Abstract
This study investigated the effects of leucine or leucine + glutamine supplementation on recovery from eccentric exercise. In a double-blind independent groups design, 23 men were randomly assigned to a leucine (0.087 g/kg; n = 8), leucine + glutamine (0.087 g/kg + glutamine 0.3 g/kg; n = 8) or placebo (0.3 g/kg maltodextrin; n = 7) group. Participants performed 5 sets of drop jumps, with each set comprising 20 repetitions. Isometric knee-extensor strength, counter-movement jump (CMJ) height, delayed-onset muscle soreness (DOMS) and creatine kinase (CK) were measured at baseline, 1, 24, 48 h and 72 h post-exercise. There was a time × group interaction for isometric strength, CMJ and CK (P < 0.05), with differences between the leucine + glutamine and placebo group at 48 h and 72 h for strength (P = 0.013; d = 1.43 and P < 0.001; d = 2.06), CMJ (P = 0.008; d = 0.87 and P = 0.019; d = 1.17) and CK at 24 h (P = 0.012; d = 0.54) and 48 h (P = 0.010; d = 1.37). The leucine group produced higher strength at 72 h compared to placebo (P = 0.007; d = 1.65) and lower CK at 24 h (P = 0.039; d = 0.63) and 48 h (P = 0.022; d = 1.03). Oral leucine or leucine + glutamine increased the rate of recovery compared to placebo after eccentric exercise. These findings highlight potential benefits of co-ingesting these amino acids to ameliorate recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Specific forms of exercise require eccentric muscle loading patterns, such as plyometric training (Twist et al. 2008), which increase the amount of mechanical stress on the muscle. During eccentrically biased exercise, the external forces applied to muscle groups overcome their internal resistance, resulting in the muscle lengthening under tension (Howatson and van Someren 2008). During eccentric contractions, lower muscle activation and preferential recruitment of fast-twitch fibres leads to greater tension per muscle fibre and a bias toward type II muscle fibre damage (Shepstone et al. 2005). Loading the lengthening muscle under tension causes greater myofibrillar damage and so-called sarcomere popping, indicating mechanical damage to the cellular structures (Lieber and Friden 1999). As a result, exercise-induced muscle damage (EIMD) is typically observed after resistance exercise but is exacerbated when eccentric exercise is performed, relative to concentric exercise at the same intensity (Proske and Morgan 2001). This can be intended by the athlete to promote muscle growth by inducing greater mechanical tension, thus disturbing the integrity of skeletal muscle and promoting microdamage in muscle fibres (Schoenfeld 2010, 2012).
In the days (24–72 h) following eccentrically biased exercise, EIMD is manifested by a transient decrease in force production, delayed-onset muscle soreness (DOMS) and leakage of intramuscular proteins into the circulation (i.e. creatine kinase; CK) (Sorichter et al. 1999). The derangement of intracellular Ca2+ homeostasis, caused by the insult of heavy resistance exercise, initiates a cascade of intracellular events that lead to the activation of proteolytic and lipolytic pathways, thus damaging cellular structures (Gissel and Clausen 2001). These processes give rise to a secondary inflammatory phase, whereby protein uptake is increased for use as an energy substrate or to mediate cell signalling pathways that are necessary for muscle and connective tissue remodelling (Nicastro et al. 2012).
Given the demands of frequent resistance training, full and rapid recovery between bouts of exercise is desirable. Therefore, interventions that help to attenuate the effects of muscle damage are beneficial to the athlete by reducing the decline in physical function and permitting greater engagement with training in the days following exercise (Cheung et al. 2003; Proske and Morgan 2001; Howatson and van Someren 2008). One type of branched-chain amino acid (BCAA), namely leucine, can be prophylactically ingested to attenuate symptoms of muscle damage (da Luz et al. 2011). Supplementation of leucine has been suggested to suppress muscle proteolysis (Zanchi et al. 2008) and reduce protein oxidation (Shimomura et al. 2009) after muscle-damaging exercise, thus helping to balance protein turnover in the cell, as well as maintaining the integrity of the muscle cell membrane. Indeed, muscle protein synthesis is directed toward the repair or remodelling of structural and contractile proteins in the days after muscle-damaging exercise (McGlory et al. 2017). This is relevant because skeletal muscle proteins, such as CK, lactate dehydrogenase (LDH) or myoglobin (Mb), are known to exit the cell and indirectly infer cellular damage, acting as surrogate markers of muscle damage. For example, Kirby et al. (2012) reported reductions in serum Mb and CK concentration 24 h following eccentrically biased exercise after subjects were supplemented with 250 mg/kg body mass of leucine 30 min before, during and immediately post-exercise and the morning of each recovery day.
Whist leucine is an effective recovery supplement when co-ingested with other BCAAs (Howatson et al. 2012; Waldron et al. 2017), it is possible that leucine is more effective for cellular recovery when it is not mixed with BCAA solutions. This may be due to the reported competition between leucine, isoleucine and valine for cellular transport (Cynober 2002). Indeed, the combination of leucine with other amino acids (AA), such as glutamine, has greater theoretical support. This relates to the putative roles of leucine during the acute inflammatory phase of muscle damage (see Rowlands et al. 2016), which relies upon the known transamination of leucine into glutamate. This process effectively contributes to the glutamate–glutamine pool (Golden et al. 1982), which is a substrate for inflammatory cells (Gleeson 2008). Indeed, given the numerous cellular interactions between glutamine and leucine (Nicastro et al. 2012), it is possible that optimal combinations of leucine and glutamine would ameliorate recovery through anti-inflammatory processes. Glutamine, ingested alone, has also been shown to reduce strength losses following eccentric exercise (Street et al. 2011). However, there is no study examining the effects of leucine, in combination with other anti-inflammatory amino acids, on the recovery from muscle-damaging exercise.
Therefore, the aim of this study was to investigate the effects of acute body-mass dependent leucine or leucine + glutamine supplementation on recovery from eccentrically biased exercise among recreational athletes. It was hypothesised that the leucine or leucine + glutamine supplementation would attenuate symptoms of muscle damage compared to the placebo group, but that the co-ingestion group would have the largest effects on recovery.
Methods
Participants
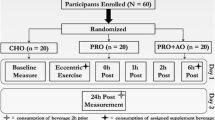
Twenty-three males (mean ± SD age 21 ± 1 years, stature 180.2 ± 6.1 cm, body mass 86.5 ± 7.9 kg) consented to take part in this study. A total sample of 18 was required, based on an effect size of 0.5 and statistical power of 0.95. Informed consent was obtained from all individual participants included in the study. All participants were recreationally resistance-trained athletes, with a minimum of 1 year training history. To be included in this study, the participants had to be injury-free and train on a weekly basis using a mixture of resistance exercises. Participants were initially screened for any recent injuries or movement compensations that may cause pain or discomfort when performing the movements to be included in the study (i.e. drop jumps). Ethical approval was granted for this study by the Institutional ethics committee. All procedures were performed in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Design
Two weeks prior to testing, participants were told to cease any use of nutritional supplements, additional to their normal diet, such as protein supplements, creatine and AA. The participants were advised to avoid any drugs with anti-inflammatory properties and not to use compression garments or seek therapeutic intervention, such as hydrotherapy treatments or forms of massage. Participants were also provided with daily diet suggestions to follow from 48 h before the study until their final testing day. This comprised a macronutrient composition of 50% carbohydrate, 15% protein (of similar amino acid content) and 35% fat. The participants visited the laboratory at the same time of day on five separate days, approximately 2 h after eating breakfast. During visit 1, the participants were familiarised with the testing procedures and were weighed for subsequent calculation of the leucine supplement. The participants were also familiarised with the muscle soreness scale and muscle function test, as well as the specific instructions for how to perform a drop jump, including intensity and technique, as this would be the mode of muscle damage during the study. Familiarisation was deemed to be sufficient after one visit as the participants were consistent in their performance on all tests and indicated that they were comfortable in performing them.
After visit 1, the participants were assigned to one of the three conditions by an independent laboratory technician (leucine, leucine + glutamine or placebo) in a double-blind, independent groups design. The participants were matched on their counter-movement jump height to ensure a baseline similarity in a functional measure of physical fitness, which was determined at familiarisation. The randomisation was carried out by assigning each participant a number and using publicly available software to allocate their group (http://www.randomization.com/).
Visit 2 was carried out 72 h after visit 1, with no other exercise performed in between. At visit 2, the participants had capillary blood samples drawn from the finger for the measurement of baseline CK and then performed a battery of baseline tests in the following order: perceived soreness, lower-limb isometric strength and counter-movement jumping. After the baseline testing, participants were given the supplement and 30 min later they were supervised through the muscle-damage protocol. Following this, a second supplement was ingested and 30 min later the battery of tests was repeated. Visits 3, 4 and 5, took place at 24, 48 and 72 h, respectively, after the initial muscle damage protocol. At each of these visits the same battery of tests was performed before and after the muscle damage protocol. At visits 3 and 4, supplements were provided 30 min before and 30 min after the muscle damage protocol. At visit 5, a morning supplement was provided, as well as the final supplement, 30 min prior to the muscle damage protocol.
Procedure
Knee-extensor isometric strength
To test the maximal isometric strength of the knee-extensor muscles, each participant sat on a custom-made, adaptable strength chair, with their back and knees fully supported. Their knee was firmly fixed at 100° and their hips at 110°, which was verified using a goniometer. Their right leg was firmly strapped to the chair across the mid-thigh, whilst their ankle (immediately above malleoli) was fixed to a strain gauge (Interface SSM-AJ-500 Force Transducer; Interface, Scottsdale, AZ; 0.05% maximum error), sampling at 1000 Hz. The strain gauge recorded force as alteration in voltage. Calibration of the strain gauge with a known mass demonstrated the relationship between voltage and Newtons as linear, allowing determination of a regression formula to convert voltage to Newtons. A second calibration was performed with the same weights at the completion of testing, producing an ICC of 0.99. The strain gauge was attached to the participant using a high-tension belt. The chair setup was replicated for each participant in subsequent trials. The participants’ upper-body was also tightly fitted to the chair with two stabilisation straps across each shoulder, which they were instructed to grip with their hands throughout the testing. A command of ‘3-2-1-GO’ was given, after which the participants performed a maximal isometric knee extension for 5 s. Non-specific verbal encouragement was provided to the participants for motivation. Participants performed three maximal tests, separated by 2 min. A maximal voluntary contraction was determined as the highest of three values and recorded for analysis. If the peak force (N) produced by participants systematically increased across the three tests, a fourth test was conducted. The reliability of this procedure was 2% (coefficient of variation; CV).
Counter-movement jumping (CMJ)
Participants performed a CMJ on a jump mat (Probiotics Inc, Huntsville, AL, USA) by standing with their feet at shoulder width, hands on hips and descending to ~ 90° before propelling themselves vertically to the highest possible height, keeping their legs fully extended. Standardised non-specific motivation and cues were provided to facilitate performance. The participants performed three jumps, separated by 2 min and the highest jump height (cm) was recorded. If the values systematically increased across the three tests, a fourth test was conducted. The test–re-test reliability of this procedure was 1.2% (CV).
Blood sampling and analysis
The index fingertip of the subject was cleaned using a sterile alcohol swab and allowed to dry. Capillary blood was drawn from the finger and a sample of whole blood (30 μL) was collected into a heparinised capillary tube. The whole blood was centrifuged at 3000 rpm (4 °C) for 5 min, and the resultant plasma was removed and stored at − 80 °C until subsequent analysis. Plasma CK was measured using a chemistry analyser (Rx Monza, Randox Laboratories Ltd., Crumlin, Antrim, UK). The intra-sample CV of the analyser is < 4% CV at high and low concentrations and the expected baseline sample range is 37–2755 IU/L for CK, according to manufacturer’s guidelines. To eliminate inter-assay variance, all samples were analysed in the same assay run.
Perceived soreness
The participants were asked to rate their perceived muscle soreness in the lower-limbs from 0 to 10 on a 200 mm Visual Analogue Scale (VAS). The numbers were concealed from the participant on the reverse of the scale, whilst the verbal anchors of no muscle soreness (0 on reverse), soreness upon movement (5 on reverse) and too sore to move (10 on reverse) were observed from the front of the scale. To do this, the participants performed a 5-s isometric squat, with their ankles, knees and hips at 90° and, after 5 s, moved a sliding scale to the number which they perceived to correspond to their level of soreness (Howatson et al. 2012).
Supplementation
All supplements were sourced from the same company (Myprotein, Cheshire, UK). Each participant was supplemented with one of three supplements: a placebo, a leucine beverage or a leucine + glutamine beverage, all of which contained 0.3 g/kg body mass of maltodextrin dissolved into 300 ml of water. This ensured that the drinks were indistinguishable in taste. The leucine drink was provided at a high dose of 0.087 g/kg (87 mg/kg) body mass (Børsheim et al. 2002). This dosage of AA has been shown to promote recovery from resistance exercise (Børsheim et al. 2002) and is between the dosages provided in previous studies, which range between 22.5 and 250 mg/kg of body mass (Stock et al. 2010; Kirby et al. 2012). The highest doses were not chosen so that the leucine + glutamine group could comfortably co-ingest with an additional 0.3 g/kg body mass of glutamine (Street et al. 2011) and without noticing the taste or difference in the drinks consistency. Drinks were consumed 30 min before and after the muscle damage protocol (Jackman et al. 2010). Over the following 72 h, the supplements were provided 30 min before and after re-testing. On the final day, the supplement was taken with breakfast and 30 min before testing to provide two doses. The supplements were prepared by an independent laboratory technician.
Muscle-damage protocol
A standardised warm-up was performed on the day, comprising walking, jogging and dynamic stretching. The participants then performed 5 sets of drop jumps from a 60 cm box, with each set comprising 20 repetitions (100 repetitions total) (Howatson et al. 2012). Participants were provided with 10 s between each jump, with 2 min rest between sets. All of the participants were able to complete the protocol.
Statistical analyses
After checks for sphericity, a two-way within and between analysis of variance was performed to evaluate the main effects of time (baseline, immediately post, 24, 48 and 72 h post-exercise) and group (placebo, leucine and leucine + glutamine) and their interactions on the dependent variables. If tests of sphericity were violated, the Greenhouse–Geisser correction was used. In the event a statistical difference was identified, a post hoc Bonferroni test was used to identify differences. The dependent variables were isometric strength, CK concentration, delayed-onset muscle soreness and counter-movement jump height (each expressed relative to baseline; %). Effect sizes (Cohen’s d) were also performed on pairwise comparisons and defined as; trivial = 0.2; small = 0.21–0.6; moderate = 0.61–1.2; large = 1.21–1.99; very large > 2.0 (Batterham and Hopkins 2006). An alpha level of P ≤ 0.05 was set for all analyses. Statistical analysis was conducted through IBM SPSS (Software V22.0, IBM, New York, USA).
Results
All absolute changes (unit-specific) are presented in Table 1. All relative changes (% baseline) are presented in Figs. 1, 2, 3 and 4.
Isometric knee-extensor force (% baseline) at baseline, immediately post-exercise and 24, 48 and 72 h post-exercise in placebo (n = 7), leucine (n = 8) and leucine + glutamine (n = 8) groups. Leu = leucine; Glu = glutamine and *sig. different between Leu + Glu and placebo; †sig. different between leucine and placebo. SD bars removed for clarity
Creatine kinase concentration (CK % baseline) at baseline, immediately post-exercise and 24, 48 and 72 h post-exercise in placebo (n = 7), leucine (n = 8) and leucine + glutamine (n = 8) groups. *sig. different between Leu + Glu and placebo; †sig. different between leucine and placebo. SD bars removed for clarity
Changes in isometric force (% baseline) are presented in Fig. 1 (mean ± SD). There were main effects of time for isometric strength (F (4,80) = 135.3; P < 0.001), with post hoc tests demonstrating differences between baseline and all subsequent time points (P < 0.001) apart from 72 h, where strength returned to baseline (P = 1.000). There was a time \( \times \) group interaction (F (8,80) = 2.161; P = 0.039), with post hoc tests identifying differences between the leucine + glutamine and the placebo group at 24 h (91.4 ± 3.4% vs. 87.5 ± 3.2%; P = 0.045; d = 1.09), at 48 h (88.1 ± 3.2% vs. 82.6 ± 2.9%; P = 0.013; d = 1.43) and at 72 h (102.7 ± 3.0% vs. 96.2 ± 3.8%; P < 0.001; d = 2.06). The leucine group also demonstrated higher strength at 72 h compared to the placebo group (100.4 ± 1.2% vs. 96.2 ± 3.8%; P = 0.007; d = 1.65).
Changes in CMJ height (% baseline) are presented in Fig. 2. There were main effects of time for CMJ height (F (4,80) = 9.538; P < 0.001), with post hoc tests demonstrating differences between baseline and all subsequent time points (P < 0.001), apart from 72 h, where CMJ height returned to baseline (P = 1.000). There was a time \( \times \) group interaction (F (8,80) = 2.734; P = 0.05), with post hoc tests identifying differences between the leucine + glutamine and the placebo group post-exercise (99.6 ± 5.6% vs. 93.6 ± 2.3%; P = 0.007; d = 1.47), at 48 h (99.5 ± 7.6% vs. 90.3 ± 5.1%; P = 0.008; d = 0.87) and at 72 h (104.6 ± 11.0% vs. 94.7 ± 6.2%; P = 0.019; d = 1.17). There were no pairwise differences (P > 0.05) between the leucine and placebo group for CMJ height.
Changes in DOMS (% baseline) are presented in Fig. 3. There were main effects of time for DOMS (F (4,80) = 84.114; P < 0.001), with post hoc tests demonstrating differences between baseline and all subsequent time points, including 72 h (P < 0.001). There was no time \( \times \) group interaction (F(8,80) = 1.473; P = 0.181), but effect size estimates demonstrated large differences between the leucine + glutamine and placebo groups (d = 1.31 and d = 1.40) and leucine and placebo groups (d = 1.21 and d = 1.38) at 24 and 48 h, respectively.
Changes in CK (% baseline) are presented in Fig. 4. There were main effects of time for CK (F (4,80) = 4.616; P = 0.009), with post hoc tests demonstrating differences between baseline and 24 h (P < 0.001). There were interactions between group and time (F (4,80) = 2.319; P = 0.046), with post hoc tests revealing differences between the leucine + glutamine and the placebo group at 24 h (437.6 ± 86.4% vs. 501.6 ± 161.8%; P = 0.012; d = 0.54) and 48 h (171.2 ± 31.7% vs. 281.3 ± 122.0%; P = 0.010; d = 1.37), as well as the leucine and placebo group at 24 h (426.8 ± 89.6% vs. 501.6 ± 161.8%; P = 0.039; d = 0.63) and 48 h (193.5 ± 54.4% vs. 281.3 ± 122.0%; P = 0.022; d = 1.03).
Discussion
All of the participants exhibited signs of muscle damage in this study and, in support of our hypothesis, co-ingestion of leucine and glutamine improved the rate of recovery after eccentrically biased exercise more than placebo and leucine alone. The effects of co-ingested leucine and glutamine were such that all of the functional variables (i.e. isometric strength, CMJ) returned to baseline at the greatest rate. The leucine group also recovered faster than the placebo group, but not by the same magnitude as the co-ingestion group. This was particularly notable for measures of isometric strength and CMJ, which are established measures of the time-course and magnitude of recovery after muscle-damaging exercise (Byrne et al. 2004). The differences between groups were predominantly noted at the 24–48 h period, with the leucine + glutamine group demonstrating ‘moderate–large’ improvements in strength, CMJ, DOMS and CK compared to placebo (Figs. 1, 2, 3, 4). These findings demonstrate a faster return to baseline values and indicate that the combination of a well-known proteinogenic amino acid (leucine), with an anti-inflammatory amino acid (glutamine), confers the greatest effects on recovery.
Acute supplementation of isolated leucine at doses of 22.5 mg/kg (Stock et al. 2010) and 250 mg/kg of body mass (Kirby et al. 2012) has been shown to ameliorate recovery from muscle-damaging exercise. For example, Kirby et al. (2012) reported an improvement in recovery of isometric strength (~ 5%) after muscle damage, using a short-term (beginning 30 min prior 0074o exercise) leucine supplementation regime, similar in timing to the current study. In combination with other BCAAs, leucine has been repeatedly shown to increase the rate of recovery from muscle-damaging exercise (Howatson et al. 2012; Jackman et al. 2010; Matsumoto et al. 2009; Waldron et al. 2017). While some have reported no change in muscle damage markers following BCAA supplementation (Kephart et al. 2016; Ra et al. 2013), this could be related to the relatively small doses (~ 3–5 g) provided compared to other studies (15–20 g; Waldron et al. 2017; Howatson et al. 2012). Whilst there are putative roles for all BCAAs in muscle protein synthesis (Blomstrand et al. 2006), leucine is known to confer the most potent anabolic signalling effects, whereas isoleucine and valine have negligible contributions (Atherton et al. 2010). This is most likely worsened by the reported competition between leucine, isoleucine and valine for cellular transport, following co-ingestion (Cynober 2002). Leucine is also known to inhibit muscle proteolysis, thus maintaining muscle protein balance (Baptista et al. 2010). Since both of the current supplements improved the recovery from eccentric exercise and each contained leucine, the role of leucine in reducing symptoms of muscle damage are apparent and support that of other studies (Kirby et al. 2012; Stock et al. 2010). Furthermore, the magnitude of change in isometric force production was similar, or greater, than previously reported with BCAA supplementation (Howatson et al. 2012; Waldron et al. 2017), providing further indirect support for the ergogenic effects of isolated leucine, relative to co-ingestion.
Glutamine can be classified as an anabolic and immunostimulatory AA, owing to its participation in myogenic signalling pathways and role as a substrate for leukocytes, respectively (Gleeson 2008). Oral glutamine supplementation (0.3 g/kg body mass) reduces strength loss following an acute bout of eccentrically biased exercise (Legault et al. 2014; Street et al. 2011), which was attributed to both its anti-inflammatory role and involvement with protein synthesis pathways. Indeed, both glutamine and leucine possess anti-inflammatory properties. For example, Cruzat et al. (2010) supplemented rats with 1.5 g/kg of glutamine for 3 weeks, reporting lower post-exercise concentrations of pro-inflammatory cytokines. Administration of leucine-rich AA has also been shown to reduce the appearance of inflammatory cytokines, whilst increasing muscle protein synthesis after both eccentric exercise in rodents (Kato et al. 2016) and endurance exercise in athletes (Rowlands et al. 2016). Rowlands et al. (2016) provided 15 g of leucine to athletes as part of a balanced macronutrient recovery meal. The authors demonstrated decreased leukocyte migration and connective tissue development, indicating the acute anti-inflammatory and proteinogenic properties of leucine-rich supplementation. These processes provide a logical explanation for the descriptive reductions in DOMS herein (ES = large), as muscle soreness is partly related to local inflammation, whereby local swelling acts to sensitise nociceptors located in the muscle (Proske and Morgan 2001). Therefore, whilst inflammation is a necessary part of the recovery process that follows acute mechanical damage of the myofibres (Howatson and van Someren 2008), its reduction could reduce the perceived limb soreness of athletes and accelerate their recovery from eccentric exercise.
Given that the co-ingestion of glutamine and leucine provided the greatest effect on recovery in this study, it is necessary to provide some speculation on their potential interaction in vivo. Leucine is an essential nitrogen donor in the synthesis of glutamine. Once inside the cell, leucine reversibly transaminates to glutamate, particularly during short periods of high-intensity exercise Henriksson (1991) thus contributing to the glutamate–glutamine pool (Aoki et al. 1981; Golden et al. 1982). The influx of leucine into the cell is also dependent on the efflux of glutamine, owing to the integrated transport systems of these AA (Nicastro et al. 2012). Indeed, under certain physiological conditions, it has been shown that glutamine transport into the cell, via its transporter SLC1A5, is rapidly used to facilitate the influx of extracellular leucine via an efflux of glutamine through a bidirectional SLC7A5/SLC3A2 transporter, which can subsequently activate the mammalian target of rapamycin complex (mTOR) complex (Nicklin et al. 2009). Therefore, it is likely that the exogenous supply of glutamine, administered herein, might have provided a greater stimulus for leucine uptake into the cell, as it is known that oral supplementation of glutamine or leucine increases plasma concentrations (Churchward-Venne et al. 2014; Rowlands et al. 2016) and transport of leucine into the cell in the post-absorptive state. The transport of leucine into the muscle cell is necessary prior to its participation in protein synthesis or before contributing to the intracellular glutamine content. Therefore, co-ingesting leucine and glutamine could (i) facilitate transport of leucine into the cell and (ii) contribute to the glutamine–glutamate pool, thereby (iii) sparing free leucine and increasing its availability.
The current study is limited by the number of experimental groups that were included. It is possible that the effects we have observed are related to the higher energy or amino acid content of the leucine + glutamine group, rather than the specific combination of amino acids. Similarly, the placebo group did not ingest any additional amino acids outside of their normal diet. We opted to investigate a fixed dose of leucine, rather than an iso-caloric dose, to establish whether the effects of the isolated leucine dose could be enhanced. This dose provided an average ~ 15/day of leucine in the current participants, which was deemed to be suitable, given that 5 g of leucine has been considered as ‘high’ and sufficient to increase muscle protein synthesis above higher doses of whey protein supplements (Churchward-Venne et al. 2014). Nevertheless, our results show that recovery from eccentric exercise, facilitated by acute doses of leucine, can be improved by adding glutamine or additional AA to the ingested supplement. Future research should consider adding additional energy- or AA-matched groups to the current research design to establish this.
Conclusion
Acute oral supplementation of leucine (0.087 g/kg) or leucine + glutamine (0.087 g/kg + 0.3 g/kg) increased the rate of recovery in isometric strength, CMJ height, DOMS and CK compared to placebo after eccentrically biased exercise. Based on a 100 kg athlete supplementing twice daily, 17.4 g of leucine, plus 30 g of glutamine would be necessary to accelerate recovery. However, further studies are required to understand whether the provision of an iso-caloric or iso-amino acid supplement would achieve the same effect.
References
Aoki TT, Brennan MF, Fitzpatrick GF, Knight DC (1981) Leucine meal increases glutamine and total nitrogen release from forearm muscle. J Clin Invest 68:1522–1528
Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ (2010) Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids 38:1533–1539
Baptista IL, Leal ML, Artioli GG, Fiamoncini J, Turri AO et al (2010) Leucine attenuates skeletal muscle wasting via inhibition of ubiquitin ligases. Muscle Nerve 41:800–808
Batterham AM, Hopkins WG (2006) Making meaningful inferences about magnitudes. Int J Sports Physiol Perform 1:50–57
Blomstrand E, Eliasson J, Karlsson HKR, Kohnke R (2006) Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J Nutr 136:269–273
Børsheim E, Tipton KD, Wolf SE, Wolfe RR (2002) Essential amino acids and muscle protein recovery from resistance exercise. Am J Phys Endocrinol Metab 283:648–657
Byrne C, Twist C, Eston R (2004) Neuromuscular function after exercise-induced muscle damage: theoretical and applied implications. Sports Med 34:49–69
Cheung K, Hume P, Maxwell L (2003) Delayed onset muscle soreness: treatment strategies and performance factors. Sports Med 33:145–164
Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR et al (2014) Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr 99:276–286
Cruzat VF, Rogero MM, Tirapegui J. (2010). Effects of supplementation with free glutamine and the dipeptide alanyl-glutamine on parameters of muscle damage and inflammation in rats submitted to prolonged exercise. Cell Biochem Funct 28: 24–30
Cynober LA (2002) Plasma amino acid levels with a note on membrane transport: characteristics, regulation, and metabolic significance. Nutrition 18:761–766
da Luz CR, Nicastro H, Zanchi NE, Chaves DFS, Lancha AH (2011) Potential therapeutic effects of branched-chain amino acids supplementation on resistance exercise-based muscle damage in humans. J Int Soc Sports Nutr 8:23
Gissel H, Clausen T (2001) Excitation-induced Ca2+ influx and skeletal muscle cell damage. Acta Physiol Scand 171:327–334
Gleeson M (2008) Dosing and efficacy of glutamine supplementation in human exercise and sport training. J Nutr 138:2045–2049
Golden MHN, Jahoor P, Jackson AA (1982) Glutamine production rate and its contribution to urinary ammonia in normal man. Clin Sci 65:299–305
Henriksson J (1991) Effect of exercise on amino acid concentrations in skeletal muscle and plasma. J Exp Biol 160:149–165
Howatson G, van Someren KA (2008) The prevention and treatment of exercise-induced muscle damage. Sport Med 38:483–503
Howatson G, Hoad M, Goodall S, Tallent J, Bell PG, French DN (2012) Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: a randomized, double-blind, placebo controlled study. J Int Soc Sports Nutr 9:2. https://doi.org/10.1186/1550-2783-9-20
Jackman SR, Witard OC, Jeukendrup AE, Tipton KD (2010) Branched-chain amino acid ingestion can ameliorate soreness from eccentric exercise. Med Sci Sports Exerc 42:962–970
Kato H, Miura K, Nakano S, Suzuki K, Bannai M, Inoue Y (2016) Leucine-enriched essential amino acids attenuate inflammation in rat muscle and enhance muscle repair after eccentric contraction. Amino Acids 48:2145–2155
Kephart WC, Mumford PW, McCloskey AE et al (2016) Post-exercise branched chain amino acid supplementation does not affect recovery markers following three consecutive high intensity resistance training bouts compared to carbohydrate supplementation. J Int Soc Sports Nutr 13:30. https://doi.org/10.1186/s12970-016-0142-y
Kirby TJ, Triplett TN, Haines TL, Skinner JW, Fairbrother KR, McBride JM (2012) Effect of leucine supplementation on indices of muscle damage following drop jumps and resistance exercise. Amino Acids 42:1987–1996
Legault Z, Bagnall N, Kimmerly DS (2014) The influence of oral l-glutamine supplementation on muscle strength recovery and soreness following unilateral knee extension eccentric exercise. Int J Sport Nutr Exerc Metab 25:417–426
Lieber RL, Friden J (1999) Mechanisms of muscle injury after eccentric contraction. J Sci Med Sport 2:253–265
Matsumoto K, Koba T, Hamada K, Sakurai M, Higuchi T, Miyata H (2009) Branched-chain amino acid supplementation attenuates muscle soreness, muscle damage and inflammation during an intensive training program. J Sports Med Phys Fitness 49:424–31
McGlory C, Devries MC, Phillips SM (2017) Skeletal muscle and resistance exercise training; the role of protein synthesis in recovery and remodeling. J Appl Phys 122:541–548
Nicastro H, Ribeiro da Luz C, Chaves D, Bechara LRG, Voltarelli VA, Rogero M et al (2012) Does branched-chain amino acids supplementation modulate skeletal muscle remodeling through inflammation modulation? possible mechanisms of action. J Nutr Metab. https://doi.org/10.1155/2012/136937
Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B et al (2009) Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136:521–534
Proske U, Morgan DL (2001) Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol 537:333–345
Ra S-G, Miyazaki T, Ishikura K et al (2013) Combined effect of branched-chain amino acids and taurine supplementation on delayed onset muscle soreness and muscle damage in high-intensity eccentric exercise. Int Soc Sports Nutr 10:51. https://doi.org/10.1186/1550-2783-10-51
Rowlands DS, Nelson AR, Raymond F, Metairon S, Mansourian R, Clarke J et al (2016) Protein-leucine ingestion activates a regenerative inflammo-myogenic transcriptome in skeletal muscle following intense endurance exercise. Phys Genomics 48:21–32
Schoenfeld BJ (2010) The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res 24:2857–2872
Schoenfeld BJ (2012) Does exercise-induced muscle damage play a role in skeletal muscle hypertrophy? J Strength Cond Res 26:1441–1453
Shepstone TN, Tang JE, Dallaire S, Schuenke MD, Staron RS, Philips SM (2005) Short-term high- vs. low-velocity isokinetic lengthening training results in greater hypertrophy of the elbow flexors in young men. J Appl Physiol 98:1768–1776
Shimomura Y, Kobayashi H, Mawatari K, Akita K, Inaguma A, Watanabe S et al (2009) Effects of squat exercise and branched-chain amino acid supplementation on plasma free amino acid concentrations in young women. J Nutr Sci Vitaminol 55:288–291
Sorichter S, Puschendorf B, Mair J (1999) Skeletal muscle injury induced by eccentric muscle action: muscle proteins as markers of muscle fiber injury. Exerc Immunol Rev 5:5–21
Stock MS, Young JC, Golding LA, Kruskall LJ, Tandy RD, Conway-Klaassen JM, Beck TW (2010) The effects of adding leucine to pre- and post-exercise carbohydrate beverages on acute muscle recovery from resistance training. J Strength Cond Res 24:2211–2219
Street B, Byrne C, Eston R (2011) Glutamine supplementation in recovery from eccentric muscle damaging exercise attenuates strength loss and muscle soreness. J Exerc Sci Fit 9:116–122
Twist C, Gleeson N, Eston R (2008) The effects of plyometric exercise on unilateral balance performance. J Sports Sci 26:1073–1080
Waldron M, Whelan K, Jeffries O, Burt D, Howe L, Patterson SD (2017) The effects of acute branched-chain amino acid supplementation on recovery from a single bout of hypertrophy exercise in resistance-trained athletes. Appl Physiol Nutr Metab 42:630–636
Zanchi NE, Nicastro H, Lancha AH (2008) Potential antiproteolytic effects of l-leucine: observations of in vitro and in vivo studies. Nutr Metab (Lond) 5:20. https://doi.org/10.1186/1743-7075-5-20
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study
Additional information
Handling Editor: E. Rawson.
Rights and permissions
About this article
Cite this article
Waldron, M., Ralph, C., Jeffries, O. et al. The effects of acute leucine or leucine–glutamine co-ingestion on recovery from eccentrically biased exercise. Amino Acids 50, 831–839 (2018). https://doi.org/10.1007/s00726-018-2565-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-018-2565-z