absorption caused by binding of Me2+. These K Ass values are the pH-dependent ones; the K Ass(pH-independent) values are 1.45 times larger than at pH 8.0. For Fe2+, buffers with pH 7.0 were used in the experiment because precipitation occurs at higher pH. In addition, all buffers were degassed and prepared directly before the experiment to minimize oxidation of Fe2+. With the formula for the pH-independent K Ass, the K Ass,MeTc value measured at pH 7.0 was used to calculate a K Ass at pH 8.0. This calculated K Ass,Fe7HTc of 115,600 M−1 is the highest among all measured association constants. K Ass,Fe7HTc should be compared with the K Ass,MeTc values of the other metals with caution, because of pH extrapolation and possible effects of the necessary buffer changes. The association constants K Ass,Me7HTc vary 500-fold between the strongest binding ions (Co2+ and Ni2+, Fe2+ is possibly stronger) and the weakest binding Me2+ ion (Ba2+). Within the group of alkaline-earth metals, the K M values decrease almost 25-fold with increasing ionic radii. In contrast, the K Ass,MeTc of the transition metals increased up to 20-fold compared with Mg2+ (almost 50-fold for Fe2+). All measured 3d transition metal ions bind tighter to 7HTc than Mg2+; the 4d transition metal ion Cd2+ has a lower K Ass,Me7HTc than Mg2+.
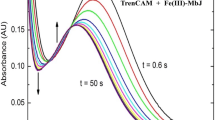
Measurement of binding constants. a Determination of K Ass,MeTc: titration of tetracycline (7HTc, 20 μM) with Me2+, here Cd2+, measured by absorption spectroscopy. CdCl2 is added in increasing amounts of 1, 10, 100, and 1,000 mM stock solutions. The absorbance has been corrected for the dilution effect. b Determination of K Ass,TetRMeTc. Method A: titration of TetR(D) at saturating Mg2+ concentration with 7HTc measured by fluorescence spectroscopy. The increase of fluorescence due to TetR/[Mg7HTc]+ formation levels off, when the 7HTc concentration reaches the starting TetR(D) concentration (0.24 μM). c Determination of K Ex(Me1 → Me2). Method B: titration of TetR(D)/[Me7HTc]+ with Mg2+ measured by fluorescence spectroscopy. Here Ba2+ in TetR(D)/[Me7HTc]+ is replaced by Mg2+ with an apparent K Ex,app(Mg → Me) of 0.40. The exchange constant, K Ex,app(Mg → Ba) can be estimated from the comparison of the starting Ba2+ concentration (1 mM) with the Mg2+ concentration at the midpoint of the exchange (approximately 0.4 mM): K Ex,app(Mg → Ba) = 0.4 mM/1 mM = 0.4. This apparent K Ex,app has to be corrected for the starting Ba2+ concentration to obtain the real K Ex = K Ex,app × 0.001 M/(0.001 M + 1/107 M−1) = 0.039. Determination of K Ex by method C results in analogous graphs. Tc tetracycline
TetR(D)/[Me7HTc]+ complex formation
We also determined the binding constants for binding of [MeTc]+ to TetR(D) to verify the influence of different metal ions on complex formation (K Ass; Table 4). All experiments were done using fluorescence measurements. K Ass,TetRMeTc values were determined either directly by titrating the protein with the [MeTc]+ complex (method A, Fig. 4b) or relative to K Ass,TetRMgTc by measuring the exchange of Me2+ by Mg2+ (method B, Fig. 4c) or Mg2+ by Me2+ (method C). As a quantitative measure, we introduced the exchange constant, K Ex, which specifies how much more strongly [MeTc]+ binds to TetR(D) compared with [MgTc]+. The measurements of the exchange constants are more precise than those of the association constants and were therefore used for calculation of the final K Ass,TetRMeTc. In contrast to simple theory, K Ex values changed with different initial metal ion concentrations (thus it was called the apparent exchange constant, K Ex,app). A corrected K Ex was calculated by an empirical formula (see “Materials and methods”). As no theoretical basis for this formula can be given, all measured K Ex,app values are given in Table 4 to enable future testing of any new model and formula. The weakest binding was revealed by the alkaline-earth metal [MeTc]+ complexes (Ca2+, Sr2+, Ba2+) which showed an up to threefold decreased K Ass,TetRMeTc compared with [MgTc]+. For the transition metals K Ass,TetRMeTc is slightly higher and very similar for most ions; [CuTc]+ binds 20% more tightly and [Zn Tc]+ 20% more weakly than [MgTc]+. The only exception seems to be [FeTc]+. Evaluation by the same method as for all other cations does not result in consistent values. The likely reasons are problems with poor solubility of Fe(OH)2 and the problems in determining K Ass,Fe7HTc discussed above.
Discussion
The interaction of tetracyclines with biological macromolecules is mediated by divalent metal ions [29]. This is also the case for the prokaryotic ribosomal target [13] and the corresponding repressor controlled tetracycline resistance mechanism of gram-negative bacteria [4]. The membrane protein TetA exports a [MeTc]+ cation through the cytoplasmic membrane coupled to the uptake of a proton [5]. The expression of this antiporter is controlled by the repressor TetR, which is induced by the antibiotic tetracycline depending on the presence of divalent metal ions [6]. Three-dimensional structures of [MgTc]+ complexes with TetR confirmed the essential role of the metal(II) ion [11]. We demonstrated that various divalent cations (Ca, Sr, Ba, Mn, Fe, Co, Ni, Cu, Zn, and Cd) can replace the Mg2+ ion in the TetR/[MgTc]+ complex, and thus potentially exhibit biological activity. In vitro [FeTc]+ can induce transcription by binding to the repressor effectively [30].
Tetracyclines are known to coordinate to a variety of metal ions as monovalent or divalent anions, but three-dimensional data are only available from tetracycline–mercuric chloride and the dipotassium salt of a tetracycline dianion [31]. These compounds were crystallized under nonphysiological conditions and their metal coordination is completely different from the situation observed in the TetR complex. The β-diketonate oxygens O11 and O12 are K+ ligands as for Mg2+, but they bind two cations; these are out of plane of the BCD chromophore of tetracycline by about 1.85 Å. Additionally, O1 and O12a also coordinate K+ and the coordination numbers of the three K+ ions are 7 and 8. Very different is the coordination geometry in the HgCl2 complex, because only functional groups of ring A of the antibiotic bind to mercury. Electronic spectra and magnetic properties of Fe2+, Co2+, and Ni2+ complexes revealed octahedral coordination of the metal ions to oxygen atoms of two tetracycline anions, completed by two aqua ligands [32]. All of these coordination schemes do not resemble the chelation of divalent metal ions in the TetR/[MeTc]+ complex. Nonphysiological conditions of concentrations and solvent cause a metal coordination unlike in the protein complex. To form [MeTc2]2− complexes, concentrations of tetracycline in the millimolar range are necessary for Mg2+ and Ca2+ [33], whereas the blood level is only 10 μM after administration of normal doses. The influence of different solvents on the interaction between metal ions and tetracycline was investigated by IR, electron paramagnetic resonance, and UV/vis spectroscopy [27]. These results favor the metal coordination to the BCD chromophore in aqueous solutions, whereas the complexation of the A chromophore is observed in nonphysiological solvents. In solution the coordination geometry of the divalent metal ions with tetracycline substituents is not exactly known, but is expected to be BCD coordination as shown in Fig. 2a [23].
To characterize the reaction of metal(II) ions with tetracycline and/or TetR we measured acidic, association, and exchange constants for the following reactions:
-
\( {\text{TcH}} + {\text{H}}_{2} {\text{O}} \leftrightharpoons {\text{Tc}}^{ - } + {\text{H}}_{3} {\text{O}}^{ + } \)
-
Neglecting other species (cationic, doubly anionic, dimeric):
-
\( K_{{\text{A}}} = {\frac{{\left[ {{\text{Tc}}^{ - } } \right]\left[ {{\text{H}}_{3} {\text{O}}^{ + } } \right]}}{{\left[ {{\text{TcH}}} \right]}}} \)
-
-
\( {\text{Tc}}^{ - } + {\text{Me}}^{{2 + }} \leftrightharpoons \left[ {{\text{MeTC}}} \right]^{ + } \)
-
Assuming all free tetracycline is in the monoanion state ([Tc−] = [Tc]free):
-
\( K_{{{\text{Ass,MeTc}}}} ({\text{pH-dependent}}) = {\frac{{\left\lfloor {\left[ {{\text{MeTc}}} \right]^{ + } } \right\rfloor }}{{\left[ {{\text{Tc}}^{ - } } \right]\left[ {{\text{Me}}^{{2 + }} } \right]}}} \)
-
Taking the protonation state of tetracycline into account [pK a(TcH) 7.65]:
-
\( K_{{{\text{Ass,MeTc}}}} ({\text{pH} \hbox{-} \text{independent}}) = {\frac{{\left\lfloor {\left[ {{\text{MeTc}}} \right]^{ + } } \right\rfloor }}{{\left[ {{\text{Tc}}^{ - } } \right]\left[ {{\text{Me}}^{{2 + }} } \right]}}}{\frac{1}{{1 + 10^{{{\text{p}}K_{{\text{A}}} - {\text{pH}}}} }}} \)
-
-
\( {\text{TetR}} + \left[ {{\text{MeTc}}} \right]^{ + } \leftrightharpoons {\text{TetR}}/\left[ {{\text{MeTc}}} \right]^{ + } \)
-
\( K_{{{\text{Ass,TetRMeTc}}}} = {\frac{{\left[ {{\text{TetRMeTc}}} \right]}}{{\left[ {{\text{TetR}}} \right]\left[ {\left[ {{\text{MeTc}}} \right]^{ + } } \right]}}} \)
-
-
\( {\text{TetR}}/\left[ {{\text{Mg}}\,{\text{Tc}}} \right]^{ + } + {\text{Me}}^{{2 + }} \leftrightharpoons {\text{TetR}}/\left[ {{\text{MeTc}}} \right]^{ + } + {\text{Mg}}^{{2 + }} \)
-
\( K_{{{\text{Ex,app}}}} = {\frac{{\left[ {{\text{TetRMeTc}}} \right]\left[ {{\text{Mg}}^{{2 + }} } \right]}}{{\left[ {{\text{TetRMgTc}}} \right]\left[ {{\text{Me}}^{{2 + }} } \right]}}}. \)
As the data show, K Ex,app is not the equilibrium constant. With an experimentally found correction term a Me2+ concentration independent equilibrium exchange constant, K Ex, is calculated which describes all measured K Ex,app values simultaneously:
\(K_{{{\text{Ex}}}} = K_{{\text{Ex,app}}} {\frac{{\left[ {{\text{Me}}^{{2 + }}} \right]}}{{\left[ {{\text{Me}}^{{2 + }} } \right] + K_{{{\text{Ass,TcMe}}}}^{{- 1}} }}} = {\frac{{\left[ {{\text{TetRMeTc}}} \right]\left[ {{\text{Mg}}^{{2 +}} } \right]}}{{\left[ {{\text{TetRMgTc}}} \right]\left[ {{\text{Me}}^{{2 + }} }\right]}}}{\frac{{\left[ {{\text{Me}}^{{2 + }} } \right]}}{{\left[{{\text{Me}}^{{2 + }} } \right] + K_{{{\text{Ass,TcMe}}}}^{{ - 1}} }}}.\)
-
pK a of tetracycline
In the course of this work pK a values of tetracycline were remeasured. They are consistent with the range of previous measurements and are not discussed further here.
K Ass,MeTc and pH dependence
In the range of physiological pH values, divalent cations are in competition with the acidic proton (pK a 7.65) of the β-diketonate (O11, O12 of rings C and B, respectively) of tetracycline [31]. This has a predictable effect on the measured association constant: at pH values below pK a, K Ass decreases. At higher pH values, K Ass levels off until tetracycline loses another proton (pK a > 9) and binds the cation even more strongly. For Mg2+ the increase in binding is observed only at pH > 8.5. For Ca2+ the same low-pH effect has been confirmed by measurements of K Ass up to pH 9.0 (data not shown).
K Ass,MeTc and dependence on type of Me2+
The association constants for [Me7HTc]+ decrease for the heavier members of the alkaline-earth metals; they are higher for the 3d transition metals and smaller for cadmium in comparison with magnesium (Table 3). All cations binding more weakly than Mg2+, i.e., Ca2+, Sr2+, Ba2+, and Cd2+, have larger ionic radii and thus lower charge densities.
The monovalent alkali metal cations also bind very weakly because of their lower charge density (ionic radii of Li+, Na+, K+, Rb+, and Cs+ are 0.90, 1.16, 1.52, 1.66, and 1.81 Å, respectively [34], association constants are 25, 14, 11, 9, and 17 M−1, respectively [35]). The importance of the charge is evident from the weak binding of Li+, even though its radius is comparable to that of Mg2+.
Among the transition metal ions the K Ass values first increase (Mn2+ < Fe2+) then decrease (more or less) with atomic number. Two properties of these transition metals, tighter binding with decreasing ionic radius on one hand and weaker binding with decreasing oxophilicity on the other hand, could explain the two competing trends. There are not enough data, though, to prove a causative relationship.
K Ass,TetRMeTc and dependence on type and concentration of Me2+
Association constants for the ternary complexes (TetR/[MeTc]+) were measured with three different methods. Titration of the repressor with tetracycline at high metal ion concentrations (method A) cannot be applied for all metals owing to solubility and quenching problems. Exchanging the metal ion in TetR/[MeTc]+ by adding higher concentrations of Mg2+ (method B) was possible for all cations analyzed. The reverse exchange reaction (method C) was also used for cross-validation. It might be regarded as a disadvantage of methods B and C that the accuracy of K Ass,TetRMeTc relies on that of K Ass,TetRMgTc, because the association constant is not measured directly. On the other hand, the relative values for the Me2+ and magnesium complexes, which are more important for our purpose of comparison, were obtained directly and more precisely. Unexpectedly, the experiments show that the measured exchange constant is not the equilibrium exchange constant and is therefore called the apparent exchange constant, K Ex,app. It decreases with increasing Me2+ concentration used at the beginning of the experiment, reaching a plateau at 0.1–10 mM depending on the Me2+ type. A correction factor was applied to the apparent exchange constant:
The molecular basis for this correction is not clear. The reason seems not to be thermodynamic, e.g., binding to other binding sites or wrong stoichiometry of the reactants, because reversing the exchange reaction (method B vs. method C) gives the same results. Slow kinetics of binary and ternary complex formation might be a reason; a quantitative model could nevertheless not be established on that basis. These corrected exchange constants provide consistent values for all concentrations measured by one method (B or C) for each cation. For experimental reasons the transition metal concentrations used in methods B and C are much higher than their free intracellular concentrations in vivo (reaction times become very slow). As the corrected K Ex are constant over an up to 100-fold range, though, we are confident that K Ex is valid at low metal concentrations. The values obtained by methods B and C compare well, but some of the values obtained by method A differ significantly from those obtained by method B. In these cases the values obtained by method B are more reliable; method A was much more sensitive to experimental errors.
Binding of Me2+ to tetracycline (K Ass,MeTc) and that of the respective [MeTc]+ complex to TetR (K Ass,TetRMeTc) does not correlate in an obvious manner. The latter is rather almost independent of the metal ion. Only [Ca7HTc]+ and [Sr7HTc]+ bind twofold to threefold more weakly. This cannot simply be explained by the ionic radii; [Ba7HTc]+ and [Cd7HTc]+ should in this case also be weaker binders. The coordination number of these metal ions must also be taken into account. Mg2+, Mn2+, Co2+, Ni2+, Cu2+, Zn2+, and Cd2+ can form stable hexaaqua complexes. Their K Ass,TetRMeTc values are very similar and the differences in ionic radii play no significant role. Mn2+ and Zn2+ form the weakest complexes among them; they are the most likely to deviate from the octahedral coordination and form tetrahedral complexes. Ca2+, Sr2+, and Ba2+ support higher coordination numbers, for instance, 7, 8, and 8, respectively, in their aqua complexes (Table 3). This is reflected by the lower K Ass,TetRMeTc for Ca2+ and Sr2+, but not for Ba2+. There is no structural information for the TetR/[CaTc]+ and TetR/[SrTc]+ complexes available, but low-resolution crystallographic data of a TetR(B)/[barium anhydrotetracycline]+ complex suggest that Ba2+ not only replaces the Mg2+ ion but also the coordinating water molecules. The tetracycline binding pocket thus needs little adjustment despite the presence of a much larger cation.
Mg–Me exchange
For formation of the ternary complex TetRMeTc from all three components, both association constants, K Ass,MeTc and K Ass,TetRMeTc, have to be considered. Owing to the preferred binding of the transition metals to tetracycline the Mg2+ → Me2+ exchange constants are in favor of the transition metal ions. Therefore, cation exchange occurs simply by soaking the crystals of the TetR/[MgTc]+ complex with other metal(II) ions. Long-term soaking with Ni2+ has produced the crystal for the TetR/[Ni7ClTc]+ structure. In a 9-h soak of an apo TetR(D) crystal with [Co7HTc]+ no tetracycline bound to the repressor. This suggests that protein–protein contacts in the crystal lattice are blocking access to the binding site since the cocrystallization experiment at soaking conditions results in formation of the expected complex.
Me2+ coordination in TetR/[MeTc]+
In our X-ray studies of TetR/[MeTc]+ complexes the divalent cations Co2+ and Ni2+ bind to the same ligands with identical coordination geometry as described for the Mg2+ ion (Fig. 2b). A detailed discussion of the coordination polyhedron is limited because metal–ligand distances are in the resolution range of the X-ray diffraction data for the Co2+, Ni2+ and Mg2+ complexes. Some rather short distances of Mg–O coordination (2TRT and 2TCT) and the very long Mg–His100 Nε distance (2TRT) are questionable. This might be explained by weak data quality at the accepted resolution limits and imprecise definitions of constraints in the former refinement protocols of the TetR(D)/[MgTc] complexes [7, 9]. The recently published structure of TetR(D) in complex with doxycycline–Mg2+ at 1.9-Å resolution (Protein Data Bank entry 2O7O) is a more suitable target to discuss the metal binding site [25]. This TetR complex shows no significant deviations in bond lengths for the metal coordination compared with the cobalt and nickel complexes (Table 2). Taking the average of metal–ligand bond lengths, we find the volume of the coordination polyhedron is very similar in these complexes. Even the distance of the Me2+ to the coordinating imidazole of His100 does not reflect higher affinity of the 3d elements. It appears that the divalent metal ion has to fit into the coordination cavity in the TetR/tetracycline complex to establish appropriate positioning of the tetracycline to induce conformational changes that abolish TetR/DNA binding. This is in agreement with the abovementioned observation that [MeTc]+ binding to TetR is almost independent of the specific metal ion.
[MeTc]+ complexes with metals other than magnesium
Clinical applications of tetracyclines in the presence of high concentrations of divalent metal ions are critical. In extracellular body fluids (for instance, blood serum, saliva), Ca2+ is present in higher concentrations (approximately 2–3 mM) and [CaTc]+ is in fact deposited in teeth and bones of children, when tetracycline is given too early and at too high doses [36]. When zinc salts are administered orally together with tetracycline (at 4–8 mM Zn2+), formation of Zn2+ complexes limits the bioavailability of the metal as well as of the antibiotic in the gastrointestinal fluid [37].
TetR/[MeTc]+ complexes with biologically relevant metals
In vitro, Fe2+ and Co2+ complexes have been described. The interpretation of the site-specific oxidative cleavage of the polypeptide chain in TetR(B)/[FeTc]+ in presence of H2O2 and ascorbate [30] is in agreement with our structural results, because Fe2+ replaces the Mg2+ ion of the [MgTc]+ complex with TetR. One of the aqua ligands will be replaced to form an Fe3+–OOH complex, which is the source of hydroxyl radicals for nucleophilic attacks to carbonyl groups of the polypeptide backbone [38]. [CoTc]+ is more efficiently transported into inverted vesicles by the membrane protein TetA than the corresponding Mg2+ or Ca2+ complexes [5]. Thus, TetA seems to bind [CoTc]+ better than [MgTc]+, whereas TetR binds both complexes equally well. In addition, it has been shown that [FeTc]+ is about 1,000-fold more active in induction of TetR(B) than the magnesium complex [30]. [FeTc]+ and [MgTc]+ bind similarly to TetR(D), and presumably this is also true for TetR(B).
The drug recognition site in TetR is evolutionarily well designed to capture the [MeTc]+ complex, but this is almost independent of the divalent metal ion involved. Does this imply that the biologically relevant metal for the TetR/[MeTc]+ complex is not known? Are Fe2+ and Co2+ complexes relevant in vivo? In the metal ion exchange experiments, transition metal Me2+ ions are significantly preferred over Mg2+. The cases described above, though, are with either nonphysiologically high metal ion concentrations (Co2+, Fe2+) or in the extracellular matrix, where there is no TetR. To evaluate the situation in the cell we searched the literature for concentrations of freely available, biologically relevant cations (Table 5).
Except for Mg2+, all divalent metal ion concentrations are tightly controlled and kept very low in the cell [39]. No explicit data for free Sr2+ and Ba2+ concentrations were available, but they are expected to be lower than the anyway low total concentrations. For some transition metals the concentrations of freely available cations are known; they are below 1 nM for the common Zn2+ and at undetectable levels for free Fe2+ and Cu2+, which are toxic in their unbound form. For the other transition metal ions, the concentrations are expected to be comparably low. In the intracellular milieu no other ion can thus compete with Mg2+ for [MeTc]+ and TetR/[MeTc]+ formation.
References
Orth P, Schnappinger D, Hillen W, Saenger W, Hinrichs W (2000) Nat Struct Biol 7:215–219
Müller G, Hecht B, Helbl V, Hinrichs W, Saenger W, Hillen W (1995) Nat Struct Biol 2:693–703
Lederer T, Takahashi M, Hillen W (1995) Anal Biochem 232:190–196
Hillen W, Berens C (1994) Annu Rev Microbiol 48:345–369
Yamaguchi A, Udagawa T, Sawai T (1990) J Biol Chem 265:4809–4813
Berens C, Hillen W (2003) Eur J Biochem 270:3109–3121
Hinrichs W, Kisker C, Düvel M, Müller A, Tovar K, Hillen W, Saenger W (1994) Science 264:418–420
Takahashi M, Altschmied L, Hillen W (1986) J Mol Biol 187:341–348
Kisker C, Hinrichs W, Tovar K, Hillen W, Saenger W (1995) J Mol Biol 247:260–280
Orth P, Cordes F, Schnappinger D, Hillen W, Saenger W, Hinrichs W (1998) J Mol Biol 279:439–447
Orth P, Saenger W, Hinrichs W (1999) Biochemistry 38:191–198
Heffron SE, Mui S, Aorora A, Abel K, Bergmann E, Jurnak F (2006) Acta Crystallogr D Biol Crystallogr 62:1392–1400
Pioletti M, Schlünzen F, Harms J, Zarivach R, Gluhmann M, Avila H, Bashan A, Bartels H, Auerbach T, Jacobi C, Hartsch T, Yonath A, Franceschi F (2001) EMBO J 20:1829–1839
Krafft C, Hinrichs W, Orth P, Saenger W, Welfle H (1998) Biophys J 74:63–71
Otwinowski Z, Minor W (1997) Methods Enzymol 276:307–325
Collaborative Computational ProjectNumber 4 (1994) Acta Crystallogr D Biol Crystallogr 50:760–763
Jones TA, Zou J, Cowan SW, Kieldgaard M (1991) Acta Crystallogr A 47:110–118
Murshudov GN, Vagin AA, Dodson EJ (1997) Acta Crystallogr D Biol Crystallogr 53:240–755
Pflugrath JW (1999) Acta Crystallogr D Biol Crystallogr 55:1718–1725
Emsley P, Cowtan K (2004) Acta Crystallogr D Biol Crystallogr 60:2126–2132
Cohen GH (1997) J Appl Crystallogr 30:1160–1161
Takahashi M, Degenkolb J, Hillen W (1991) Anal Biochem 199:197–202
Schneider S (2001) In: Nelson M (ed) Tetracyclines in biology, chemistry and medicine. Birkhäuser, Basel, pp 65–106
Stezowski JJ (1976) J Am Chem Soc 98:6012–6018
Aleksandrov A, Proft J, Hinrichs W, Simonson T (2007) Chembiochem 8(6):675–685
Aleksandrov A, Simonson T (2006) J Comp Chem 27:1517–1533
Stephens C, Murai K, Brunings K, Woodward R (1956) J Am Chem Soc 78:4155–4158
Schnarr M, Matthies M, Lohmann W (1979) Z Naturforsch C 34:1156–1161
Kohn KW (1961) Nature 191:1156–1158
Ettner N, Metzger JW, Lederer T, Hulmes JD, Kisker C, Hinrichs W, Ellestad GA, Hillen W (1995) Biochemistry 22:22–31
Jogun KH, Stezowski JJ (1976) J Am Chem Soc 98:6018–6026
Baker WA Jr, Brown PA (1966) J Am Chem Soc 88:1314–1317
Berthon G, Brion M, Lambs L (1983) J Inorg Biochem 19:1–18
Huheey JE, Keiter EA, Keiter RL (1993) Inorganic chemistry: principles of structure and reactivity, 4th edn. Harper Collins, New York
Coibion C, Laszlo P (1979) Biochem Pharmacol 28:1367–1372
Cohlan SQ, Bevelander G, Tiamsic T (1963) Am J Dis Child 20:275–290
Brion M, Lambs L, Berthon G (1985) Inflamm Res 17:229–242
Lubben M, Meetsma A, Wilkinson EC, Fering B, Que L Jr (1995) Angew Chem Int Ed Engl 34:1512–1514
Finney LA, O’Halloran TV (2003) Science 300:931–936
Acknowledgments
We thank Leona Berndt, Britta Girbardt, and Tony Lassak for assistance in protein preparation and fluorescence measurements. We are grateful for detailed comments and efforts by the referees.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palm, G.J., Lederer, T., Orth, P. et al. Specific binding of divalent metal ions to tetracycline and to the Tet repressor/tetracycline complex. J Biol Inorg Chem 13, 1097–1110 (2008). https://doi.org/10.1007/s00775-008-0395-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-008-0395-2