Abstract
In heart failure (HF), metabolic disturbances represent functional perturbations in peripheral tissues and also predict patient outcomes. This study developed a simplified essential amino acid-based profile and tested whether it could improve prognostication. Plasma essential amino acids and lipidomics were measured on 1084 participants. The initial cohort included 94 normal controls and 599 patients hospitalized due to acute/decompensated HF. The validation cohort included 391 HF patients. Patients were followed for composite events (death/HF related re-hospitalization) and were categorized into three groups: high risk type 1 (leucine ≥145 μM and phenylalanine ≥ 88.9 μM), high risk type 2 (leucine < 81.2 μM), and low risk (other). Types 1 and 2 were associated with higher event rates [hazard ratio (95% confidence intervals) = 1.88 (1.27–2.79) and 7.71 (4.97–11.9), respectively, p < 0.001]. Compared to the low-risk group, both types of high-risk patients were older and had lower blood pressure and estimated glomerular filtration rates, but higher B-type natriuretic peptides (BNP). In addition, type 1 was associated with more incompletely metabolized lipids in the blood; type 2 patients had lower body mass indexes, rates of using guideline-based medications, and levels of cholesterol, hemoglobin, and albumin. The prognostic value of types 1 and 2 remained significant after adjusting for age, BNP and other risk factors. The value of using high-risk types for prognosis was confirmed in the validation cohort. In conclusion, simplified essential amino acid-based profiling identified two high-risk populations and provided metabolic information and prognostic value additive to traditional risk factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) is the leading cause of hospitalization and mortality worldwide (Savarese and Lund 2017; Rajadurai et al. 2017). Although advanced new pharmacological therapies and devices have improved outcomes, functional capacity and quality of life for HF patients remain poor, especially in the advanced stages (Lavie et al. 2013; Sperry et al. 2015). B-type natriuretic peptide (BNP) is a robust biomarker for diagnosing acute HF (Maisel et al. 2004). However, BNP represents cardiac wall stress rather than global functioning status. HF care involves the function of multiple end organs, such as the liver and skeletal muscles, and patients’ nutritional state and metabolic energy systems. New assessment tools that compensate for the weakness of BNP are needed to improve HF care and prognostication (Chioncel et al. 2016).
Circulating amino acid profiles are regarded as systemic metabolic biosignatures that represent the metabolic balance among disease-related pathophysiology, food intake, absorption, and tissue synthesis and breakdown (Hakuno et al. 2015; Griffin et al. 2011). Our previous metabolomics study demonstrated several amino acids as the best metabolites for identifying HF. We also found that the amount of total essential amino acids indicated nutritional status and provided significant prognostic value (Cheng et al. 2015). Exploring the dynamics of essential amino acids in plasma may help physicians better interpret HF-related metabolism disturbances, guide future nutritional interventions, and lead to development of new medications and devices.
Although metabolomics provides copious information about metabolism status, the limited availability of mass spectrometry and the complexity of multi-metabolite measurement present obstacles for its clinical application. Tools such as ultra-performance liquid chromatography (UPLC) make quantification of essential amino acids feasible. Aiming for clinical application, this study sought to develop a simplified plasma essential amino acid-based profile by measuring only two amino acids to risk-stratify patients after acute/decompensated HF. We tested whether the metabolism information the profiles provide has prognostic value additive to traditional risk factors and BNP.
Methods
Patients and study design
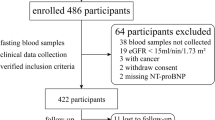
From January 2011 to May 2014, patients hospitalized due to acute or decompensated chronic HF (the American College of Cardiology and the American Heart Association HF classification system stage C) with a left ventricular ejection fraction (LVEF) of < 50%, and aged 20–85 years, were consecutively enrolled. Exclusion criteria included (i) the presence of disorders other than HF that might compromise survival within 6 months; (ii) patients being bed-ridden for > 3 months and/or unable to stand alone; (iii) the presence of systemic diseases such as hypothyroidism, decompensated liver cirrhosis, and systemic lupus erythematosus; (iv) patients with severe coronary artery disease without complete revascularization therapy; and (v) patients with a serum creatinine of > 3 mg/dl. Normal controls were aged 20–85 years, and had no significant systemic disease, such as hypertension, diabetes mellitus, or coronary artery disease. They were not on any medications, and had an LVEF of > 60%. Informed consent was obtained from all patients. The study was designed and carried out in accordance with the principles of the Declaration of Helsinki and with approval from the Ethics Review Board of Chang Gung Memorial Hospital.
To validate the prognostic value of the simplified metabolic profile, a second independent cohort, including 391 HF patients as defined above, was recruited from July 2013 to March 2016. (Study flow diagram is provided in Supplemental Fig. 1).
Prognostic value of simplified essential amino acid profile. a Blood leucine levels in patients with and without events. Dotted line boxes indicate leucine level at 145 μM; b Association between leucine level and risk of the composite events in the additive Cox regression models; c The profile for defining high-risk types 1 and 2 based on leucine (Leu) and phenylalanine (Phe) levels; d and e The Kaplan–Meier curves for high-risk types 1 and 2, and for low-risk patients in the initial and the validation cohorts, respectively (for composite events). Amino acids were measured by UPLC
Blood sampling and examination
For HF patients, blood samples for metabolomics were collected in the early morning after fasting for 8 h before discharge from hospital in EDTA-containing tubes. Plasma was analyzed by UPLC workflow. BNP was measured in triplicate with the Triage BNP Test (Biosite, San Diego, CA), which was a fluorescence immunoassay for quantitative determination of plasma BNP. Precision, analytical sensitivity and stability characteristics of the assay were previously described (Maisel et al. 2004). For normal controls, blood samples for UPLC analysis and BNP were collected at enrollment. Measurement of other parameters, including estimated glomerular filtration rate (eGFR), hemoglobin, and albumin, was conducted in the central core laboratory.
Follow-up program
Follow-up data were prospectively obtained every month from hospital records, personal communication with the patients’ physicians, telephone interviews with patients, and patients’ regular visits to staff physician outpatient clinics (followed for up to 3 years until they died, the study ended, or they were lost to follow-up). “Re-hospitalization” was defined as HF-related re-hospitalizations. A committee of three cardiologists adjudicated all hospitalizations without knowledge of patients’ clinical variables to determine whether the events were related to worsening HF. “All-cause death” was chosen as an endpoint because of the interrelationship of HF with other comorbidities in the patient cohort. The composite event of HF-related re-hospitalization and all-cause death (time to the first event) was analyzed for prognostic purposes. We also performed a competing risks analysis. For validation, patients in the second independent population were followed up for one year.
Ultra-performance liquid chromatography-based amino acid measurement
EDTA plasma samples were collected and stored at − 80 °C until assayed. The plasma samples (100 μl) were precipitated by adding an equal volume (100 μl) of 10% sulfosalicylic acid containing an internal standard (norvaline 200 µM). After protein precipitation, the samples were vortexed and centrifuged at 12,000 g for 10 min at room temp. After the samples were centrifuged, 20 μl of the supernatant was mixed with 60 μl working buffer (borate buffer, pH 8.8). The derivatization was initiated by the addition of 20 μl of 10 mM AQC in acetonitrile. After 10 min incubation, the mixture was added with an equal volume Eluent A (20 mM ammonium formate/0.6% Formic acid/1% acetonitrile) and analyzed using the ACQUITY UPLC System (Frank and Powers 2007; Pappa-Louisi et al. 2007). AQC derivatization reagent was obtained from Waters Corporation (Milford, MA, USA). An aqueous amino acid standard mixture was prepared at different concentrations (0, 25, 50, 100, 250, 500 μM) for each amino acid and was done by the same procedure. The Waters ACQUITY UPLC® System consisted of a Binary Solvent Manager (BSM), a Sample Manager fitted with a 10 μl loop, and a Tunable UV (TUV) detector. The system was controlled and data collected using Empower™ 2 Software. Separations were performed on a 2.1 × 100 mm ACQUITY BEH C18 column at a flow rate of 0.70 mL/min. The average intra-assay coefficient of variation was 4.5% for leucine and 4.6% for phenylalanine. A total coefficient of variation was 4.1% for leucine and 3.7% for phenylalanine. The limit of detection was 0.9 μM for leucine and 3.3 μM for phenylalanine. The linear range was 25–500 μM for these amino acids. For other amino acids, the average intra-assay coefficient of variation was 4.3–4.6% and the limit of detection was 0.1–4.7 μM. Leucine and isoleucine were separated by UPLC.
Quantification of plasma acylcarnitines by tandem mass spectrometry
The acylcarnitine analyses were carried out with the AbsoluteIDQ® p180 Kit (Biocrates Life Science AG, Innsbruck, Austria) (Cheng et al. 2015). The kit enabled us to identify and quantify 40 acylcarnitines. 10 μL aliquot of each plasma sample was mixed with isotopically labeled internal standards in a multititer plate, and dried under nitrogen. The samples were derivatized with 5% phenylisothiocyanate (PITC) for the amino acids and biogenic amines, and dried again. 300 μL of extraction solvent (5 mM ammonium acetate in methanol) was added, and after 30 min incubation, it was centrifuged for 2 min at 100 × g. The filtrate of sample extracts was diluted with kit running solvent (Biocrates Life Sciences AG) for flow injection analysis coupled with tandem mass spectrometric analysis (FIA-MS/MS), Xevo TQ mass spectrometer (Waters Corporation). The analysis was performed in positive electrospray ionization mode. Identification and quantification were achieved by multiple reactions monitoring (MRM). The FIA-MS/MS method was used (100% organic running solvent) with varying flow conditions (0 min, 30 μL/min; 1.6 min 30 μL/min; 2.4 min, 200 μL/min; 2.8 min, 200 μL/min; 3 min 30 μL/min). The corresponding MS settings were as follows: dwell time of 0.019–0.025 s; capillary voltage at 3.92 kV for positive mode; capillary voltage at 1.5 kV for negative mode; nitrogen as collision gas medium; source temperature at 150 °C. It was standardized by spiking in of isotopically labeled standards. For example, deuterium-labeled acylcarnitine standards, including D3-C2, D3-C3, D3-C4, D9-C5, D3-C6, D3-C8, D3-C10, D3-C16, and D3-C18 carnitines, were used for C2, C3, C4, C5, C6, C8, C10, C16, and C18, respectively. For a subset of acylcarnitines, measurements were semi-quantitative since specific standards were not commercially available. A detailed overview of the measured acylcarnitines is provided in Supplemental Table I.
Statistical analyses
Results are expressed as the mean ± SD for variables with normal distribution, and median (interquartile range) for variables with skewed distribution, and as the number (percentage) for categorical variables. Data were compared by two-sample t tests, ANOVA (subgroup analysis was conducted by Bonferroni correction) and Chi square (multiple comparison with Bonferroni-adjusted p values) when appropriate. Receiver operating characteristic (ROC) curves were estimated, and we used Youden’s index to identify the cutoff value of variables. Follow-up data were collected as scheduled or at the last available visit. We used Cox proportional hazards models with stepwise analysis to determine independent predictors of the first defined events (death, or HF-related re-hospitalization). Variables with p value < 0.05 in the univariate analysis were selected for the multivariable analysis. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. To compare the time-dependent outcomes, we performed Kaplan–Meier analyses with a log-rank test. Furthermore, since all-cause death and HF-related re-hospitalizations were also competing risks, we used a competing risks analysis model to estimate the risk of each of these events independent of each other. Since we have finished the validation study, we used R software to calculate the observed power. Based on the data of the control group (n = 257, event rate = 0.202 at 1 year) and the high-risk type 2 group (n = 45, event rate = 0.556 at 1 year, with a hazard ratio of 3.62) in the validation cohort, the observed power was found to be 99.9%, indicating adequate sample size. All statistical analyses were two-sided and performed using SPSS software (version 22.0, SPSS, Chicago, IL, USA) and R software (version 3.5.1). A p value of < 0.05 was considered significant.
Results
Baseline characteristics
The study enrolled a total of 599 HF patients and 94 normal controls. We observed 164 (27.4%) composite events among the HF patients over a follow-up period of 3 years, including 72 (11.9%) deaths and 132 (22.7%) HF-related re-hospitalizations. Comparisons of baseline characteristics and laboratory data between patients with and without composite events are shown in Table 1. Patients who experienced events were older, more often female, had higher New York Heart Association functional classes, incidence of diabetes mellitus, chronic kidney disease, hypertension and use of diuretics, and higher BNP levels compared to the patients with no events. They also had lower systolic blood pressure and incidence of using beta-blockers, as well as lower total cholesterol, triglyceride, hemoglobin, albumin, and eGFR. No significant difference in left ventricular ejection fraction was noted between the two groups.
Identification of amino acids with potential for assessing prognosis
Comparisons of all amino acids between patients with and without composite events are shown in Table 2 (measured by UPLC). Plasma levels of all essential amino acids were lower in patients who experienced events compared to those who did not, except for phenylalanine, which increased in patients with events. To identify amino acids that might be useful for prognosis, we performed Cox univariate and multivariable analysis. Results showed that four essential amino acids—leucine, methionine, phenylalanine and tryptophan—have strongly significant prognostic value, independent of others. In addition, leucine had the best correlation with the total amount of essential amino acids (r = 0.83, p < 0.001), followed by methionine, phenylalanine and tryptophan (r = 0.42, 0.35 and 0.10, respectively). We, therefore, used leucine as the main essential amino acid for risk stratification. To keep the quality of measuring amino acids stable, we used UPLC to measure these amino acids for statistical analysis in the initial and the validation studies.
Leucine levels were poorly correlated with albumin levels (r = 0.26, p < 0.001). Although lower leucine levels were significantly associated with higher event rates, a significant portion of events also happened in patients with high leucine levels (Fig. 1a), indicating a reverse J shaped relationship (Fig. 1b). We performed the ROC curve analyses using a cutoff value based on Youden’s index (supplemental Fig. II). Based on the cutoff, patients were separated into two subgroups (patients with leucine levels ≥145 μM and < 145 μM). In these two subgroups, we performed multivariable analyses to identify the amino acids that were independently significant for prognosis. In patients with leucine levels ≥ 145 μM and < 145 μM, phenylalanine (HR 1.87, 95% CI 1.26–2.78) and leucine (HR 2.80, 95% CI 2.25–3.48) were found to be the best prognostic amino acids, respectively; including other amino acids did not provide additional prognostic value. Based on ROC curves, the cutoff points for phenylalanine and leucine were set at 88.9 and 81.2 μM, respectively. On this basis, we created a profiling method and categorized patients into low risk or high risk types 1 and 2 (Fig. 1c).
Confirming the value of the profile for stratifying risk, Cox univariate analysis showed significant prognostic value for high-risk types 1 and 2 (Table 3). Further Cox multivariable analysis showed that both high-risk types 1 and 2 retained significant prognostic value after adjusting for age, sex, diabetes mellitus, chronic kidney disease, hypertension, hemoglobin, albumin, and BNP (model 1) and after cumulative adjustment for New York Heart Association functional class, diastolic blood pressure, body mass index, medications and cholesterol (model 2). Kaplan–Meier curves showed that both type 1 and 2 high-risk patients had lower three-year event-free survival rates compared to patients classified as low risk (Log rank = 9.98 and 118.7, p = 0.002 and < 0.001, respectively) (Fig. 1d). The competing risks analysis also demonstrated that the high-risk types had value for predicting each of these events independent of each other (p < 0.001; Supplemental Fig. III and Table II). The prognostic value of the metabolic profile was further validated in 391 stage C patients. During the 1-year follow-up period, 111 (28.4%) composite events were noted. Kaplan–Meier curves show that both type 1 and 2 high-risk patients had lower 1-year event-free survival rates compared to low-risk patients (Fig. 1e). The demographic data for the validation cohort are shown in Supplemental Table III.
Since patients who suffered an event had a significantly higher rate of diabetes mellitus and hypertension, we conducted subgroup analysis for patients with diabetes mellitus and hypertension (supplemental Fig. IV). In the different subgroups, the cutoff values for leucine and phenylalanine were slightly different. However, the prognostic value of high-risk types 1 and 2 remained significant in these subgroups.
Characteristics of patients at high risk
Compared to the low-risk group, both types of high-risk patients were older, had lower blood pressure and eGFR, but higher New York Heart Association functional classes, and BNP. In addition, high-risk type 2 patients were more often female, but had lower body mass indexes; they also had a lower percentage of using guideline-based medications, and lower levels of cholesterol, hemoglobin, and albumin (Table 4).
Lipidomics in patients of different risk types
In this study, in addition to our amino acid-based definition of high risk, we would like to provide additional supportive data based on other metabolites as well. Since the value of acylcarnitines in assessing the severity of HF has been demonstrated (Ahmad et al. 2016), we investigated lipidomics by measuring a variety of acylcarnitines in normal controls and patients with different risk types (Table 5) (measured by tandem mass spectrometry). Compared to the normal controls, HF patients at low risk of events had higher levels of long-chain (C18:1, C18:2, C16, and C10:2) and short-chain acylcarnitines (C5 and C4). It is noteworthy that compared to the low-risk group, high-risk type 1 was associated with higher levels of long- (C18, C18:1, C14, C14:2, C12, C12:1, C10, and C10:1), medium- (C9, C8, and C6:1) and short-chain acylcarnitines (C5, C5:1, C4, C3 and C2). High-risk type 2 patients had similar levels of long-, medium- and short-chain acylcarnitines, but they had lower levels of C3 compared to the low risk group.
Discussion
This study developed a novel risk stratification strategy for patients with acute/decompensated HF based on a simplified essential amino acid-based profile. Our profile defined two different types of patients at high risk for HF-related events, namely, high risk types 1 and 2. The different risk types demonstrated different metabolic and nutritional statuses associated with HF. Both high-risk types provided prognostic value additive to BNP and traditional risk factors.
BNP is a powerful diagnostic biomarker for HF, with ST2 and galectin-3 providing additional prognostic information (Maisel et al. 2004; Wang et al. 2016; Rehman et al. 2008). However, all these biomarkers offer limited information beyond wall stress and activation of global fibrosis. Previous studies have shown that metabolic assessment provided superior prognostic value for HF patients compared to BNP and galectin-3 (Cheng et al. 2015; Wang et al. 2017). This study revealed that our simplified amino acid profile had prognostic value independent of albumin, BNP and clinical risk factors. The profile provides a quantifiable readout with information that is often not obvious from assessing cardiac wall stress and traditional nutritional biomarkers.
Based on the amino acid profile, high-risk type 1 was defined by high circulating leucine and phenylalanine levels. Of all essential amino acids, our data showed that leucine, phenylalanine, tryptophan and methionine were independently significant for identifying HF patients at high risk of composite events. Additionally, leucine had the best correlation with the total amount of essential amino acids that have been shown to predict HF-related events (Cheng et al. 2015). Thus, we used leucine levels as the main biomarker for risk stratification.
Interestingly, we noted a reverse J shaped relationship between leucine levels and event rates. Although lower leucine levels predicted higher event rates, event rates were also increased in patients with high leucine and phenylalanine levels. Phenylalanine was the only essential amino acid that was elevated in HF patients compared to the normal controls, suggesting a pathophysiological link. Nitric oxide synthesis dysregulation and tetrahydrobiopterin depletion were probably related to impaired conversion of phenylalanine to tyrosine, followed by accumulation of phenylalanine (Nishijima et al. 2011; Ziolo et al. 2004). Furthermore, the increase of phenylalanine levels may also be associated with increased muscular protein breakdown (Aquilani et al. 2012) and impaired liver function caused by HF. Accordingly, the increased leucine and phenylalanine levels in type 1 high-risk patients indicate substantial tissue breakdown, which is probably related to insufficient tissue perfusion, increased insulin resistance, and dysfunctional energy production machinery associated with HF. Accumulated circulating long-chain and medium-chain acylcarnitines point to mitochondrial β-oxidation dysfunction in processing lipids for energy production, which may further cause insulin resistance (Hunter et al. 2016; Schooneman et al. 2013). Short-chain acylcarnitines are partially derived from the metabolism of amino acids; elevated levels are thus compatible with the suggested phenomena of tissue protein breakdown for energy production. However, the formal evaluation of muscle protein dynamics involves complex calculation based on isotope infusion studies (Nagabhushan and Narasinga Rao 1978; Liu and Barrett 2002), which are not applicable to patients with HF.
Intriguingly, high-risk type 2 is associated with remarkable malnutrition as indicated by extremely low amounts of circulating amino acids; such low levels could shut down a variety of protein and enzyme functions, causing HF deterioration. The type 2 high-risk population presented circulating acylcarnitine levels similar to those of the low-risk population—even lower for some short-chain acylcarnitines. It is worth noting that type 2 high-risk patients were characterized by a variety of clinical risk factors indicating poor prognosis. One advantage of amino acid-based risk stratification is its potential for providing information about nutritional deficiency as part of risk assessment, as well as enabling clinicians to propose plans for intervention. It remains to be elucidated whether patient outcomes can be improved if the type 2 risk pattern is identified and corrected earlier.
Although researchers search enthusiastically for a nutritional regimen specific for HF, our findings do not point to a universal solution. Nutritional supplementation as recommended by current guidelines may not be adequate for managing the metabolic changes associated with HF from moment to moment. Both HF patients and patients in critical condition are commonly subjected to over- and under-feeding (Reid 2006). Both practices are harmful, yet poorly defined. A strategic approach to providing appropriate calorie and protein supply would attempt to switch the catabolic state of amino acids to an anabolic state, benefitting patients by shifting them away from the high-risk profiles, while maintaining appropriate metabolic condition.
Study limitations
This study has a few limitations. Although we have identified essential amino acids critical for prognosis, the causal mechanisms to explain why they work are speculative and not well established. To clarify the value of amino acid profiling, serial measurement is needed to understand the dynamics of the amino acid profile over time and to track changes associated with nutrition and medications.
Conclusions
Our study shows that a simplified amino acid-based risk stratification profile provides information on amino acid and lipid metabolism in HF patients with robust prognostic value additive to BNP and traditional risk factors. High-risk type 1 represents patients with substantial metabolism disturbance and accumulated circulating waste of incompletely metabolized lipids. High-risk type 2 represents patients characterized by severe malnutrition and multiple clinical factors signaling poor prognosis. Future research is needed to investigate whether multidisciplinary interventions based on such profiles can improve outcomes among patients who are already receiving guideline-based medications.
Abbreviations
- BNP:
-
B-type natriuretic peptide
- CI:
-
Confidence interval
- eGFR:
-
Estimated glomerular filtration rate
- HR:
-
Hazard ratio
- HF:
-
Heart failure
- Leu:
-
Leucine
- LVEF:
-
Left ventricular ejection fraction
- Phe:
-
Phenylalanine
- ROC:
-
Receiver operating characteristic curve
- UPLC:
-
Ultra-performance liquid chromatography
References
Ahmad T, Kelly JP et al (2016) Prognostic implications of long-chain acylcarnitines in heart failure and reversibility with mechanical circulatory support. J Am Coll Cardiol 67(3):291–299
Aquilani R, La Rovere MT et al (2012) Preserved muscle protein metabolism in obese patients with chronic heart failure. Int J Cardiol 160(2):102–108
Cheng ML, Wang CH et al (2015) Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure: diagnostic and prognostic value of metabolomics. J Am Coll Cardiol 65(15):1509–1520
Chioncel O, Collins SP et al (2016) Natriuretic peptide-guided management in heart failure. J Cardiovasc Med 17(8):556–568
Frank MP, Powers RW (2007) Simple and rapid quantitative high-performance liquid chromatographic analysis of plasma amino acids. J Chromatogr B Analyt Technol Biomed Life Sci 852(1–2):646–649
Griffin JL, Atherton H et al (2011) Metabolomics as a tool for cardiac research. Nat Rev Cardiol 8(11):630–643
Hakuno D, Hamba Y et al (2015) Plasma amino acid profiling identifies specific amino acid associations with cardiovascular function in patients with systolic heart failure. PLoS One 10(2):e0117325
Hunter WG, Kelly JP et al (2016) Metabolic dysfunction in heart failure: diagnostic, prognostic, and pathophysiologic insights from metabolomic profiling. Curr Heart Fail Rep 13(3):119–131
Lavie CJ, Berra K et al (2013) Formal cardiac rehabilitation and exercise training programs in heart failure: evidence for substantial clinical benefits. J Cardiopulm Rehabil Prev 33(4):209–211
Liu Z, Barrett EJ (2002) Human protein metabolism: its measurement and regulation. Am J Physiol Endocrinol Metab 283(6):E1105–E1112
Maisel AS, Clopton P et al (2004) Impact of age, race, and sex on the ability of B-type natriuretic peptide to aid in the emergency diagnosis of heart failure: results from the breathing not properly (BNP) multinational study. Am Heart J 147(6):1078–1084
Nagabhushan VS, Narasinga Rao BS (1978) Studies on 3-methylhistidine metabolism in children with protein-energy malnutrition. Am J Clin Nutr 31(8):1322–1327
Nishijima Y, Sridhar A et al (2011) Tetrahydrobiopterin depletion and NOS2 uncoupling contribute to heart failure-induced alterations in atrial electrophysiology. Cardiovasc Res 91(1):71–79
Pappa-Louisi A, Nikitas P et al (2007) Optimization of separation and detection of 6-aminoquinolyl derivatives of amino acids by using reversed-phase liquid chromatography with on line UV, fluorescence and electrochemical detection. Anal Chim Acta 593(1):92–97
Rajadurai J, Tse HF et al (2017) Understanding the epidemiology of heart failure to improve management practices: An Asia-Pacific Perspective. J Card Fail 23(4):327–339
Rehman SU, Mueller T et al (2008) Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol 52(18):1458–1465
Reid C (2006) Frequency of under- and overfeeding in mechanically ventilated ICU patients: cause and possible consequences. J Hum Nutr Diet 19(1):13–22
Savarese G, Lund LH (2017) Global public health burden of heart failure. Card Fail Rev 3(1):7–11
Schooneman MG, Vaz FM et al (2013) Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes 62(1):1–8
Sperry BW, Ruiz G et al (2015) Hospital readmission in heart failure, a novel analysis of a longstanding problem. Heart Fail Rev 20(3):251–258
Wang CH, Yang NI et al (2016) Estimating systemic fibrosis by combining galectin-3 and ST2 provides powerful risk stratification value for patients after acute decompensated heart failure. Cardiol J 23(5):563–572
Wang CH, Cheng ML et al (2017) Metabolic profile provides prognostic value better than galectin-3 in patients with heart failure. J Cardiol 70(1):92–98
Ziolo MT, Maier LS et al (2004) Myocyte nitric oxide synthase 2 contributes to blunted beta-adrenergic response in failing human hearts by decreasing Ca2+ transients. Circulation 109(15):1886–1891
Acknowledgement
The authors thank Cardiology Section, Department of Internal Medicine, Chang Gung Memorial Hospital, Keeling, Taiwan for providing samples from patients and normal controls. We also thank Healthy Aging Research Center, Chang Gung University from the Featured Areas Research Center Program within the Framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan.
Funding
This study was supported in part by the Ministry of Science and Technology of Taiwan (MOST105-2314-B-182-046-MY2, 107-2314-B-182-071-MY2); Chang Gung Memorial Hospital (CMRPG2C0313, G2E0351, G2G0601, G2G0581); and the Ministry of Education of Taiwan (EMRPD1G0251, EMRPD1H0401).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Handling Editor: D. Tsikas.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, CH., Cheng, ML. & Liu, MH. Simplified plasma essential amino acid-based profiling provides metabolic information and prognostic value additive to traditional risk factors in heart failure. Amino Acids 50, 1739–1748 (2018). https://doi.org/10.1007/s00726-018-2649-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-018-2649-9