Abstract
Introduction
The trabecular bone score (TBS) has emerged as a convenient measure for assessing the microstructure of trabecular bone in the second through fourth lumbar vertebrae (L2–4) and can be conducted concurrently with bone mineral density (BMD) assessment. This study was performed to evaluate changes in BMD and the TBS during ADT for prostate cancer.
Materials and Methods
Consecutive patients who had prostate cancer without bone metastases at Kobe University Hospital were studied from March 2020 to December 2021. BMD and TBS were measured every 6 months from the start of treatment using Hologic Horizon devices (Hologic, Inc., Marlborough, MA, USA).
Results
Thirty-four patients were followed for 2 years. Significant declines in BMD (−3.8% for femoral neck, −4.2% for total hip, and −6.1% for lumbar spine) and TBS (−16.6%) were noted after 2 years of ADT. Correlation analyses revealed a weak correlation between lumbar spine BMD and TBS at ADT initiation, but this correlation strengthened after 2 years. The multiple regression analysis results suggested that the rate of BMD loss may be slower in patients with a preserved pretreatment TBS.
Conclusion
In patients without bone metastases undergoing ADT for prostate cancer, notable decreases were found in both BMD and TBS over a 2-year treatment period. Factors influencing the TBS decline remain unclear; however, patients with a lower pretreatment TBS exhibited a more rapid decline in BMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Androgen deprivation therapy (ADT) is not only associated with an improved prognosis for patients with metastatic prostate cancer, but it has also been administered to a wide range of patients with localized cancer [1, 2]. However, various adverse events have been documented, including decreased bone density, fragility fractures, reduced sexual function, muscle weakness, and abnormal lipid metabolism associated with androgen removal [3,4,5]. Guidelines emphasize the importance of preserving bone mineral density (BMD) during hormonal therapy, with dual-energy X-ray absorptiometry (DXA) being the recommended bone densitometry technique [6, 7].
The significance of both bone quality and density in fortifying bone strength and resistance to fractures has been underscored in recent years [8, 9]. The trabecular bone score (TBS) has emerged as a convenient measure with which to assess the microstructure of trabecular bone in the first through fourth lumbar vertebrae (L2–4) [10]. This assessment can be concurrently conducted with DXA, the gold standard bone densitometry technique, offering a more expeditious procedure with reduced radiation exposure. The TBS is considered to provide more accurate assessment of the fracture risk [11].
Purpose
No studies to date have focused on changes in the TBS during ADT for prostate cancer. In this single-center, prospective, observational study, we measured the TBS in addition to periodic BMD and examined the changes in these parameters during ADT.
Materials and methods
BMD and TBS measurements were conducted following the Declaration of Helsinki principles on consecutive patients who had prostate cancer without bone metastases at Kobe University Hospital from March 2020 to December 2021. The patients underwent initial histological diagnosis, bone scintigraphy, and thoracoabdominal computed tomography to confirm the absence of bone metastases. Throughout the therapeutic course, the patients underwent prostate-specific antigen measurement every 3 months and were followed up with a computed tomography scan of the chest and abdomen approximately once a year. Patients whose condition progressed to castration-resistant prostate cancer within 2 years after administration of hormone treatments were excluded from the study. All patients continued to receive bicalutamide as a combined androgen blockade for 2 years, along with a Luteinizing Hormone-Releasing Hormone agonist or antagonist.
BMD was measured at the femoral neck, total hip, and lumbar spine (L2–4) using a Hologic Horizon A device (Hologic, Inc., Marlborough, MA, USA) before hormone treatment and at 6, 12, 18, and 24 months thereafter. Measurements were allowed to be taken within 1 month before or after the scheduled date. TBS measurements were simultaneously performed in the lumbar spine (L2–4). DXA was performed using a Hologic Horizon device with APEX software version 13.6.0.7 (Hologic, Inc.), and the TBS was measured using TBS iNsight® software version 3.0.3.0 (Medimaps SASU, Pessac, France).
Osteoporosis was diagnosed and treated in accordance with the 2012 revision of the diagnostic criteria for primary osteoporosis [12]. The diagnostic criteria were involvement of the lumbar spine or proximal femur, presence of another fragility fracture, or BMD equal to or below − 2.5 SD. Each patient was evaluated by an orthopedic specialist (N.H.) for the presence of fragility fractures prior to and during treatment, and their medical history and two-way X-rays of the lumbar region were acquired. Patients diagnosed with osteoporosis were treated with bisphosphonate and anti-receptor activator of nuclear factor-kappa B ligand antibody. Patients who were diagnosed with osteoporosis before and within 2 years after the start of ADT were excluded from the study.
Prophylactic treatment for secondary osteoporosis was not administered in any of the cases.
Statistical analysis
All statistical analyses were performed with EZR version 1.55 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [13], and a p value of < 0.05 was considered statistically significant. Multiple regression analysis, with bone density as the criterion variable, was performed to examine the impact of each explanatory variable on bone density. The variables in this analysis were age (continuous), body mass index (BMI) (continuous), habitual drinking, smoking, diabetes, Gleason grade, prostate-specific antigen concentration (continuous), bone-type alkaline phosphatase concentration (continuous), collagen type I trimeric cross-linked peptide concentration (continuous), tartrate-resistant acid phosphatase 5b concentration (continuous), 25-hydroxyvitamin D concentration (continuous), testosterone concentration (continuous), TBS (continuous), BMD at each site (continuous), and estimated glomerular filtration rate (continuous). Model selection was guided by the Bayesian information criterion. Pearson correlation analysis was performed to calculate the correlation coefficients.
Results
Of 48 patients who had prostate cancer without bone metastases, 9 were excluded because of an osteoporosis diagnosis at the initial assessment. The remaining 34 patients were enrolled in the study after accounting for deaths, transfers, progression to castration-resistant prostate cancer, and initiation of osteoporosis treatment during the 2-year follow-up. Bone densitometry was unattainable for one patient at 18 months. All patients maintained testosterone levels within the castration range throughout the study. No patients developed fragility fractures.
The patients’ background characteristics at the start of ADT are shown in Table 1. Their median age was 75 years, and their median BMI was 23.8 kg/m2. All patients underwent combined androgen blockade during the study period. The median BMD was 0.706, 0.914, and 1.083 g/cm2 in the femoral neck, total hip, and lumbar spine, respectively. The median TBS was 1.31.
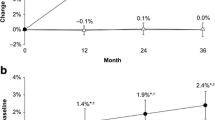
The median change in BMD was − 0.013 g/cm2 (− 1.8%) for the femoral neck, − 0.015 g/cm2 (− 1.6%) for the total hip, and − 0.029 g/cm2 (− 2.7%) for the lumbar spine at 12 months, progressing to − 0.027 g/cm2 (− 3.8%), − 0.038 g/cm2 (− 4.2%), and − 0.066 g/cm2 (− 6.1%) at 24 months, respectively, indicating a temporal decline (Fig. 1a–c). The median change in the TBS was − 0.052 (− 4.0%) and − 0.218 (− 16.6%) at 12 and 24 months, respectively (Fig. 1d).
In the Pearson correlation analysis, the lumbar spine BMD and TBS at the initiation of hormone therapy displayed a weak but nonsignificant correlation (R = 0.332, 95% confidence interval = − 0.01 to 0.602, p = 0.055). Conversely, a robust correlation was observed between BMD and TBS in the lumbar spine 2 years after initiation of treatment (R = 0.753, 95% confidence interval = − 0.528 to 0.879, p < 0.001). No correlation was discerned between BMD and TBS at each site throughout the 2-year period (Fig. 2a–c).
The multiple regression analysis of factors influencing BMD loss at 2 years showed that BMI, pretreatment femoral neck BMD, collagen type I trimeric cross-linked peptide, and TBS were influential factors for the femoral neck (multiple R2 = 0.481, adjusted R2 = 0.394, p = 0.003) (Table 2); that age was an influential factor for the total hip (multiple R2 = 0.13, adjusted R2 = 0.098, p = 0.055) (Table 3); and that BMI, testosterone, and TBS were influential factors for the lumbar spine (multiple R2 = 0.295, adjusted R2 = 0.21, p = 0.031) (Table 4). The multiple regression analysis revealed no factors predicting the change in the TBS at 2 years (data not shown). Histograms of the residuals showed that the deviations were normally distributed. The variance inflation factors were consistently < 5, signifying an absence of multicollinearity.
Discussion
This study is the first to investigate changes in BMD and the TBS throughout a 2-year period in patients undergoing ADT for prostate cancer. The TBS exhibited a significant decrease during this treatment period. A strength of this study is that the data were prospectively collected from consecutive patients.
In contrast to DXA, which quantifies bone mass, the TBS provides an indirect assessment of bone microstructure, which correlates with bone quality [14]. Numerous studies have suggested that the TBS can identify risks not captured by BMD when predicting fragility fractures [15,16,17]. In addition, it has been stated that the TBS of the lumbar spine is a predictor of fractures not only in the lumbar spine but also in other areas, regardless of gender [18,19,20]. In prostate cancer patients without bone metastases, who have a high potential for long-term prognosis, measuring the TBS is considered important from the perspective of bone health.
Parallel to the temporal trends in bone density observed in men undergoing hormone therapy in the current study, the TBS exhibited a declining trajectory. Prior research demonstrated a 0.18% to 1.08% annual decrease in the TBS in older men aged 66 to 75 years without factors affecting bone metabolism [21]. Another report showed a − 0.25% annual decline in older men with a median age of 72.8 years [22]. The rate of decline in the TBS in the present study was 4.0% at 12 months and 16.6% at 24 months, which is a particularly rapid rate of decline compared with these previous reports. The rate of decline in the TBS in postmenopausal women during a 1-year period was − 0.31% [23] or − 0.16% [24], and the TBS in our study declined at a comparatively rapid rate. A similar phenomenon has been reported for changes in bone density over time. Analogously, the rate of bone density loss in patients with prostate cancer undergoing hormone therapy, generally ranging from 4 to 8% [25, 26], exceeds the typical rate in postmenopausal women (approximately 2% annually) [27].
The pivotal role of both testosterone and estrogen in sustaining bone density is well-established. In addition to its direct effect on maintaining bone density, testosterone is metabolized by aromatase to estrogen, resulting in estrogen’s ability to maintain bone density. Therefore, testosterone exerts a dual effect on bone density [28]. The findings presented herein suggest a potent impact of testosterone on bone quality as evidenced by the TBS. The study suggests that testosterone may be involved in the tendency of TBS, which reflects the trabecular bone structure, to show a delayed decline compared to BMD, which reflects the bone mass of both cortical and trabecular bones. It has been reported that due to differences in androgen receptor expression levels, cortical bone is more influenced by male hormones than trabecular bone [29]. In fact, it has been demonstrated that testosterone replacement therapy in patients with hypopituitarism significantly improves BMD compared to TBS [30]. However, one study showed that testosterone replacement in patients with low testosterone concentrations had no effect on the TBS [31]. Therefore, further investigations are needed to elucidate the underlying mechanisms.
Previous studies have shown a correlation between lumbar spine BMD and the TBS in healthy older men (R = 0.555–0.576) [32, 33], and in the present study, a weak correlation was found between these parameters prior to treatment. Conversely, no correlation was seen between the reductions in BMD and TBS throughout the 2 years following treatment initiation. This is consistent with previous reports [34, 35]. The stronger correlation observed after 2 years can be attributed to the fact that patients with higher TBS are more likely to maintain their BMD, while patients with lower TBS are more likely to experience a decline in BMD, as demonstrated by the multiple regression analysis.
For further investigation, a multiple regression analysis was performed to examine the factors affecting the 2-year changes in BMD and TBS. Although the correlation coefficient was small and did not appear to be an effective predictor, patients with a high TBS at the initial examination tended to have a slower rate of BMD loss at the femoral neck and the lumbar spine (Tables 2, 3, 4), but this was not observed at the total hip. Generally, the rate of bone remodeling varies depending on the bone site and race [36, 37], but there have been no consistent reports explaining why the total hip is less affected by TBS compared to the femoral neck and lumbar spine. Further investigation is needed to understand this phenomenon. By contrast, factors affecting changes in the TBS could not be identified. This finding explains the stronger correlation between the lumbar spine BMD and TBS at 2 years than at baseline. Although Miyazawa et al. indicated that patients who were undergoing hormone therapy for prostate cancer and had impaired renal function experienced more rapid BMD loss [38], renal function did not seem to have a significant effect in the present study. In our study, patients with higher BMD at the femoral neck before ADT treatment experienced a faster rate of BMD loss. Although there are not many reports examining the impact of pretreatment BMD, Miyazawa et al. reported that pre-ADT treatment BMD does not affect the rate of BMD loss, which contrasts with our findings. We believe these differences may be due to variations in statistical methods or measurement sites.
This study had several limitations. First, the number of patients was small and the analysis lacked statistical power. Second, the use of the Bayesian information criterion in the multiple regression analysis may not have accurately encompassed all factors. Third, an adverse event associated with ADT treatment is an increase in body fat [39], which is considered an important factor in the reduction of TBS [40]. In this study, data on changes in body weight and body fat percentage over time were not collected, so it is necessary to fully acknowledge that these factors may have influenced the results. Finally, there was a lack of attention to fracture events in this study. A decrease in the TBS has not previously been shown to lead to fractures in patients with hormone-treated prostate cancer, but associations between the TBS and fracture events have been well documented in patients with other conditions.
Conclusion
The study revealed decreases in BMD and the TBS over a 2-year period during ADT in patients who had prostate cancer without bone metastases. The factors precipitating the decline in the TBS remain unclear; however, patients with a lower pretreatment TBS tended to experience a faster rate of bone density decline.
Abbreviations
- ADT:
-
Androgen deprivation therapy
- BMD:
-
Bone mineral density
- BMI:
-
Body mass index
- DXA:
-
Dual-energy X-ray absorptiometry
- TBS:
-
Trabecular bone score
References
Desai K, McManus JM, Sharifi N (2021) Hormonal therapy for prostate cancer. Endocr Rev 42:354–373
Gómez Rivas J, Fernandez L, Abad-Lopez P, Moreno-Sierra J (2023) Androgen deprivation therapy in localized prostate cancer. Current status and future trends. ActasUrol Esp 47:398–407
Nguyen PL, Alibhai SMH, Smith MR et al (2015) Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol 67:825–836
Greenspan SL, Coates P, Sereika SM, Nelson JB, Trump DL, Resnick NM (2005) Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab 90:6410–6417
Wang A, Obertová Z, Brown C, Lawrenson R et al (2015) Risk of fracture in men with prostate cancer on androgen deprivation therapy: a population-based cohort study in New Zealand. BMC Cancer 15:837
Fukumoto S, Soen S, Matsumoto T, Subcommittee for CTIBL in the JSBMR et al (2020) Management manual for cancer treatment-induced bone loss (CTIBL): position statement of the JSBMR. J Bone Miner Metab 38:141–144
Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J, ESMO Guidelines Working Group (2014) Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 25:124–137
Licata A (2009) Bone density vs bone quality: what’s a clinician to do? Cleve Clin J Med 76:331–336
Compston J (2006) Bone quality: what is it and how is it measured? Arq Bras Endocrinol Metabol 50:579–585
Rajan R, Cherian KE, Kapoor N, Paul TV (2020) Trabecular bone score-an emerging tool in the management of osteoporosis. Ind J Endocrinol Metab 24:237–243
Pothuaud L, Barthe N, Krieg M-A, Mehsen N, Carceller P, Hans D (2009) Evaluation of the potential use of trabecular bone score to complement bone mineral density in the diagnosis of osteoporosis: a preliminary spine BMD-matched, case-control study. J Clin Densitom 12:170–176
Soen S, Fukunaga M, Sugimoto T et al (2013) Diagnostic criteria for primary osteoporosis: year 2012 revision. J Bone Miner Metab 31:247–257
Kanda Y (2013) Investigation of the freely-available easy-to-use software “EZR” (Easy R) for medical statistics. Bone Marrow Transplant 48:452–458
Pothuaud L, Carceller P, Hans D (2008) Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone 42:775–787
Pothuaud L, Barthe N, Krieg M-A, Mehsen N, Carceller P, Hans D (2009) Evaluation of the potential use of trabecular bone score to complement bone mineral density in the diagnosis of osteoporosis: apreliminary spine BMD-matched, case-control study. J Clin Densitom 12:170–176
Choi YJ, Chung Y-S, Suh C-H, Jung J-Y, Kim H-A (2017) Trabecular bone score as a supplementary tool for the discrimination of osteoporotic fractures in postmenopausal women with rheumatoid arthritis. Medicine (Baltimore) 96:e8661
Kim HA, Lee HY, Jung JY, Suh CH, Chung YS, Choi YJ (2020) Trabecular bone score is a useful parameter for the prediction of vertebral fractures in patients with polymyalgia Rheumatica. J Clin Densitom 23:373–380
Leslie WD, Aubry-Rozier B, Lix LM, Morin SN, Majumdar SR, Hans D (2014) Spine bone texture assessed by trabecular bone score (TBS) predicts osteoporotic fractures in men: the Manitoba bone density program. Bone 67:10–14
Martineau P, Leslie WD, Kanis JA et al (2018) In which patients does lumbar spine trabecular bone score (TBS) have the largest effect? Bone 113:161–168
Iki M, Tamaki J, Yoneshima H et al (2014) Trabecular bone score (TBS) predicts vertebral fractures in Japanese women over 10 years independently of bone density and prevalent vertebral deformity: the Japanese population-based osteoporosis (JPOS) cohort study. J Bone Miner Res 29:399–407
Tang H, Di W, Cai J et al (2022) Age-related changes in trabecular bone score and bone mineral density in chinese men: a cross-sectional and longitudinal study. Clin Interv Aging 17:429–437
Schousboe JT, Vo TN, Schwartz AV et al (2018) Predictors of change of trabecular bone score (TBS) in older men: results from the Osteoporotic Fractures in Men (MrOS) Study. Osteoporos Int 29:49–59
Krieg MA, Aubry-Rozier B, Hans D, Leslie WD (2013) Manitoba Bone Density P. Effects of antiresorptive agents on trabecular bone score (TBS) in older women. Osteoporos Int 24:1073–1078
Popp AW, Guler S, Lippuner K et al (2013) Effects of zoledronate versus placebo on spine bone mineral density and microarchitecture assessed by the trabecular bone score in postmenopausal women with osteoporosis: a three-year study. J Bone Miner Res 28:449–454
Berruti A, Dogliotti L, Tarabuzzi R et al (2002) Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol 167:2361–2367
Maillefert JF, Sibilia J, Michel F, Saussine C, Javier RM, Tavernier C (1999) Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol 161:1219–1222
Finkelstein JS, Brockwell SE, Neer RM et al (2008) Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 93:861–868
Shigehara K, Izumi K, Kadono Y, Mizokami A (2021) Testosterone and bone health in men: a narrative review. J Clin Med 10:530
Wiren KM, Chapman Evans A, Zhang XW (2002) Osteoblast differentiation influences androgen and estrogen receptor-alpha and -beta expression. J Endocrinol 175:683–694
Leifke E, Körner HC, Link TM, Behre HM, Peters PE, Nieschlag E (1998) Effects of testosterone replacement therapy on cortical and trabecular bone mineral density, vertebral body area and paraspinal muscle area in hypogonadal men. Eur J Endocrinol 138:51–58
Cauley JA, Ellenberg SS, Schwartz AV, Ensrud KE, Keaveny TM, Snyder PJ (2021) Effect of testosterone treatment on the trabecular bone score in older men with low serum testosterone. Osteoporos Int 32:2371–2375
Bazzocchi A, Ponti F, Guglielmi G et al (2015) Trabecular bone score in healthy ageing. Br J Radiol 88:20140865
Rajaei A, Amiri A, Farsad F, Dehghan P (2019) The correlation between trabecular bone score and lumbar spine bone mineral density in patients with normal and high body mass index. Iran J Med Sci 44:374–381
Schousboe JT, Vo TN, Ensrud KE et al (2018) Predictors of change of trabecular bone score (TBS) in older men: results from the Osteoporotic Fractures in Men (MrOS) Study. Osteoporos Int 29:49–59
Padlina I, Gonzalez-Rodriguez E, Lamy O et al (2017) The lumbar spine age-related degenerative disease influences the BMD not the TBS: the Osteolaus cohort. Osteoporos Int 28:909–915
Franco F, Borau Zamora C, Martín Campana D, Berli ME (2023) Computational analysis of the influence of menopause and ageing on bone mineral density, exploring the impact of bone turnover and focal bone balance—a study on overload and underload scenarios. Life (Basel) 13:2155
Seeman E (1997) From density to structure: growing up and growing old on the surfaces of bone. J Bone Miner Res 12:509–521
Miyazawa Y, Sekine Y, Suzuki K et al (2018) Effect of androgen-deprivation therapy on bone mineral density in Japanese patients with prostate cancer. In Vivo 32:409–412
van Londen GJ, Levy ME, Perera S, Nelson JB, Greenspan SL (2008) Body composition changes during androgen deprivation therapy for prostate cancer: a 2-year prospective study. Crit Rev Oncol Hematol 68:172–177
Romagnoli E, Lubrano C, Lenzi A et al (2016) Assessment of trabecular bone score (TBS) in overweight/obese men: effect of metabolic and anthropometric factors. Endocrine 54:342–347
Acknowledgements
We thank Angela Morben, DVM, ELS, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
Takuto Hara: conceptualization, data curation, formal analysis, investigation, methodology, writing—original draft and review and editing. Hanako Nishimoto: conceptualization, data curation, methodology, writing—review and editing. TomoakiTerakawa: conceptualization, data curation, formal analysis, investigation, writing—review and editing. Yasuyoshi Okamura: data curation, writing—review and editing. Kotaro Suzuki: data curation, writing—review and editing. Yukari Bando: data curation, writing—review and editing. Koji Chiba: writing—review and editing. Yoji Hyodo: writing—review and editing. Yuzo Nakano: writing—review and editing. Ryosuke Kuroda: writing—review and editing. Hideaki Miyake: supervision.
Corresponding author
Ethics declarations
Conflict of interest
Hideaki Miyake has received payments for lectures from Merck, Janssen, Takeda.
Takuto Hara, Hanako Nishimoto, Tomoaki Terakawa, Yasuyoshi Okamura, Kotaro Suzuki, Yukari Bando, Koji Chiba, Yoji Hyodo, Jun Teishima, Yuzo Nakano, and Ryosuke Kuroda declare that no competing interests exist.
Ethics approval
This study was approved by the ethics committee of Kobe University (IRB No. B190287).
Informed consent
All patients provided verbal informed consent.
Registration of trial
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Hara, T., Nishimoto, H., Terakawa, T. et al. Temporal declines in bone mineral density and trabecular bone score during androgen deprivation therapy. J Bone Miner Metab (2024). https://doi.org/10.1007/s00774-024-01537-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00774-024-01537-z