Abstract
Parathyroid hormone (1–34, PTH) combined β-tricalcium phosphate (β-TCP) achieves stable bone regeneration without cell transplantation in previous studies. Recently, with the development of tissue engineering slow release technology, PTH used locally to promote bone defect healing become possible. This study by virtue of collagen with a combination of drugs and has a slow release properties, and investigated bone regeneration by β-TCP/collagen (β-TCP/COL) with the single local administration of PTH. After the creation of a rodent critical-sized femoral metaphyseal bone defect, β-TCP/COL was prepared by mixing sieved granules of β-TCP and atelocollagen for medical use, then β-TCP/COL with dripped PTH solution (1.0 µg) was implanted into the defect of OVX rats until death at 4 and 8 weeks. The defected area in distal femurs of rats was harvested for evaluation by histology, micro-CT, and biomechanics. The results of our study show that single-dose local administration of PTH combined local usage of β-TCP/COL can increase the healing of defects in OVX rats. Furthermore, treatments with single-dose local administration of PTH and β-TCP/COL showed a stronger effect on accelerating the local bone formation than β-TCP/COL used alone. The results from our study demonstrate that combination of single-dose local administration of PTH and β-TCP/COL had an additive effect on local bone formation in osteoporosis rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis, the most common bone disease characterized by microarchitectural deterioration, low bone mass, and bone fragility leading to an increased risk of fractures, has become a major health problem worldwide [1,2,3]. Although an age-related decline in bone mineral density (BMD) is observed in both men and women, a greater loss is observed in postmenopausal women [4]. In the US, ~ 9 million women are currently diagnosed with osteoporosis and this number is expected to surpass 10 million by 2020 [5]. The incidence of postmenopausal osteoporosis is growing due to changing demographics and increasing life expectancy. According to previous researches, osteoporotic bone shows a prolonged and impaired healing process compared with normal bone [6, 7]. β-tricalcium phosphate (β-TCP) is frequently used for clinical treatment, it is free of imperfections of many biogenic repairing materials and possessed of good function as a filler and support and of good bone conduction, but it does not have good osteoinductivity especially for osteoporotic condition [8].
To shorten healing time and improve callus quality in osteoporotic animals, various anti-resorptive or anabolic drugs have been investigated to inhibit excessive bone resorption or promote new bone formation during defects healing, such as bisphosphonates (BPs), and intermittently administered human parathyroid hormone (PTH) [9,10,11]. However, excessive inhibition of osteoclasts by anticatabolic agents might lead to delayed bone remodeling at the late healing period. PTH is an important physiological regulator of mineral homeostasis and has bone anabolic and catabolic actions. When administered intermittently, PTH induces substantial increases in osteoblast surface, osteoid surface, and osteoid volume in animals [10, 12]. Intermittently administered parathyroid hormone has been the only anabolic drug that could promote defects healing in osteoporotic subjects [13, 14]. Our previous researches have demonstrated that the combination of PTH and β-TCP brings better effect to bone tissue repair in osteoporosis status [13, 14]. Further researches show that local incorporated of PTH can accelerate bone formation and osseointegration [15].
All the evidences above consolidate the notion that β-TCP and PTH represents a promising therapeutic approach for the treatment of osteoporotic defect induced by postmenopause. However, little is known about the effect of combined treatment with local administration of PTH and β-TCP/COL composite on osteoporotic defect in bone loss induced by ovariectomized (OVX). Thus, we hypothesized that combined treatment with local administration of PTH and β-TCP/COL composite treatment would enhance healing of metaphyseal defects in OVX rats via enhanced stimulation of bone formation and significantly better than the effect of using β-TCP/COL composite alone. The purpose of this study is to observe the effects of combined treatment with local administration of PTH and β-TCP/COL composite on defect healing in OVX rats.
Materials and methods
Animals
Seventy female Sprague–Dawley (SD) rats (3 months old) with an average weight of 230 g were included in this study. Every four animals were kept in one cage with climate-controlled conditions (25 °C; 55% humidity; 12 h of light alternating with 12 h of darkness). Free access to standard laboratory diet and tap water were permitted. Principles of laboratory animal care were followed, and the study protocol was approved by the Animal Research Committee of the university.
Preparation of β-TCP/COL composite
β-TCP/COL composite was prepared based on the procedures described by Mahmoud et al. [16]. At first, collagen (Sigma-Aldrich, St. Louis, MO, USA) suspension was prepared in aqueous alkali solution (pH 12) at room temperature. Then β-TCP(Olympus Terumo Biomaterials, Tokyo, Japan) powder was slowly added into the collagen suspension (2:1) while stirring, after a homogenous suspension was formed, the glutaraldehyde solution was added as a cross-linking agent. The mixture froze in a refrigerator for 5 h at – 40 °C. Porous composites were obtained after further lyophilization.
Surgery and treatment
After bilateral ovariectomy (N = 65) or sham operation (N = 5) according to previous reports [17, 18], 12 weeks were allowed to pass before defect surgery for the establishment of standard osteoporotic animal models. Each five randomly selected OVX rats and the five sham-operated ones were euthanized. The distal femurs were harvested for bone mineral density (BMD) evaluation to confirm the establishment of osteoporosis. Afterwards, femoral cylindrical defects were created from anterior to posterior direction in the distal femur, which were standardized at 3 mm in diameter, penetrated internal and external about 5 mm in length under generous irrigation with NaCl 0.9%, and lay above the distal epiphyseal growth plate as previously described [13, 19], then all animals were randomly divided into three groups, control group(CON), β-TCP/COL composite group(TPC) and local administration of PTH and β-TCP/COL composite group(PTPC); The defects from group TPC and PTPC were filled with β-TCP/collagene composite material, and the defects from group PTPC were dripped 1.0 µg PTH solution. The subcutaneous and skin layers were closed, using resorbable polyglactin (Vicryl, 4-0; Ethicon, USA) and resorbable monocryl (4-0; Ethicon, USA) sutures, respectively. 800,000 IU penicillin sodium (North China Pharmaceutical Group Corporation, China) was injected intramuscularly before and after operation for 3 days to prevent infection. The retrieval procedure was performed at 28 and 56 days (10 rats at each time point), when the animals were sacrificed using an overdose of barbiturate (Mebumal, ACO L € akemedel AB, Solna, Sweden). For the subsequent analytical technique at 28 and 56 days, the skin was carefully reopened and the bone defect site with the overlying membrane and soft tissue were harvested en bloc and preserved in formalin.
Micro-CT evaluation
The samples were fixed in 4% formaldehyde for 24 h at room temperature. A Micro-CT imaging system (μCT 50, Scanco Medical AG, Bassersdorf, Switzerland) was used to evaluate new bone formation within the defect region. All samples were placed in a custom-made holder to ensure that the long axis of the drilled channel was oriented perpendicular to the axis of X-ray beam. Scanning was performed at 55 kV and 114 μA with a thickness of 0.048 mm per slice in medium resolution mode, 1024 reconstruction matrix, and 200 ms integration time. For detection of trabecular bone filling the defect, segmentation parameters were set to sigma: 0.8 voxels, support: 1, and threshold: 3.08 cm−1. For analysis of the bone regeneration process within the defect, the central 2.5-mm-diameter region of the 3-mm-diameter defect and in the middle third in length was defined by drawing circular contour as area of measurement per slice, thus to obtain a consistent volume of interest (VOI) and to avoid including the native bone margins. The parameters comput0ed from these data included bone volume/tissue volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp) as previously described [20].
Biomechanical testing
Compression testing of the distal femoral metaphysis was conducted as previously described [21]. The end of the dorsal distal femur (both condyles) was placed in a 5 mm wide and 2 mm-deep notch of an aluminum alloy base, which was fixed to the mechanical testing system (MTS Landmark Systems, USA). This position resulted in stable three-point contact with the base so that the femur could not slip during the breaking test. A 1 N preload was applied to the ventral aspect of the condyles and then compressed until failure at a rate of 2 mm/min. The ultimate load at failure was determined as the strength of the condyles.
Histological examination
At every time point after X-ray analysis, the bones were fixed in 4% paraformaldehyde/PBS overnight at 4 °C, and then immediately decalcified with formic acid for 48 h. Decalcified specimens were dehydrated in an ethanol series (80, 85, 80, 90, 95, and 100%), embedded in paraffin, and cut into 5-mm thick sections in the middle third in length of the distal femoral metaphysis, stained with hematoxylin and eosin (HE) and evaluated qualitatively by light microscopy (Zeiss Aixoplan with Spot RT digital camera, Zeiss, German). The image was quantitatively analyzed by Image Pro Plus Software (Media Cybernetics, Silver Spring, MD).
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Statistical analyses were performed using the statistics package SPSS 19.0 (SPSS, Chicago, IL, USA). Multiple comparisons between groups were carried out using one-way ANOVA and Tukey’s post hoc test. The significance level of 0.05 was applied for all analyses.
Results
Clinical observation
Five rats in total were excluded from analysis due to anesthetic accident, infection, and there were 5 animals left for evaluation in each group at each observation time. The BMD of the tibial metaphysic was measured in vivo by dual-energy X-ray absorptiometry (Lunar Prodigy Advance, GE Lunar, Madison, WI, USA). The BMD of the tibial metaphysis from the Sham and OVX groups was 231.67 ± 30.14 (mg/cm2) and 165.30 ± 27.65 (mg/cm2). In quantitative analysis, the BMD of the tibial metaphysis from sham-operated rats were 28.5% higher than those of OVX rats (t test, P < 0.05). These results confirmed the establishment of osteoporosis in OVX rats.
Microstructure parameters
The images of the three-dimensional reconstructions of the trabecular in the defects were clearly shown in Fig. 1 and the quantitative results were expressed as BV/TV, Tb.Th, Tb.N and Tb.Sp (Fig. 2). After treatment with PTH and β-TCP/collagene composite for 4 and 8 weeks, the microarchitecture parameters BV/TV, Tb.Th, Tb.N were significantly lower, and the microarchitecture parameters Tb.Sp were significantly higher than the control group (P < 0.05), and notable difference in BV/TV, Tb.Th, Tb.N and Tb.Sp was observed between group TPC and PTPC (P < 0.05). Moreover, PTH plus β-TCP/collagene composite treatment presented the strongest effect on BV/TV, Tb.Th, Tb.N and Tb.Sp regardless of treatment groups.
Biomechanical test
Results of biomechanical test of femoral condyles were expressed as ultimate load (Fig. 3). After treatment for 4 and 8 weeks, the biomechanical strength increased with time in all groups. At each observation time, the strongest effects on strength of femoral condyles were observed in the PTPC group. After treatment with PTH and β-TCP/collagene composite for 4 and 8 weeks, the ultimate load of CON, TPC and PTPC groups were significantly increased by 1.70-, 1.58- and 1.52-fold, and significant was observed among three groups (P < 0.05).
Histology analysis
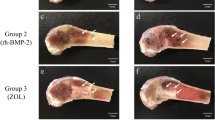
At 4 and 8 weeks postsurgery, defects were filled with a thin, loose connective tissue with minimal new bone formation and much fatty marrow from group CON. At 4 weeks postsurgery, the defect sites were filled with little new bone formation and a greater number of residual biomaterials particles from group TPC, and more new bone formation and less residual biomaterials particles at 8 weeks. At 8 weeks, defect sites exhibited more bone formation from the defect margin to the center than was observed at 4 weeks from group PTPC, and much biomaterials appear to be resorbed (Fig. 4).
The percentage of remaining biomaterials and bone fill in the defects
The quantitative results were shown as the percentage of remaining biomaterials and bone fill in the defects (Fig. 5a, b). At 4 and 8 weeks, PTH decreased the percentage of remaining biomaterials from groups TPC and PTPC by 1.09- and 1.5-fold, respectively (P < 0.05); for analysis of the percentage of bone fill in the defects, the percentages of bone fill from groups TPC and PTPC were significantly decreasing by 1.61- and 2.07-fold (P < 0.05) at 4 weeks, and by 1.52- and 2.14-fold at 8 weeks, respectively, as compared to group CON at the same time points.
a Result of the remaining percentage of biomaterials by HE examination. b Result of bone fill in the defects by HE examination. Data were expressed as mean ± SD; error bars in the figure are presented as SD, N = 5 specimens/group. *P < 0.05 versus CON group, #P < 0.05 versus TPC group (by one-way ANOVA and Tukey’s post hoc test)
Discussion
In this study, 12 weeks were allowed to pass for the establishment of standard osteoporotic animal models by bilateral ovariectomy, and then we evaluated the bone-forming capacity of using β-TCP/COL composite and PTH on the distal femurs defect for 4 and 8 weeks; Histology and Micro-CT Analysis results showed combined application of single-dose local administration of PTH and β-TCP/COL composite showed stronger effects than β-TCP/COL composite alone on bone formation in osteopenic rats, with the strongest effects on defect healing and callus formation, and microarchitecture as well as biomechanical parameters. PTH or β-TCP/collagene composite alone also increased defect healing compared to group control, although less than combination therapy. Our findings regarding the effect on new bone formation in the critical-sized osseous defects indicate that using a single-dose local administration of PTH together with β-TCP/COL composite would be promising as a method of achieving rapid and bone regeneration of osseous defects.
A marked increase in stochastic remodeling at menopause causes bone loss, severe deterioration of bone microarchitecture, and reduction in bone quality. This is the most important etiologic factor in the pathogenesis of postmenopausal osteoporosis. These increases in remodeling are associated with estrogen deprivation at menopause and are reversed by hormone replacement therapy [22]. Several previous studies have investigated the influence of osteoporosis on bone healing using ovariectomized rats as an experimental model; this is a well-studied method that simulates the changes in postmenopausal women, and needs an average of 3 months for the osteopenic condition to onset [13, 14, 18, 23]. As expected, OVX resulted in a significant decrease in the tibial metaphysis BMD after 3 months. Femur metaphysis was chosen as the defect site because it is a clinically relevant region representing a frequently fractured area in osteoporotic patients and after model is successfully developed parameters of all bones change and the change of femur is most obvious [24]. Because of this, femur is selected as experiment object.
The structure and mechanical properties of these materials used for the preparation of bone grafts should be similar to those of natural bones, as well as must be biocompatible, osteoinductive, and osteoconductive [25]. Among the different kinds of calcium phosphate-based materials, hydroxyapatite and tricalcium phosphate have been widely explored for use as scaffolds in bone regeneration [26]. Due to long-term resorption, hydroxyapatite has limited use in bone tissue engineering, while tricalcium phosphate can form a porous structure and release calcium and phosphorus ions by rapid degradation, which contributes to osteogenic activity, and thus to new bone formation [27]. Collagen is the most abundant protein in the extracellular matrix and plays an important role in maintaining the biological and structural integrity of extracellular matrix and provides physical support to tissues. In addition, collagen offers low immunogenicity, a porous structure, permeability, good biocompatibility, and biodegradability and has functions to regulate the morphology, adhesion, migration, and differentiation of cells [28, 29]. In recent years, a composite of synthetic β-TCP and collagene (β-TCP/Col) has been developed and β-TCP/Col significantly increases bone regeneration more than the implantation of β-TCP alone when implanted into a critical-sized calvarial defect rat model [30]. At 4 and 8 weeks, when bone regeneration at the defects sites was assessed in the present study, β-TCP/Col produced stronger effects on defects healing than in control group rats, which seemed to suggest the additive effects of β-TCP/Col on bone formation in defects.
Perfect bone biomaterials are osteoconductive scaffold that promotes the attachment, migration and proliferation of host cells at the implanting site; osteoinductive proteins or growth factors, which stimulate osteoprogenitor cells to differentiate into and synthesize mineralized bone matrix; and osteogenic cells that can synthesize bone tissue [31]. β-TCP/Col is free of imperfections of many biogenic repairing materials and possessed of good function as a filler and support and of good bone conduction, but it does not have good osteoinductivity [26]. The anabolic drug PTH could increase bone remodeling with a greater effect on bone formation than bone resorption and lead to increased bone mass and improved bone microarchitecture [32], and it has also been reported to enhance defects healing in both intact and ovariectomized (OVX) rats [33]. The total callus area was larger and the time of the earliest bridging was not delayed, callus and cortical density, biomechanical properties, rate of endosteal callus formation were higher compared with those measured in control group. The beneficial effect of intermittent PTH administration on osteoporotic defect healing has been demonstrated for enhanced cortical and cancellous bone formation by the early stimulation of proliferation and differentiation of osteoprogenitor cells increasing production of bone matrix proteins [34,35,36]. Our previous studies have shown that systemic administration of PTH can significantly improve β-TCP degradation and osteogenic ability, suggesting that PTH can be used as a drug to improve the ability of β-TCP bone induction [10, 14].
In the present study, we observed significant improvements in osteoporotic femora defect following combination therapy with a single-dose local administration of PTH and β-TCP/Col. In adjunctive therapy with β-TCP/Col, PTH enhanced the effects of β-TCP/Col by increasing bone mass, bone strength and bone formation at the defect region. Obvious significant improvements in biomechanical or structural properties were observed with either single therapy of PTH or β-TCP/Col, or combination therapy. Why did the single-dose local administration of PTH treatment show a stronger effect on bone formation in defected area? Specific mechanism not yet known, it is mainly owing to the increase of local bone formation by PTH local function and the sustained PTH release from β-TCP/Col [15]. Thus, the present study has concluded that combination therapy has obvious significant influence on osteoporotic defect and does enhance the osteogenesis effects of β-TCP/Col.
In summary, our study suggests that using a single-dose local administration of PTH together with β-TCP/collagene composite would be promising as a method of achieving rapid and bone regeneration of osseous defects.
References
Ström O, Borgström F, Kanis John A, Compston Juliet, Cooper Cyrus, McCloskey Eugene V, Jönsson B (2011) Osteoporosis: burden, health care provision and opportunities in the EU: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 6:59–155
Prevention NCDPoO (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA J Am Med Assoc 285:785–795
Kanis JA, Burlet N, Cooper C, Delmas PD, Reginster JY, Borgstrom F, Rizzoli R (2008) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 19:399–428
Reginster JY (2011) Antifracture efficacy of currently available therapies for postmenopausal osteoporosis. Drugs 71:65–78
Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R (2010) Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the american society for bone and mineral research. J Bone Miner Res 25:2267–2294
Mccann RM, Colleary G, Geddis C, Clarke SA, Jordan GR, Dickson GR, Marsh D (2008) Effect of osteoporosis on bone mineral density and fracture repair in a rat femoral fracture model. J Orthop Res 26:384–393
Hao YJ, Zhang G, Wang YS, Qin L, Hung WY, Leung K, Pei FX (2007) Changes of microstructure and mineralized tissue in the middle and late phase of osteoporotic fracture healing in rats. Bone 41:631–638. https://doi.org/10.1016/j.bone.2007.06.006
Tao ZS, Zhou WS, Tu KK, Huang ZL, Zhou Q, Sun T, Lv YX, Cui W, Yang L (2015) The effects of combined human parathyroid hormone (1–34) and simvastatin treatment on osseous integration of hydroxyapatite-coated titanium implants in the femur of ovariectomized rats. Injury 46:2164–2169. https://doi.org/10.1016/j.injury.2015.08.034
Tao ZS, Lv YX, Cui W, Huang ZL, Tu KK, Zhou Q, Sun T, Yang L (2016) Effect of teriparatide on repair of femoral metaphyseal defect in ovariectomized rats. Z Gerontol Geriatr 49:423–428
Tao ZS, Zhou WS, Tu KK, Huang ZL, Zhou Q, Sun T, Lv YX, Cui W, Yang L (2015) Effect exerted by teriparatide upon repair function of beta-tricalcium phosphate to ovariectomised rat’s femoral metaphysis defect caused by osteoporosis. Injury 46:2134–2141. https://doi.org/10.1016/j.injury.2015.07.042
Zacchetti G, Dayer R, Rizzoli R, Ammann P (2014) Systemic treatment with strontium ranelate accelerates the filling of a bone defect and improves the material level properties of the healing bone. Biomed Res Int 2014:549785. https://doi.org/10.1155/2014/549785
Tao ZS, Tu KK, Huang ZL, Zhou Q, Sun T, Xu HM, Zhou YL, Lv YX, Cui W, Yang L (2015) Combined treatment with parathyroid hormone (1–34) and beta-tricalcium phosphate had an additive effect on local bone formation in a rat defect model. Med Biol Eng Comput. https://doi.org/10.1007/s11517-015-1402-8
Tao ZS, Zhou WS, Tu KK, Huang ZL, Zhou Q, Sun T, Lv YX, Cui W, Yang L (2015) Treatment study of distal femur for parathyroid hormone (1–34) and beta-tricalcium phosphate on bone formation in critical-sized defects in osteopenic rats. J Craniomaxillofac Surg Off Publ Eur Assoc Craniomaxillofac Surg 43:2136–2143. https://doi.org/10.1016/j.jcms.2015.09.004
Tao ZS, Qiang Z, Tu KK, Huang ZL, Xu HM, Sun T, Lv YX, Cui W, Yang L (2015) Treatment study of distal femur for parathyroid hormone (1–34) and beta-tricalcium phosphate on bone formation in critical size defects in rats. J Biomater Appl 30:484–491. https://doi.org/10.1177/0885328215592854
Yu X, Wang L, Jiang X, Rowe D, Wei M (2012) Biomimetic CaP coating incorporated with parathyroid hormone improves the osseointegration of titanium implant. J Mater Sci Mater Med 23:2177–2186
Mohseni M, Jahandideh A, Abedi G, Akbarzadeh A, Hesaraki S (2017) Assessment of tricalcium phosphate/collagen (TCP/collagene)nanocomposite scaffold compared with hydroxyapatite (HA) on healing of segmental femur bone defect in rabbits. Artif Cells Nanomed Biotechnol. https://doi.org/10.1080/21691401.2017.1324463
Tao ZS, Bai BL, He XW, Liu W, Li H, Zhou Q, Sun T, Huang ZL, Tu KK, Lv YX, Cui W, Yang L (2016) A comparative study of strontium-substituted hydroxyapatite coating on implant’s osseointegration for osteopenic rats. Med Biol Eng Comput. https://doi.org/10.1007/s11517-016-1494-9
Tao ZS, Zhou WS, He XW, Liu W, Bai BL, Zhou Q, Huang ZL, Tu KK, Li H, Sun T, Lv YX, Cui W, Yang L (2016) A comparative study of zinc, magnesium, strontium-incorporated hydroxyapatite-coated titanium implants for osseointegration of osteopenic rats. Mater Sci Eng C Mater Biol Appl 62:226–232. https://doi.org/10.1016/j.msec.2016.01.034
Zhang Y, Cheng N, Miron R, Shi B, Cheng X (2012) Delivery of PDGF-B and BMP-7 by mesoporous bioglass/silk fibrin scaffolds for the repair of osteoporotic defects. Biomaterials 33:6698–6708
Zhang Y, Wu C, Luo T, Li S, Cheng X, Miron RJ (2012) Synthesis and inflammatory response of a novel silk fibroin scaffold containing BMP7 adenovirus for bone regeneration. Bone 51:704–713
Yang N, Cui Y, Tan J, Fu X, Han X, Leng H, Song C (2014) Local injection of a single dose of simvastatin augments osteoporotic bone mass in ovariectomized rats. J Bone Miner Metab 32:252–260. https://doi.org/10.1007/s00774-013-0496-z
Bone HG, Greenspan SL, Mckeever C, Bell N, Davidson M, Downs RW, Emkey R, Meunier PJ, Miller SS, Mulloy AL (2000) Alendronate and estrogen effects in postmenopausal women with low bone mineral density. Alendronate/Estrogen Study Group. J Clin Endocr Metab 85:720–726
Tao ZS, Lv YX, Cui W, Huang ZL, Tu KK, Zhou Q, Sun T, Yang L (2015) Effect of teriparatide on repair of femoral metaphyseal defect in ovariectomized rats. Z Gerontol Geriatr. https://doi.org/10.1007/s00391-015-0949-1
Comelekoglu U, Bagis S, Yalin S, Ogenler O, Yildiz A, Sahin NO, Oguz I, Hatungil R (2007) Biomechanical evaluation in osteoporosis: ovariectomized rat model. Clin Rheumatol 26:380–384. https://doi.org/10.1007/s10067-006-0367-2
Hutmacher DW (2000) Scaffolds in tissue engineering bone and cartilage. Biomaterials 21:2529
Li Q, Wang T, Zhang G, Yu X, Zhang J, Zhou G, Tang Z (2016) A comparative evaluation of the mechanical properties of two calcium phosphate/collagen composite materials and their osteogenic effects on adipose-derived stem cells. Stem Cells Int 1–12. https://doi.org/10.1155/2016/6409546
Dorozhkin SV (2013) Calcium orthophosphates in dentistry. J Mater Sci Mater Med 24:1335
Chevallay B, Herbage D (2000) Collagen-based biomaterials as 3D scaffold for cell cultures: applications for tissue engineering and gene therapy. Med Biol Eng Comput 38:211–218
Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, Van RJ, Deryugina E, Friedl P (2009) Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol 20:931–941
Kanda N, Matsui K, Kawai T, Edamatsu H, Tanuma Y, Suzuki O, Takahashi T, Kamakura S (2016) Implantation of octacalcium phosphate collagen composites (OCP/Col) after extraction of canine deciduous teeth achieved undisturbed permanent tooth eruption. Arch Oral Biol 72:179
Beaman FD, Bancroft LW, Peterson JJ, Kransdorf MJ (2006) Bone graft materials and synthetic substitutes. Radiol Clin N Am 44:451–461
Meganck JA, Koh AJ, Keller ET (2008) Parathyroid hormone mediates bone growth through the regulation of osteoblast proliferation and differentiation. Bone 42:806–818
Komrakova M, Stuermer EK, Werner C, Wicke M, Kolios L, Sehmisch S, Tezval M, Daub F, Martens T, Witzenhausen P (2010) Effect of human parathyroid hormone hPTH (1–34) applied at different regimes on fracture healing and muscle in ovariectomized and healthy rats. Bone 47:480–492
Andreassen TT, Fledelius C, Ejersted C, Oxlund H (2001) Increases in callus formation and mechanical strength of healing fractures in old rats treated with parathyroid hormone. Acta Orthop Scand 72:304–307
Nozaka K, Miyakoshi N, Kasukawa Y, Maekawa S, Noguchi H, Shimada Y (2008) Intermittent administration of human parathyroid hormone enhances bone formation and union at the site of cancellous bone osteotomy in normal and ovariectomized rats. Bone 42:90–97
Nakajima A, Shimoji N, Shiomi K, Shimizu S, Moriya H, Einhorn TA (2002) Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1–34). J Bone Miner Res 17:2038–2047
Acknowledgements
This study was supported by a grant from the natural science foundation for education department of Anhui Province (Grant no. KJ2017A266).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest.
About this article
Cite this article
Tao, ZS., Zhou, WS., Wu, XJ. et al. Single-dose local administration of parathyroid hormone (1–34, PTH) with β-tricalcium phosphate/collagen (β-TCP/COL) enhances bone defect healing in ovariectomized rats. J Bone Miner Metab 37, 28–35 (2019). https://doi.org/10.1007/s00774-018-0906-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-018-0906-3