Abstract
Parathyroid hormone (PTH) is a potent anabolic agent, but the cellular mechanisms by which it increases bone mass are not fully understood. Dickkopf 1 (Dkk1) is an endogenous inhibitor of Wnt signaling and suppresses bone formation in vivo. We sought to determine if Dkk1 and anabolic PTH treatment interact in regulating bone mass. PTH treatment of primary murine osteoblasts for 24 h reduced Dkk1 expression by 90% as quantified by real-time PCR, whereas PTH treatment in vivo reduced Dkk1 expression by 30% when given as a single daily subcutaneous dose. To directly determine whether Dkk1 modulates the anabolic response of PTH in vivo, we engineered transgenic (TG) mice expressing murine Dkk1 under the control of the 2.3-kb rat collagen alpha-1 promoter. TG mice had significantly reduced bone mass, which was accompanied by reduced histomorphometric parameters of bone formation (reduced OV/TV, ObS/OS, and NOb/TAR). Treatment of TG mice and wild-type (WT) littermates with 95 ng/g body weight of human (1–34) PTH daily for 34 days resulted in comparable increases in bone mass at all skeletal sites. Histomorphometric analyses indicated that PTH treatment increased the numbers of both osteoblasts and osteoclasts in WT mice but only increased the numbers of osteoblasts in TG mice. We conclude that overexpression of Dkk1 does not attenuate the anabolic response to PTH in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cell-surface receptors known as low-density lipoprotein receptor-related proteins 5/6 (LRP5 and LRP6) are key regulators of bone mass [1]. Available data indicate that members of the Wnt family bind both LRP5/6 and a seven-transmembrane spanning receptor, Frizzled, to form a functional ligand–receptor complex that then activates the canonical Wnt/β-catenin intracellular signaling pathway [1]. The signaling functions of LRP5 and LRP6 are tightly regulated by a large number of extracellular proteins, including members of the Dickkopf (Dkk) family of proteins. Dkks are encoded by a four-member gene family in vertebrates, three of which (Dkk1, -2, and -4) are reported to interact with LRP5 or LRP6 [2]. Gain-of-function mutations in LRP5 increase bone mass in part by conferring resistance to the inhibitory actions of Dkk1 [3]. Increased expression of Dkk1 by malignant plasma cells has been reported as the basis for reduced bone formation in multiple myeloma [4, 5]. A neutralizing antibody to Dkk1 prevents suppression of osteoblast function in a mouse model of myeloma [6]. Dkk1 TG mice have been reported to develop osteopenia [7]. Haploinsufficiency of the Dkk1 gene leads to an increase in bone mass in mice [8, 9]. Together, these findings suggest that Dkk1 suppresses bone formation in vivo.
PTH is a potent anabolic agent when administered as a single daily dose, but the cellular mechanisms by which it increases bone mass are incompletely understood. Kulkarni et al. [10] reported that PTH treatment of osteoblast-like cells increased expression of LRP6 and Frizzled 1 and suppressed Dkk1 expression. These effects were associated with an increase in cellular levels of β-catenin, suggesting that the actions of PTH in bone could be mediated at least in part by modulating the activity of the Wnt signaling pathway [10]. Consistent with that hypothesis, inhibition of protein kinase A suppressed PTH-induced activation of a Wnt-activated TCF/LEF reporter gene whereas forskolin mimicked the effects of PTH. Wan et al. [11] have recently reported that PTH induces phosphorylation of LRP6, which results in recruitment of axin to LRP6 and stabilization of β-catenin. Tobimatsu et al. [12] have also reported that PTH increased levels of β-catenin in the mouse osteoblastic MC3T3-E1 cell line.
Although these findings suggest cross-talk between the PTH and the Wnt-β-catenin signaling cascades, there is less evidence for a direct interaction between these two pathways at the level of cell-surface receptors in vivo. Thus, a 4-week course of intermittent PTH treatment increased skeletal mass to the same extent in LRP5−/− and LRP5+/+ mice, indicating that the anabolic effects of PTH do not require LRP5 signaling [13]. Consistent with this finding, Iwaniec et al. [14] reported that treatment with PTH for 6 weeks resulted in similar increases in osteoblast surface and osteoclast surface in both LRP5−/− and LRP5+/+ mice, although curiously PTH did not augment cancellous bone volume in either genotype. However, femur cortical thickness was 11% higher in PTH-treated mice in comparison with vehicle-treated mice, regardless of genotype [14]. These investigators concluded that LRP5 is not essential for the anabolic effects of PTH in bone [14]. Interestingly, Wan et al. have reported that single doses of PTH, administered daily for 28 days to mice, increased cellular levels of β-catenin and phosphorylated LRP5/6 in osteoblasts resident on trabecular bone [11, 15]. In contrast, continuous PTH treatment for the same period of time did not change levels of β-catenin or phosphorylated LRP5/6 [15]. Where and to what extent the PTH signaling cascades and those activated by Wnts intersect in bone cells is still being clarified.
To further explore the role of Dkk1 in bone as well as its effects on the anabolic response to PTH treatment, we engineered TG mice in which Dkk1 was expressed under the control of the 2.3-kb rat collagen type Iα promoter. We characterized the skeletal phenotype of these animals and their response to an anabolic PTH regimen.
Materials and methods
Generation and identification of Dkk1 TG mice
The Dkk1 transgene was created using the same strategy previously reported for generating the transgene used to target expression of membrane-bound colony stimulating factor-1 to osteoblasts [16]. The 1.1-kb cDNA for mouse Dkk1 was amplified by real-time PCR (RT-PCR) using mRNA isolated from primary murine osteoblasts [17]. The forward primer was GAAGTTGAGGTTCCGCAGTC; the reverse primer was CAGGGGAGTTCCATCAAGAA. The Dkk1 cDNA was cloned 3′ of the 2.3-kb rat collagen type Iα promoter. A 2.2-kb segment of the human growth hormone gene containing exons 1–5 and the intervening introns were added downstream of the cDNA to provide termination/polyadenylation signals and to increase expression efficiency.
The assembled transgene was microinjected into fertilized C57BL/6 × SJLF2 oocytes, and the resultant TG mice were identified by PCR amplification of a 171-bp sequence within exon 5 of the HGH portion of the transgene [18]. The integrity of genomic DNA was assessed by coamplification of a 259-bp segment of the endogenous murine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene [18]. Two TG founders were identified. TG lines were generated by mating founder animals to CD-1 WT mice; the TG animals in subsequent generations were identified by PCR. All animals used for this study were 12–14 weeks old unless otherwise indicated. The use of mice in this study was approved by the Yale Animal Care and Use Committee.
Measuring Dkk1 transgene expression
Transgene expression was assessed by quantitative RT-PCR (qRT-PCR) using the DNA Engine Opticon 2 System from M.J. Research (Waltham, MA, USA) and the Brilliant QRT-PCR Master Mix Kit from Stratagene (La Jolla, CA, USA). To prepare total cellular RNA, bone and other tissues were rapidly dissected and snap frozen in liquid nitrogen, pulverized with a mortar and pestle in the presence of powdered dry ice to keep the tissue frozen, and then solubilized in Trizol reagent (GIBCO-BRL). The thermal cycling conditions comprised an initial 50ºC for 30 min and a denaturation step at 95ºC for 3 min, 40 cycles at 95ºC for 30 s, 60ºC for 1 min, and 72ºC for 1 min. The forward primer for Dkk1 was CCATCAAGCCAGCA ATTCTT; the reverse primer for HGH was GATTGGCCATACTGGGCTTA. This primer pair amplifies only the transgene. The transgene was detected in bone but not in lung, heart, skin, spleen, liver, or kidney. The founder line expressing the highest level of Dkk1 was chosen for further study.
Dkk1 expression in bone was determined by qRT-PCR using the forward primer TCCCAGAAGAACCACACTGACTTC and the reverse primer TCTTGGACCAGAAGT CTCTTGCAC. This primer pair amplifies both endogenous Dkk1 and the Dkk1 transgene and was therefore used to detect the total level of Dkk1 transcript expression in TG mice and WT littermates.
Bone density measurements
In vivo bone density measurements were performed by dual-energy X-ray absorptiometry (DXA) using a PIXImus densitometer (Lunar Corporation, Madison, WI, USA). Anesthetized mice (ketamine, 30 mg/kg body weight, and xylazine, 3 mg/kg body weight, given IP) were placed in the prone position and scans performed with a 1.270-mm-diameter collimator, 0.762-mm line spacing, 0.380-mm point resolution, and an acquisition time of 5 min. The spine window is a rectangle spanning a length of the spine from T1 to the beginning of the sacrum. The femur window encompasses the entire right femur of each mouse. The coefficient of variation for total body BMD is approximately 1.5%.
Bone histomorphometry
Histomorphometry was performed as previously reported [19–21]. At the time of death the tibiae were removed, stripped of soft tissue, and fixed in 70% ethanol. Tibiae were then dehydrated through graded ethanol, cleared in toluene, infiltrated with increasing concentrations of methylmethacrylate, and embedded in methylmethacrylate according to previously described methods [19, 20]. Analyses were performed on 5-μM-thick sections stained with toluidine blue, pH 3.7, using a Nikon microscope interfaced with the Osteomeasure system software and hardware (Osteometrics, Atlanta, GA, USA). Measurements were obtained in an area of cancellous bone that measured approximately 2.5 mm2, containing only secondary spongiosa, and located 0.5–2.5 mm distal to the epiphyseal growth cartilage. Longitudinal sections (5 μm thick) taken in the frontal plane through the cancellous bone of the proximal tibia were prepared with a Leica RM2165 microtome, mounted on chrom–alum-coated glass slides, and stained with toluidine blue, pH 3.7. All indices were defined according to the American Society of Bone and Mineral Research histomorphometry nomenclature [22].
Effects of PTH treatment on Dkk1 expression in vitro and in vivo
Primary murine osteoblasts were prepared from calvariae of 2- to 4-day-old mice by collagenase–dispase digestion as described previously [17]. Cells were grown in alpha-minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS), penicillin, streptomycin, l-glutamine, and 20 mM HEPES pH 7.4. At 75% confluence, cells were treated with 10−8 M human (1–34) PTH. The cells were harvested at the indicated times after PTH treatment; RNA was isolated and used for qRT-PCR.
For anabolic PTH treatment in vivo, human (1–34) PTH was reconstituted in 10 mM acetic acid containing 2% heat-inactivated mouse serum and given at 95 ng/g body weight daily for 34 days, as we have previously reported [21]. Vehicle-treated animals received 10 mM acetic acid containing 2% heat-inactivated mouse serum. Animals were weighed every 7 days and the dose of PTH was adjusted for changes in weight. On days 1 and 34 of treatment, total body and regional bone mineral density (BMD) were determined by DXA. The left tibiae were then harvested and processed for histomorphometric analyses.
Results
High-level expression of Dkk1 in bone from TG mice
The level of total Dkk1 transcript expression (endogenous plus transgene) was examined by qRT-PCR as described in “Materials and methods”. The pattern and level of Dkk1 transcript expression in heart, liver, spleen, lung, and kidney were not significantly different in the transgenic line reported in this article when compared to WT littermates. In contrast, there was a ninefold increase in Dkk1 transcript expression (combined transgene and endogenous Dkk1) in the bone of the transgenic line described in this report.
Overexpression of Dkk1 reduces BMD and suppresses bone formation
Dkk1 TG mice showed 6% (P < 0.01), 6% (P < 0.01), and 4% (P < 0.05) reductions in femoral, spinal, and total BMD, respectively, compared to their WT littermates (Fig. 1). Histomorphometric analyses revealed a significant reduction in trabecular bone volume, consistent with the densitometric findings. As shown in Table 1, there was also suppression in measures of osteoblast function with reductions in osteoblast surface per bone surface and in the number of osteoblasts per total bone area in the TG animals compared to controls. In contrast, overexpression of Dkk1 did not affect osteoclast surface per bone surface or the number of osteoclasts per total bone area.
Dickkopf 1 (Dkk1) transgenic (TG) mice (Dkk1 Tg) have lower bone density compared to wild-type (WT) mice. Spinal, femur, and total body bone mineral density (BMD) of 43 Dkk1 transgenic mice and 44 age-matched WT littermates was measured by dual-energy X-ray absorptiometry (DXA) (PIXImus). *P < 0.05, **P < 0.01 compared to WT
PTH suppresses Dkk1 expression in osteoblasts in vitro and in vivo
As normal bone remodeling is regulated by PTH, we wondered if there is an interaction between PTH and Dkk1 in vitro and in vivo. When murine primary osteoblasts were cultured in the presence of 10−8 M PTH for 24 h, there was a time-dependent significant decline in the level of Dkk1 expression as quantified by qRT-PCR. Figure 2 shows greater than 90% suppression of Dkk1 expression after 24 h PTH treatment. We next examined the effects of an in vivo anabolic PTH treatment regimen on Dkk1 expression in WT animals. After 34 days of single daily PTH administration, there was a moderate, but significant, 33% reduction in Dkk1 transcript expression in bone from PTH-treated as compared to vehicle-treated WT animals (Fig. 3). In the TG animals, there was no change in the level of total Dkk1 with PTH treatment, indicating that expression of the Dkk1 transgene (which largely dictates Dkk1 levels in the TG animals) was not affected by administration of PTH (Fig. 3).
Parathyroid hormone (PTH) suppresses Dkk1 mRNA expression in vitro in osteoblasts. Total RNA was isolated from murine primary osteoblasts treated with 10−8 M PTH for the indicated times. Levels of Dkk1 expression were quantified by quantitative real-time polymerase chain reaction (qRT-PCR) and expressed as a percent (%) of the time 0 value. The results represent the mean ± SEM of four separate experiments
PTH suppresses Dkk1 mRNA expression in vivo in WT mice. Eight WT (Wt) and eight TG (Dkk1 Tg) mice were treated with either single daily doses of human (1–34) PTH (95 ng/g) or vehicle for 34 days (four animals/group); femurs were harvested and RNA extracted as described previously [16]. Levels of Dkk1 expression were quantified by qRT-PCR and expressed as a percent (% change) of the value in vehicle-treated animals
The anabolic response to PTH is not impaired in Dkk1 TG mice
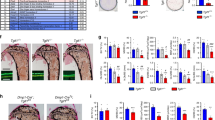
Daily subcutaneous PTH administration led to a significant increase in spinal, femur, and total body BMD in TG and WT mice (Fig. 4a). Interestingly, TG overexpression of Dkk1 did not impair the anabolic response to PTH. At the end of 34 days of PTH treatment, spinal BMD had increased by 12.9% over baseline in the WT animals and by an equivalent 14.9% in the PTH-treated TG mice. Similarly, femur BMD increased by 10.9% and total body BMD by 8.4% over baseline in the WT animals and increased by 18.9% and 11.9%, respectively, in the TG animals. Further, the net increase in spinal BMD (mean PTH-treated value minus the mean vehicle-treated value) was 8.5% in the WT animals and 9.7% in the TG mice. The mean percent increases in femur and total body BMD were 4.1% and 10.7% in WT animals and 5.9% and 6.2% in TG. As shown in Fig. 4b, although there is a lower trabecular bone mass in the transgenic animals at baseline, both TG and WT animals showed robust increases in bone mass with PTH treatment.
Anabolic effect of PTH in WT and TG (Tg) mice. a Percent change from baseline in spine, femur, and total body BMD determined by dual-energy X-ray absorptiometry (DXA) (PIXImus) in 40 WT (20 PTH-treated and 20 vehicle-treated) and 39 TG (18 PTH-treated and 21 vehicle-treated) mice. The data are the mean values after 34 days of treatment with 95 ng/g of either human (1–34) PTH or vehicle. b Distal tibiae of WT (Wt; upper two panels) and TG (Tg; lower two panels) mice treated with vehicle (Veh) or PTH for 34 days. Arrowheads point to trabeculae, which are increased in number and/or thickness with PTH treatment; arrows point to cortical bone, which is increased in thickness with PTH treatment. ×4
Mean body weights were comparable at the end of the treatment period in all four groups: WT-vehicle, 34.6 ± 1.6 g; WT-PTH, 32.0 ± 1.4 g; TG-vehicle, 35.8 ± 1.6 g; TG-PTH, 35.6 ± 1.6 g.
Consistent with the bone density results, histomorphometric analyses showed that PTH increased trabecular bone volume (BV/TV) in Dkk1 Tg mice and their WT littermates by a comparable extent (38.6% vs. 35.1%, respectively). Osteoblast surface (ObS/BS) and osteoblast numbers/bone perimeter (NOb/BPm) increased significantly in response to PTH treatment in both groups, indicating an ability of PTH to overcome or bypass the suppression in bone formation induced by the transgene (Fig. 5a). In contrast to the similarity in changes induced by PTH in histomorphometric parameters of bone formation in the two groups, there was a clear difference in parameters of bone resorption. Although PTH induced increases in osteoclast surface (OcS/BS), osteoclast number per total area (Noc/TAR), and osteoclasts numbers/bone perimeter (Noc/BPm) in WT mice, none of these parameters increased significantly in the TG animals (Fig. 5b).
Effect of daily PTH administration on cellular parameters of osteoblast and osteoclast activity in WT (left graphs) (a, b) and TG (right graphs) (a, b) mice. Histomorphometric analyses were performed in 16 PTH-treated and 17 vehicle-treated TG animals as well as 16 PTH-treated and 14 vehicle-treated WT mice. Results are expressed as mean ± SEM. *P < 0.05. ObS/BS osteoblast surface, NOb/BPm osteoblast numbers/bone perimeter, OcS/BS osteoclast surface, Noc/TAR osteoclast number per total area, Noc/BPm osteoclasts numbers/bone perimeter
Discussion
We found that selective overexpression of Dkk1 in osteoblasts leads to low bone mass. This observation is consistent with the report of Li et al. [7], who observed low bone density in transgenic mice in which Dkk1 was expressed under the control of the rat collagen 1A1 promoter. Our data indicate this effect appears to be caused by suppression of bone formation with a trend toward increased resorptive activity. As shown in Table 1, ObS/BS and NOb/TAR were suppressed in TG animals as compared to WT controls, changes also observed by Li and colleagues [7]. In our mice the suppression in bone formation was accompanied by a trend toward an increase in resorptive activity as reflected by an increased OcS/BS. However, despite the baseline suppression in bone formation, the anabolic response to PTH was not attenuated in Dkk1 TG animals.
The relationship between the PTH and the Wnt signaling cascades has been an area of intense recent investigative interest, and a number of observations have suggested an interaction between these two pathways [10–12]. Although these data strongly suggest an interaction between the PTH and canonical Wnt signaling pathways, the transmembrane Wnt receptor LRP5 does not seem to be required for the anabolic effect of PTH. Two groups have reported that mice with genetic absence of LRP5 have a normal anabolic response to single daily subcutaneous administration of parathyroid hormone in vivo [13, 14]. Thus, the level at which the PTH and Wnt signaling cascades intersect remains somewhat uncertain.
Dkk1 is an important inhibitor of Wnt signaling and acts to interdict the interaction of LRP5/6 and Frizzled with Wnt ligand. Another transmembrane protein, Kremin, is thought to be necessary for Dkk1 to effect this inhibition, although this model has recently been called into question [23]. The importance of Dkk1 as a regulator of bone mass in vivo has been unequivocally established by studies in mice with haploinsufficiency of the Dkk1 gene. These mice show a marked increase in bone mass [8, 9]. Underscoring the relevance of these findings in mice to humans is the observation that Dkk1 appears to play a central role mediating the inhibitory effect of myeloma cells on bone formation [4]. To determine whether Dkk1 and PTH interact in any important way in vivo, we initially explored the effects of PTH on Dkk1 expression in osteoblasts. Consistent with the findings of Onyia et al. [24], we showed that PTH profoundly suppressed Dkk1 expression in osteoblasts continuously exposed to the hormone (see Fig. 2). We also found that intermittent administration of PTH inhibited Dkk1 expression, albeit by a more modest 33% (see Fig. 3). These data indicate that one potentially important mechanism by which PTH might induce bone anabolism is by suppressing Dkk1 expression in vivo. We reasoned that if this were the case, transgenic overexpression of Dkk1 in bone should abrogate the anabolic effects of PTH because downregulation of Dkk1 would not occur. Our Dkk1 transgene achieved high-level expression in bone and was indeed unresponsive to PTH (Fig. 3), allowing us to test this hypothesis. Our data demonstrate that there is no attenuation in the anabolic response to PTH in Dkk1 TG animals. In two independent experiments; both the increase from baseline and the net change in BMD (mean BMD value after PTH minus mean BMD value after vehicle) at all three skeletal sites were comparable in the WT and TG animals (see Fig. 4). These data clearly indicate that suppression of canonical Wnt signaling at the level of the LRP5/6 receptors does not attenuate the anabolic response to PTH. This finding suggests that if there is a nonredundant interaction between the PTH and Wnt signaling cascades it must occur at a postreceptor site.
Kramer et al. [25] have recently reported that transgenic overexpression of sclerostin attenuated the anabolic response to PTH. Because sclerostin, like DKK1, is also an inhibitor of LRP5\6, this would imply a role for LRP5\6 in PTH-dependent bone anabolism. It may be that sclerostin is a more potent inhibitor. It is also possible that sclerostin could affect the anabolic effect of PTH via mechanisms other than inhibition of LRP5\6. In this context it is worth noting that the anabolic response to PTH is not attenuated in LRP5 knockout mice despite the fact that these animals are markedly osteopenic [13]. Finally, there are methodological differences in the two studies. Our transgenic animals were 2 months old, and we treated them with PTH for 1 month; Kramer et al. studied 6-month old transgenic animals and treated them for 2 months. Further studies using carefully controlled protocols will be needed to reconcile these findings.
A recent report indicating that the site of LRP5 action in regulating bone may not be in osteoblasts but rather in the enterochromaffin cells of the duodenum make our findings all the more interesting [26]. The work of Yadav et al. would suggest that because the principal site of action of LRP5 is the enterochromaffin cell, the Dkk1 overexpressed by our TG animals is acting systemically to suppress Wnt signaling in those target cells. This pathway would lead to an increase in serotonin secretion, which in turn suppresses osteoblast function. If this is the case, then anabolic PTH would be circumventing the suppressive effect of serotonin in osteoblasts in our Dkk1 TG animals. However, it is still possible that Dkk1 is acting directly to suppress osteoblasts in the local environment of bone. Our findings do not distinguish between these two possibilities.
The mechanism by which PTH achieves an anabolic response in WT animals has been extensively studied, and our results are consistent with previous findings from our laboratory as well as those from a number of other groups: namely, the anabolic response is characterized by an increase in parameters of both bone formation and resorption. It is presumed that the formative response precedes the increase in resorptive activity of osteoclasts and that it is this “uncoupling” of bone turnover which leads to the initial increase in bone mass during treatment. It is speculated that the eventual increase in osteoclastic activity attenuates the anabolic response to PTH with continued treatment. Surprisingly, in the Dkk1 TG animals, anabolic PTH treatment did not result in an increase in osteoclastic activity. Baseline osteoclastic activity was higher in the TG animals. However, it seems unlikely that this somewhat higher baseline resorptive activity is the explanation why osteoclast activity did not increase further with PTH treatment. In contrast to the histomorphometric findings in osteoclasts, indices of bone formation, ObS/Bs and NOb/Bpm, did increase significantly with anabolic PTH treatment in the TG animals.
This change, in conjunction with the lack of an effect on osteoclasts, suggests that the combined effects were the cellular bases for the increase in bone mass in the Dkk1 TG animals. Indeed, one might have anticipated a more robust increase in bone mass given that there was no change in osteoclast activity. The reason this was not the case may be that PTH-induced increment in bone formation was somewhat less in the Dkk1 TG animals, although it was still statistically significant.
The fact that osteoclastic activity did not increase in the TG animals raises the interesting possibility that Dkk1 could be used therapeutically to augment the anabolic response to PTH, albeit this seems somewhat counterintuitive. Nonetheless, as noted, the increase in resorptive activity that attends the anabolic response to PTH has been suggested as one of the factors limiting the therapeutic response to this drug. If Dkk1 in some fashion suppresses the resorptive response to PTH, it may be that sequential use of these two agents could result in a more sustained anabolic response to PTH.
References
Williams BO, Insogna KL (2009) Where Wnts went: the exploding field of Lrp5 and Lrp6 signaling in bone. J Bone Miner Res 24:171
Niehrs C (2006) Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25:7469–7481
Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP (2002) High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 346:1513–1521
Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD Jr (2003) The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 349:2483–2494
Qiang Y-W, Barlogie B, Rudikoff S, Shaughnessy JD Jr (2008) Dkk1-induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma. Bone (NY) 42:669–680
Heath DJ, Chantry AD, Buckle CH, Coulton L, Shaughnessy JD, Evans HR, Snowden JA, Stover DR, Vanderkerken K, Croucher PI (2009) Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J Bone Miner Res 24:425–436
Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Zhu Ke H, Li X, Richards WG (2006) Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone (NY) 39:754–766
Morvan F, Boulukos K, Clément-Lacroix P, Roman Roman S, Suc-Royer I, Vayssière Ba, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G (2006) Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res 21:934
MacDonald BT, Joiner DM, Oyserman SM, Sharma P, Goldstein SA, He X, Hauschka PV (2007) Bone mass is inversely proportional to Dkk1 levels in mice. Bone (NY) 41:331–339
Kulkarni NH, Halladay DL, Miles RR, Gilbert LM, Frolik CA, Galvin RJ, Martin TJ, Gillespie MT, Onyia JE (2005) Effects of parathyroid hormone on Wnt signaling pathway in bone. J Cell Biochem 95:1178–1190
Wan M, Yang C, Li J, Wu X, Yuan H, Ma H, He X, Nie S, Chang C, Cao X (2008) Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev 22:2968–2979
Tobimatsu T, Kaji H, Sowa H, Naito J, Canaff L, Hendy GN, Sugimoto T, Chihara K (2006) Parathyroid hormone increases beta-catenin levels through Smad3 in mouse osteoblastic cells. Endocrinology 147:2583–2590
Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, Turner CH (2006) The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem 281:23698–23711
Iwaniec UT, Wronski TJ, Liu J, Rivera MF, Arzaga RR, Hansen G, Brommage R (2007) PTH stimulates bone formation in mice deficient in Lrp5. J Bone Miner Res 22:394–402
Wan M, Yang C, Yuan H, Wu X, Lu C, Chang C, Cao X (2007) Parathyroid hormone activates beta-catenin signaling through LRP5/6. J Bone Miner Res 22:S83
Yao GQ, Wu JJ, Sun BH, Troiano N, Mitnick MA, Insogna K (2003) The cell surface form of colony-stimulating factor-1 is biologically active in bone in vivo. Endocrinology 144:3677–3682
Yao GQ, Sun BH, Weir EC, Insogna KL (2002) A role for cell-surface CSF-1 in osteoblast-mediated osteoclastogenesis. Calcif Tissue Int 70:339–346
Weir EC, Philbrick WM, Amling M, Neff LA, Baron R, Broadus AE (1996) Targeted overexpression of parathyroid hormone-related peptide in chondrocytes causes chondrodysplasia and delayed endochondral bone formation. Proc Natl Acad Sci USA 93:10240–10245
Baron R, Vignery A, Neff L, Silvergate A, Santa Maria A (1983) Bone histomorphometry. In: Recker R (ed) Techniques and interpretation. CRC Press, Boca Raton, pp 31–32
Insogna KL, Stewart AF, Vignery AM, Weir EC, Namnum PA, Baron RE, Kirkwood JM, Deftos LM, Broadus AE (1984) Biochemical and histomorphometric characterization of a rat model for humoral hypercalcemia of malignancy. Endocrinology 114:888–896
Knopp E, Troiano N, Bouxsein M, Sun BH, Lostritto K, Gundberg C, Dziura J, Insogna K (2005) The effect of aging on the skeletal response to intermittent treatment with parathyroid hormone. Endocrinology 146:1983–1990
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Semenov MV, Zhang X, He X (2008) DKK1 antagonizes Wnt signaling without promotion of LRP6 internalization and degradation. J Biol Chem 283:21427–21432
Onyia JE, Helvering LM, Gelbert L, Wei T, Huang S, Chen P, Dow ER, Maran A, Zhang M, Lotinun S, Lin X, Halladay DL, Miles RR, Kulkarni NH, Ambrose EM, Ma YL, Frolik CA, Sato M, Bryant HU, Turner RT (2005) Molecular profile of catabolic versus anabolic treatment regimens of parathyroid hormone (PTH) in rat bone: an analysis by DNA microarray. J Cell Biochem 95:403–418
Kramer I, Loots GG, Studer A, Keller H, Kneissel M (2010) Parathyroid hormone (PTH) induced bone gain is blunted in SOST overexpressing and deficient mice. J Bone Miner Res 25:178–189
Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G (2008) Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135:825–837 (see comment)
Acknowledgments
This work was supported by grants from the National Institutes of Health DK45228 and DE12459 (to K.L.I.) and in part by the Yale Core Center for Musculoskeletal Disorders (P30; AR46032).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yao, GQ., Wu, JJ., Troiano, N. et al. Targeted overexpression of Dkk1 in osteoblasts reduces bone mass but does not impair the anabolic response to intermittent PTH treatment in mice. J Bone Miner Metab 29, 141–148 (2011). https://doi.org/10.1007/s00774-010-0202-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-010-0202-3